Effect of Spherical Adsorptive Carbon Among Chronic Kidney Disease Patients: A Nationwide Cohort Study
Abstract
1. Introduction
2. Materials and Methods
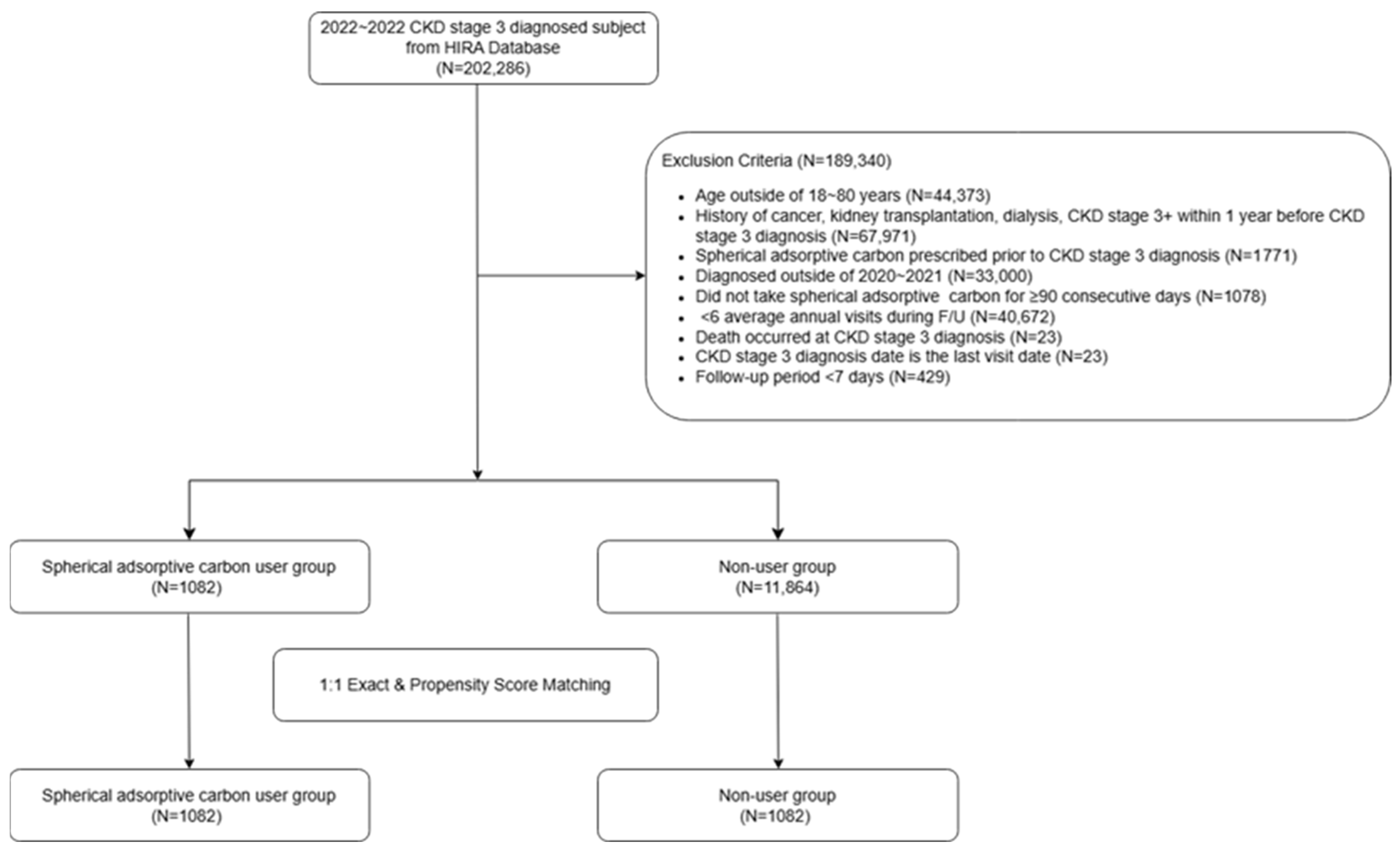
2.1. Study Population
2.2. Propensity Score Matching
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Baseline Characteristics
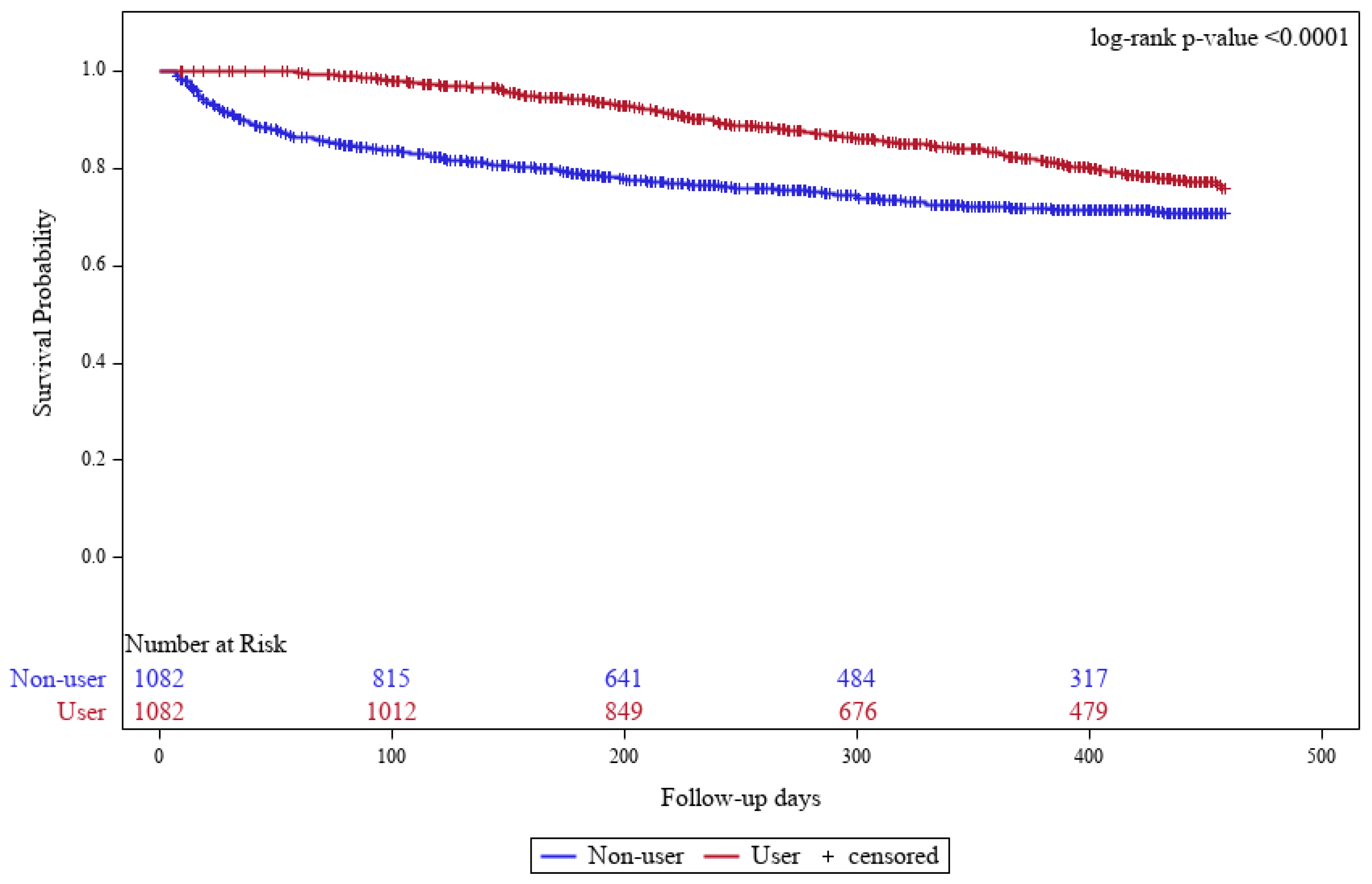
3.2. Comparison of Clinical Outcomes Between the SAC and Non-User Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease–a systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef]
- Murphy, D.; McCulloch, C.E.; Lin, F.; Banerjee, T.; Bragg-Gresham, J.L.; Eberhardt, M.S.; Morgenstern, H.; Pavkov, M.E.; Saran, R.; Powe, N.R.; et al. Trends in prevalence of chronic kidney disease in the United States. Ann. Intern. Med. 2016, 165, 473–481. [Google Scholar] [CrossRef]
- Lee, M.-J.; Ha, K.H.; Kim, D.J.; Park, I. Trends in the Incidence, Prevalence, and Mortality of End-Stage Kidney Disease in South Korea. Diabetes Metab. J. 2020, 44, 933–937. [Google Scholar] [CrossRef]
- Sarafidis, P.; Ferro, C.J.; Morales, E.; Ortiz, A.; Malyszko, J.; Hojs, R.; Khazim, K.; Ekart, R.; Valdivielso, J.; Fouque, D.; et al. SGLT-2 inhibitors and GLP-1 receptor agonists for nephroprotection and cardioprotection in patients with diabetes mellitus and chronic kidney disease. A consensus statement by the EURECA-m and the DIABESITY working groups of the ERA-EDTA. Nephrol. Dial. Transplant. 2020, 35, 1825. [Google Scholar] [CrossRef] [PubMed]
- Tarun, T.; Ghanta, S.N.; Ong, V.; Kore, R.; Menon, L.; Kovesdy, C.; Mehta, J.L.; Jain, N. Updates on New Therapies for Patients with CKD. Kidney Int. Rep. 2024, 9, 16–28. [Google Scholar] [CrossRef]
- Asai, M.; Kumakura, S.; Kikuchi, M. Review of the efficacy of AST-120 (KREMEZIN®) on renal function in chronic kidney disease patients. Ren. Fail. 2019, 41, 47–56. [Google Scholar] [CrossRef]
- Niwa, T.; Nomura, T.; Sugiyama, S.; Miyazaki, T.; Tsukushi, S.; Tsutsui, S. The protein metabolite hypothesis, a model for the progression of renal failure: An oral adsorbent lowers indoxyl sulfate levels in undialyzed uremic patients. Kidney Int. Suppl. 1997, 62, S23–S28. [Google Scholar] [PubMed]
- Toyoda, S.; Kikuchi, M.; Komatsu, T.; Hori, Y.; Nakahara, S.; Kobayashi, S.; Sakai, Y.; Inoue, T.; Taguchi, I. Impact of the oral adsorbent AST-120 on oxidative stress and uremic toxins in high-risk chronic kidney disease patients. Int. J. Cardiol. 2014, 177, 705–707. [Google Scholar] [CrossRef] [PubMed]
- Niwa, T.; Ise, M.; Miyazaki, T. Progression of glomerular sclerosis in experimental uremic rats by administration of indole, a precursor of indoxyl sulfate. Am. J. Nephrol. 1994, 14, 207–212. [Google Scholar] [CrossRef]
- Schulman, G.; Agarwal, R.; Acharya, M.; Berl, T.; Blumenthal, S.; Kopyt, N. A multicenter, randomized, double-blind, placebo-controlled, dose-ranging study of AST-120 (Kremezin) in patients with moderate to severe CKD. Am. J. Kidney Dis. 2006, 47, 565–577. [Google Scholar] [CrossRef]
- Lee, C.-T.; Hsu, C.-Y.; Tain, Y.-L.; Ng, H.-Y.; Cheng, B.-C.; Yang, C.-C.; Wu, C.-H.; Chiou, T.T.-Y.; Lee, Y.-T.; Liao, S.-C. Effects of AST-120 on blood concentrations of protein-bound uremic toxins and biomarkers of cardiovascular risk in chronic dialysis patients. Blood Purif. 2014, 37, 76–83. [Google Scholar] [CrossRef]
- Nakamura, T.; Sato, E.; Fujiwara, N.; Kawagoe, Y.; Suzuki, T.; Ueda, Y.; Yamagishi, S.-I. Oral adsorbent AST-120 ameliorates tubular injury in chronic renal failure patients by reducing proteinuria and oxidative stress generation. Metabolism 2011, 60, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Cha, R.-H.; Kang, S.W.; Park, C.W.; Cha, D.R.; Na, K.Y.; Kim, S.G.; Yoon, S.A.; Han, S.Y.; Chang, J.H.; Park, S.K.; et al. A Randomized, Controlled Trial of Oral Intestinal Sorbent AST-120 on Renal Function Deterioration in Patients with Advanced Renal Dysfunction. Clin. J. Am. Soc. Nephrol. 2016, 11, 559–567. [Google Scholar] [CrossRef]
- Kim, L.; Kim, J.-A.; Kim, S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health 2014, 36, e2014008. [Google Scholar] [CrossRef] [PubMed]
- Okada, K.; Takahashi, S.; Nagura, Y.; Hatano, M.; Shimamura, T. Early morphological changes of tubules in rats with chronic renal failure. Nihon Jinzo Gakkai Shi 1992, 34, 65–70. [Google Scholar] [PubMed]
- Owada, S.; Goto, S.; Bannai, K.; Hayashi, H.; Nishijima, F.; Niwa, T. Indoxyl sulfate reduces superoxide scavenging activity in the kidneys of normal and uremic rats. Am. J. Nephrol. 2008, 28, 446–454. [Google Scholar] [CrossRef]
- Gelasco, A.K.; Raymond, J.R. Indoxyl sulfate induces complex redox alterations in mesangial cells. Am. J. Physiol. Renal. Physiol. 2006, 290, F1551–F1558. [Google Scholar] [CrossRef]
- Motojima, M.; Hosokawa, A.; Yamato, H.; Muraki, T.; Yoshioka, T. Uremic toxins of organic anions up-regulate PAI-1 expression by induction of NF-kappaB and free radical in proximal tubular cells. Kidney Int. 2003, 63, 1671–1680. [Google Scholar] [CrossRef]
- Shimizu, H.; Bolati, D.; Adijiang, A.; Adelibieke, Y.; Muteliefu, G.; Enomoto, A.; Higashiyama, Y.; Higuchi, Y.; Nishijima, F.; Niwa, T. Indoxyl sulfate downregulates renal expression of Klotho through production of ROS and activation of nuclear factor-kB. Am. J. Nephrol. 2011, 33, 319–324. [Google Scholar] [CrossRef]
- Niwa, T.; Tsukushi, S.; Ise, M.; Miyazaki, T.; Tsubakihara, Y.; Owada, A.; Shiigai, T. Indoxyl sulfate and progression of renal failure: Effects of a low-protein diet and oral sorbent on indoxyl sulfate production in uremic rats and undialyzed uremic patients. Miner. Electrolyte Metab. 1997, 23, 179–184. [Google Scholar]
- Ueda, S.; Yamagishi, S.-I.; Takeuchi, M.; Kohno, K.; Shibata, R.; Matsumoto, Y.; Kaneyuki, U.; Fujimura, T.; Hayashida, A.; Okuda, S. Oral adsorbent AST-120 decreases serum levels of AGEs in patients with chronic renal failure. Mol. Med. 2006, 12, 180–184. [Google Scholar] [CrossRef]
- Niwa, T.; Yazawa, T.; Ise, M.; Sugano, M.; Kodama, T.; Uehara, Y.; Maeda, K. Inhibitory effect of oral sorbent on accumulation of albumin-bound indoxyl sulfate in serum of experimental uremic rats. Nephron 1991, 57, 84–88. [Google Scholar] [CrossRef]
- Okada, K.; Shibahara, H.; Takahashi, S. Effect of oral adsorbent on the progression of diabetic nephropathy. Nephron 1997, 76, 489–490. [Google Scholar] [CrossRef]
- Kobayashi, N.; Maeda, A.; Horikoshi, S.; Shirato, I.; Tomino, Y.; Ise, M. Effects of oral adsorbent AST-120 (Kremezin) on renal function and glomerular injury in early-stage renal failure of subtotal nephrectomized rats. Nephron 2002, 91, 480–485. [Google Scholar] [CrossRef]
- Owada, A.; Nakao, M.; Koike, J.; Ujiie, K.; Tomita, K.; Shiigai, T. Effects of oral adsorbent AST-120 on the progression of chronic renal failure: A randomized controlled study. Kidney Int. Suppl. 1997, 63, S188–S190. [Google Scholar]
- Fujii, H.; Nishijima, F.; Goto, S.; Sugano, M.; Yamato, H.; Kitazawa, R.; Kitazawa, S.; Fukagawa, M. Oral charcoal adsorbent (AST-120) prevents progression of cardiac damage in chronic kidney disease through suppression of oxidative stress. Nephrol. Dial. Transplant. 2009, 24, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Higuchi, Y.; Yagi, Y.; Nishijima, F.; Yamato, H.; Ishii, H.; Osaka, M.; Yoshida, M. Reduction of indoxyl sulfate by AST-120 attenuates monocyte inflammation related to chronic kidney disease. J. Leukoc. Biol. 2013, 93, 837–845. [Google Scholar] [CrossRef]
- Nakamura, T.; Kawagoe, Y.; Matsuda, T.; Ueda, Y.; Shimada, N.; Ebihara, I.; Koide, H. Oral ADSORBENT AST-120 decreases carotid intima-media thickness and arterial stiffness in patients with chronic renal failure. Kidney Blood Press. Res. 2004, 27, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Sato, E.; Tanaka, A.; Oyama, J.-I.; Yamasaki, A.; Shimomura, M.; Hiwatashi, A.; Ueda, Y.; Amaha, M.; Nomura, M.; Matsumura, D.; et al. Long-term effects of AST-120 on the progression and prognosis of pre-dialysis chronic kidney disease: A 5-year retrospective study. Hear. Vessel. 2016, 31, 1625–1632. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Liu, W.; Tsai, S.-F. Effects of AST-120 on mortality in patients with chronic kidney disease modeled by artificial intelligence or traditional statistical analysis. Sci. Rep. 2024, 14, 738. [Google Scholar] [CrossRef]
- Ueda, H.; Shibahara, N.; Takagi, S.; Inoue, T.; Katsuoka, Y. AST-120 treatment in pre-dialysis period affects the prognosis in patients on hemodialysis. Ren. Fail. 2008, 30, 856–860. [Google Scholar] [CrossRef]
- Hatakeyama, S.; Yamamoto, H.; Okamoto, A.; Imanishi, K.; Tokui, N.; Okamoto, T.; Suzuki, Y.; Sugiyama, N.; Imai, A.; Kudo, S.; et al. Effect of an oral adsorbent, AST-120, on dialysis initiation and survival in patients with chronic kidney disease. Int. J. Nephrol. 2012, 2012, 376128. [Google Scholar] [CrossRef]
- Akizawa, T.; Asano, Y.; Morita, S.; Wakita, T.; Onishi, Y.; Fukuhara, S.; Gejyo, F.; Matsuo, S.; Yorioka, N.; Kurokawa, K. Effect of a carbonaceous oral adsorbent on the progression of CKD: A multicenter, randomized, controlled trial. Am. J. Kidney Dis. 2009, 54, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Schulman, G.; Berl, T.; Beck, G.J.; Remuzzi, G.; Ritz, E.; Arita, K.; Kato, A.; Shimizu, M. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. J. Am. Soc. Nephrol. 2015, 26, 1732–1746. [Google Scholar] [CrossRef] [PubMed]
- Tomino, Y.; Hisada-Urita, A.; Seki, T.; Watanabe, T.; Kanda, R.; Takahashi, T. Importance of AST-120 (Kremezin®) Adherence in a Chronic Kidney Disease Patient with Diabetes. Case Rep. Nephrol. Dial. 2018, 8, 107–111. [Google Scholar] [CrossRef] [PubMed]
| Before Matching (N = 12,946) | After Matching (N = 2164) | |||||
|---|---|---|---|---|---|---|
| SAC Group | Non-User Group | SMD | SAC Group | Non-User Group | SMD | |
| (N = 1082) | (N = 11,864) | (N = 1082) | (N = 1082) | |||
| Quarter of CKD stage 3 | 0.085 | 0.000 | ||||
| 202001 | 103 (9.52) | 1100 (9.27) | 103 (9.52) | 103 (9.52) | ||
| 202002 | 124 (11.46) | 1231 (10.38) | 124 (11.46) | 124 (11.46) | ||
| 202003 | 132 (12.20) | 1401 (11.81) | 132 (12.20) | 132 (12.20) | ||
| 202004 | 113 (10.44) | 1406 (11.85) | 113 (10.44) | 113 (10.44) | ||
| 202101 | 116 (10.72) | 1483 (12.50) | 116 (10.72) | 116 (10.72) | ||
| 202102 | 156 (14.42) | 1556 (13.12) | 156 (14.42) | 156 (14.42) | ||
| 202103 | 152 (14.05) | 1613 (13.60) | 152 (14.05) | 152 (14.05) | ||
| 202104 | 186 (17.19) | 2074 (17.48) | 186 (17.19) | 186 (17.19) | ||
| Duration from CKD stage 3 to index date | 0.810 | 0.000 | ||||
| Months | 2.98 (3.90) | 6.38 (4.47) | 2.98 (3.90) | 2.98 (3.90) | ||
| Year of index date | 0.462 | 0.000 | ||||
| 2020 | 335 (30.96) | 2221 (18.72) | 335 (30.96) | 335 (30.96) | ||
| 2021 | 579 (53.51) | 5704 (48.08) | 579 (53.51) | 579 (53.51) | ||
| 2022 | 167 (15.43) | 3769 (31.77) | 167 (15.43) | 167 (15.43) | ||
| 2023 | 1 (0.09) | 170 (1.43) | 1 (0.09) | 1 (0.09) | ||
| Age, years | 63.09 (12.23) | 65.85 (11.80) | 0.229 | 63.09 (12.23) | 64.11 (12.05) | 0.084 |
| Sex, male (%) | 827 (76.43) | 7595 (64.02) | 0.274 | 827 (76.43) | 834 (77.08) | 0.015 |
| Prior medication, n (%) | ||||||
| NSAID | 926 (85.58) | 10,312 (86.92) | 0.039 | 926 (85.58) | 951 (87.89) | 0.068 |
| Steroid | 733 (67.74) | 8204 (69.15) | 0.030 | 733 (67.74) | 740 (68.39) | 0.014 |
| ACE Inhibitor or ARBs | 825 (76.25) | 8398 (70.79) | 0.124 | 825 (76.25) | 840 (77.63) | 0.033 |
| Cyclosporine or Tacrolimus | 36 (3.33) | 363 (3.06) | 0.015 | 36 (3.33) | 31 (2.87) | 0.027 |
| SGLT2 Inhibitor | 227 (20.98) | 2313 (19.5) | 0.037 | 227 (20.98) | 230 (21.26) | 0.007 |
| CCI Group, n (%) | 0.073 | |||||
| 0~2 | 310 (28.65) | 3756 (31.66) | 310 (28.65) | 279 (25.79) | ||
| 3~5 | 492 (45.47) | 5129 (43.23) | 492 (45.47) | 496 (45.84) | ||
| 6~8 | 234 (21.63) | 2441 (20.57) | 234 (21.63) | 258 (23.84) | ||
| ≥9 | 46 (4.25) | 538 (4.53) | 46 (4.25) | 49 (4.53) | ||
| Other comorbidity, n (%) | ||||||
| Hypertension | 1043 (96.40) | 11,060 (93.22) | 0.143 | 1043 (96.40) | 1048 (96.86) | 0.026 |
| Diabetes mellitus | 950 (87.80) | 9573 (80.59) | 0.196 | 950 (87.80) | 973 (89.93) | 0.068 |
| Dyslipidemia | 1040 (96.12) | 11,164 (94.1) | 0.094 | 1040 (96.12) | 1048 (96.86) | 0.040 |
| SAC User Group (N = 1082) | Non-User Group (N = 1082) | p-Value | |
|---|---|---|---|
| Events, No. (%) | 153 (14.14) | 115 (10.63) | |
| Time to events, days | |||
| Mean(SD) | 246.8 (106.0) | 118.6 (109.2) | <0.0001 * |
| Q1, Q3 | 98, 388 | 18, 118 | |
| Incidence rate, per 100 PY | 15.75 | 15.35 | |
| Crude HR (95% CI) | 0.37 (0.29–0.48) | Ref. | <0.0001 ** |
| SAC User Group | Non-User Group | Crude HR (95% CI) | p-Value | |
|---|---|---|---|---|
| (N = 1082) | (N = 1082) | |||
| Dialysis | ||||
| Events, No.(%) | 5 (2.65) | 8 (2.96) | ||
| Incidence rate, per 100 PY | 0.51 | 1.07 | 0.83 (0.27–2.59) | 0.7519 |
| Death | ||||
| Events, No.(%) | 31 (16.4) | 147 (54.44) | ||
| Incidence rate, per 100 PY | 3.19 | 19.62 | 0.32 (0.21–0.48) | <0.0001 |
| SAC Prescription Days | HR * (95% CI) | p-Value |
|---|---|---|
| 90–179 days | 1.50 (0.75–3.43) | 0.2237 |
| 180–364 days | 0.36 (0.11–1.23) | 0.1035 |
| 365 days | 0.25 (0.08–0.87) | 0.0285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, D.H.; Park, K.; Yang, J.W.; Lee, J.Y. Effect of Spherical Adsorptive Carbon Among Chronic Kidney Disease Patients: A Nationwide Cohort Study. Int. J. Environ. Res. Public Health 2025, 22, 1365. https://doi.org/10.3390/ijerph22091365
Shin DH, Park K, Yang JW, Lee JY. Effect of Spherical Adsorptive Carbon Among Chronic Kidney Disease Patients: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health. 2025; 22(9):1365. https://doi.org/10.3390/ijerph22091365
Chicago/Turabian StyleShin, Dong Hui, Keunryul Park, Jae Won Yang, and Jun Young Lee. 2025. "Effect of Spherical Adsorptive Carbon Among Chronic Kidney Disease Patients: A Nationwide Cohort Study" International Journal of Environmental Research and Public Health 22, no. 9: 1365. https://doi.org/10.3390/ijerph22091365
APA StyleShin, D. H., Park, K., Yang, J. W., & Lee, J. Y. (2025). Effect of Spherical Adsorptive Carbon Among Chronic Kidney Disease Patients: A Nationwide Cohort Study. International Journal of Environmental Research and Public Health, 22(9), 1365. https://doi.org/10.3390/ijerph22091365






