Air Pollution and Respiratory System Responses in Healthy Adults Engaging in Outdoor Physical Exercise in Urban Environments: A Scoping Review
Abstract
1. Introduction
2. Methodology
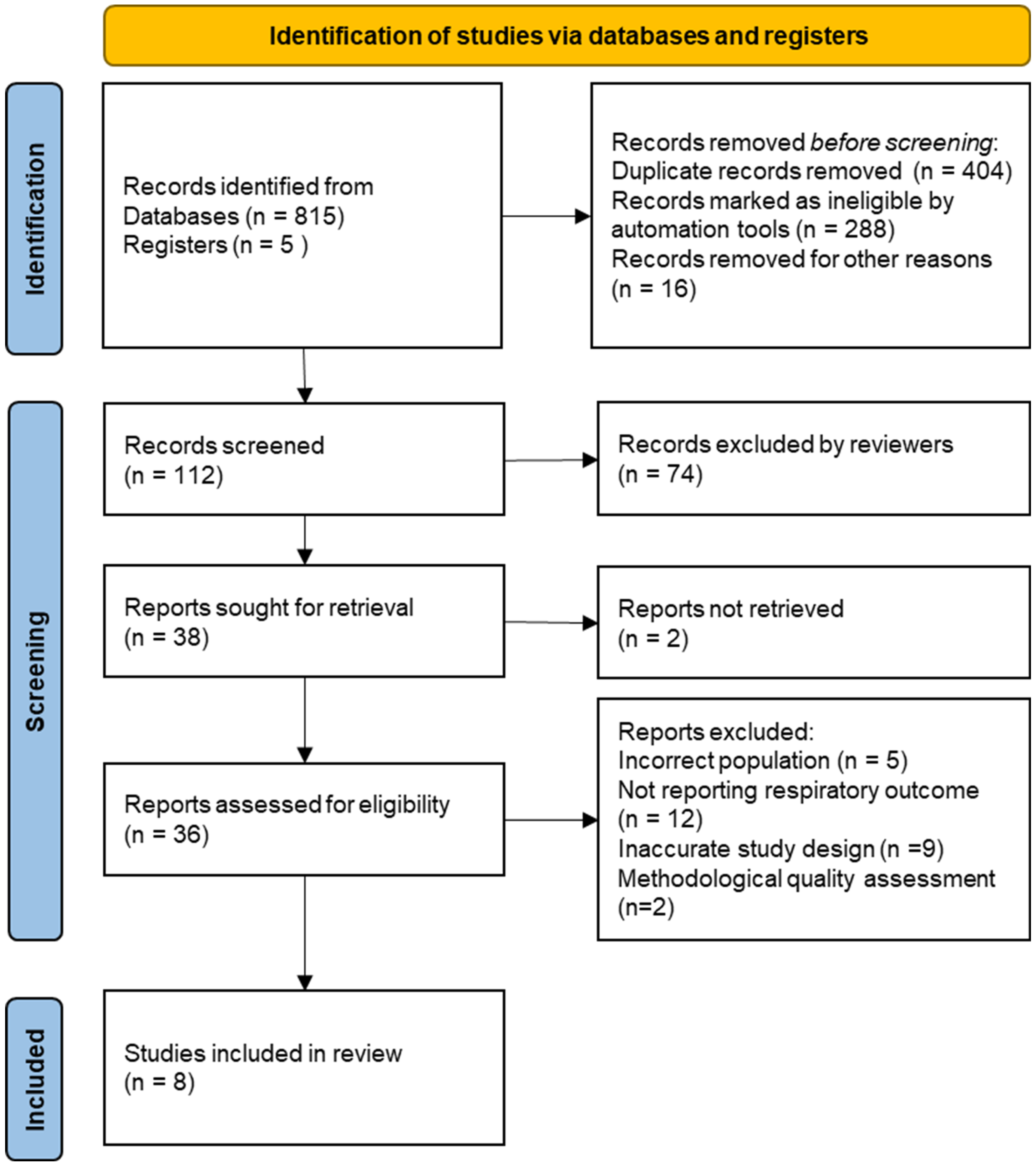
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oyarzún, G.; Valdivia, M.; Gonzalo, C. Impactos en la salud de la contaminación del aire. Rev. Chil. Enferm. Respir. 2021, 37, 103–106. [Google Scholar] [CrossRef]
- Wu, P.; Guo, Q.; Zhao, Y.; Bian, M.; Cao, S.; Zhang, J.J.; Duan, X. Emerging concern on air pollution and health: Trade-off between air pollution exposure and physical activity. Eco Environ. Health 2024, 3, 202–207. [Google Scholar] [CrossRef] [PubMed]
- López, I.L.M.; Veloz, M.F.V.; López, G.A.C.; Ipiales, D.S.R.; Barceló, N.M. Afecciones respiratorias y contaminación ambiental en Riobamba, Ecuador. Correo Científico Médico Holguín 2020, 24, 117–132. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1560-43812020000100117&lng=es (accessed on 9 April 2024).
- Romero Placeres, M.; Diego Olite, F.; Álvarez Toste, M. La contaminación del aire: Su repercusión como problema de salud. Rev. Cuba. Hig. Epidemiol. 2006, 44, 1–14. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-30032006000200008&lng=es (accessed on 14 May 2024).
- Huang, X. The impact of PM10 and other airborne particulate matter on the cardiopulmonary and respiratory systems of sports personnel under atmospheric exposure. Atmosphere 2023, 14, 1697. [Google Scholar] [CrossRef]
- Hvidtfeldt, U.A.; Severi, G.; Andersen, Z.J.; Atkinson, R.; Bauwelinck, M.; Bellander, T.; Brunekreef, B.; Strak, M.; Raaschou-Nielsen, O. Long-term low-level ambient air pollution exposure and risk of lung cancer: A pooled analysis of seven European cohorts. Environ Int. 2020, 146, 106249. [Google Scholar] [CrossRef]
- Deng, Q.; Ou, C.; Shen, Y.M.; Xiang, Y.; Miao, Y.; Li, Y. Health effects of physical activity as predicted by particle deposition in the human respiratory tract. Sci. Total Environ. 2019, 657, 819–826. [Google Scholar] [CrossRef]
- OMS. La OMS Revela las Principales Causas de Muerte y Discapacidad en el Mundo: 2000–2019; OMS: Geneva, Switzerland, 2020; Available online: https://www.who.int/es/news/item/09-12-2020-who-reveals-leading-causes-of-death-and-disability-worldwide-2000-2019 (accessed on 9 April 2024).
- Cardiovascular Diseases. Available online: https://www.who.int/health-topics/cardiovascular-diseases (accessed on 9 April 2024).
- Marmett, B.; Carvalho, R.B.; Dorneles, G.P.; Nunes, R.B.; Rhoden, C.R. Should I stay or should I go: Can air pollution reduce the health benefits of physical exercise? Med. Hypotheses 2020, 144, 109993. [Google Scholar] [CrossRef] [PubMed]
- Tescione, A.; Misiti, F.; Digennaro, S. Practicing outdoor physical activity: Is it really a good choice? Short- and long-term health effects of exercising in a polluted environment. Sustainability 2022, 14, 15790. [Google Scholar] [CrossRef]
- WHO. Global Action Plan on Physical Activity 2018–2030: More Active People for a Healthier World; WHO: Geneva, Switzerland, 2019; Available online: https://repository.gheli.harvard.edu/repository/12490/ (accessed on 9 April 2024).
- Nowak, A.S.; Kennelley, G.E.; Krabak, B.J.; Roberts, W.O.; Tenforde, K.M.; Tenforde, A.S. Endurance athletes and climate change. J. Clim. Chang. Health 2022, 6, 100118. [Google Scholar] [CrossRef]
- Oravisjärvi, K.; Pietikäinen, M.; Ruuskanen, J.; Rautio, A.; Voutilainen, A.; Keiski, R.L. Effects of physical activity on the deposition of traffic-related particles into the human lungs in silico. Sci. Total Environ. 2011, 409, 4511–4518. [Google Scholar] [CrossRef]
- Guarnieri, M.; Balmes, J.R. Outdoor air pollution and asthma. Lancet 2014, 383, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of air pollution on asthma outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Eguiluz-Gracia, I.; Mathioudakis, A.G.; Bartel, S.; Vijverberg, S.J.H.; Fuertes, E.; Comberiati, P.; Cai, Y.S.; Tomazic, P.V.; Diamant, Z.; Vestbo, J.; et al. The need for clean air: The way air pollution and climate change affect allergic rhinitis and asthma. Allergy 2020, 75, 2170–2184. [Google Scholar] [CrossRef]
- Hansel, N.N.; McCormack, M.C.; Kim, V. The effects of air pollution and temperature on COPD. COPD 2016, 13, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Azanza, D.X.C.; Chango-Sigüenza, M.; Sigüenza, G.; Vanegas, A.; Romero, A.; Ávila, E.; Chango, O. Hallazgos desde la ecocardiografía y su correlación con la capacidad respiratoria en atletas de alto rendimiento entrenados a gran altura. Rev. Ecuat. Cardiol. 2021, 4, 45–54. [Google Scholar]
- Scoping—PRISMA Statement. Available online: https://www.prisma-statement.org/scoping (accessed on 1 May 2024).
- Nih. Available online: https://pubmed.ncbi.nlm.nih.gov/ (accessed on 1 May 2024).
- Redalyc. Available online: https://www.redalyc.org/ (accessed on 1 May 2024).
- Scielo. Available online: https://scielo.org/en (accessed on 1 May 2024).
- Web of Science Group, a Clarivate Company. Web of Science Master Journal List WoS MJL by Clarivate. Clarivate.com. Available online: https://mjl.clarivate.com/search-results (accessed on 1 May 2024).
- Jbi. Available online: https://jbi.global/critical-appraisal-tools (accessed on 1 May 2024).
- Sinharay, R.; Gong, J.; Barratt, B.; Ohman-Strickland, P.; Ernst, S.; Kelly, F.J.; Zhang, J.J.; Collins, P.; Cullinan, P.; Chung, K.F. Respiratory and cardiovascular responses to walking down a traffic-polluted road compared with walking in a traffic-free area in participants aged 60 years and older with chronic lung or heart disease and age-matched healthy controls: A randomised, crossover study. Lancet 2018, 391, 339–349. [Google Scholar] [CrossRef]
- Matt, F.; Cole-Hunter, T.; Donaire-Gonzalez, D.; Kubesch, N.; Martínez, D.; Carrasco-Turigas, G.; Nieuwenhuijsen, M. Acute respiratory response to traffic-related air pollution during physical activity performance. Environ. Int. 2016, 97, 45–55. [Google Scholar] [CrossRef]
- Kocot, K.; Zejda, J.E. Acute cardiorespiratory response to ambient air pollution exposure during short-term physical exercise in young males. Environ. Res. 2021, 195, 110746. [Google Scholar] [CrossRef]
- Pagani, L.G.; Santos, J.M.B.; Foster, R.; Rossi, M.; Luna Junior, L.A.; Katekaru, C.M.; de Sá, M.C.; Jonckheere, A.C.; Almeida, F.M.; Amaral, J.B.; et al. The effect of particulate matter exposure on the inflammatory airway response of street runners and sedentary people. Atmosphere 2019, 11, 43. [Google Scholar] [CrossRef]
- Strak, M.; Boogaard, H.; Meliefste, K.; Oldenwening, M.; Zuurbier, M.; Brunekreef, B.; Hoek, G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2010, 67, 118–124. [Google Scholar] [CrossRef]
- Kocot, K.; Barański, K.; Melaniuk-Wolny, E.; Zajusz-Zubek, E.; Kowalska, M. Exercise under Exposure to Air Pollution and Spirometry in Healthy Adults with and without Allergy. Atmosphere 2021, 12, 1168. [Google Scholar] [CrossRef]
- Kesavachandran, C.N.; Kamal, R.; Bihari, V.; Pathak, M.K.; Singh, A. Particulate matter in ambient air and its association with alterations in lung functions and respiratory health problems among outdoor exercisers in National Capital Region, India. Atmos. Pollut. 2015, 6, 618–625. [Google Scholar] [CrossRef]
- Marmett, B.; Carvalho, R.B.; da Silva, G.N.; Dorneles, G.P.; Romão, P.R.T.; Nunes, R.B.; Rhoden, C.R. The role of O3 exposure and physical activity status on redox state, inflammation, and pulmonary toxicity of young men: A cross-sectional study. Environ. Res. 2023, 231, 116020. [Google Scholar] [CrossRef]
- Madureira, J.; Brancher, E.A.; Costa, C.; de Pinho, R.A.; Teixeira, J.P. Cardio-respiratory health effects of exposure to traffic-related air pollutants while exercising outdoors: A systematic review. Environ. Res. 2019, 178, 108647. [Google Scholar] [CrossRef]
- Andrade, A.; Dominski, F.H.; Vilarino, G.T. Outdoor air quality of environments used for exercise and sports practice: An analysis of scientific production through bibliometric analysis. Appl. Sci. 2021, 11, 4540. [Google Scholar] [CrossRef]
- Ribalta, C.; Garrandes, F.; Bermon, S.; Adami, P.E.; Ibarrola-Ulzurrun, E.; Rivas, I.; Viana, M. Dynamic and stationary monitoring of air pollutant exposures and dose during marathons. Sci. Total Environ. 2024, 927, 171997. [Google Scholar] [CrossRef]
- Chen, Y.; He, K.; Deveci, M.; Coffman, D. Health impacts of bike sharing system—A case study of Shanghai. J. Transp. Health 2023, 30, 101611. [Google Scholar] [CrossRef]
- Ramírez, A.; Sarmiento, O.L.; Duperly, J.; Wai Wong, T.; Rojas, N.; Arango, C.M.; Maldonado, A.; Aristizabal, G.; Pérez, L.; Lobelo, F. Should they play outside? Cardiorespiratory fitness and air pollution among schoolchildren in Bogotá. Rev. Salud Pública 2012, 14, 570–583. [Google Scholar]
- Qin, F.; Xu, M.-X.; Wang, Z.-W.; Han, Z.-N.; Dong, Y.-N.; Zhao, J.-X. Effect of aerobic exercise and different levels of fine particulate matter (PM2.5) on pulmonary response in Wistar rats. Life Sci. 2020, 254, 117355. [Google Scholar] [CrossRef]
- Peng, M.; Zhang, F.; Yuan, Y.; Yang, Z.; Wang, K.; Wang, Y.; Tang, Z.; Zhang, Y. Long-term ozone exposure and all-cause mortality: Cohort evidence in China and global heterogeneity by region. Ecotoxicol. Environ. Saf. 2024, 270, 115843. [Google Scholar] [CrossRef]
- McConnell, R.; Berhane, K.; Gilliland, F.; London, S.J.; Islam, T.; Gauderman, W.J.; Avol, E.; Margolis, H.G.; Peters, J.M. Asthma in exercising children exposed to ozone: A cohort study. Lancet 2002, 359, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yan, M. Association of physical activity and PM2.5-attributable cardiovascular disease mortality in the United States. Front. Public Health 2023, 11, 1224338. [Google Scholar] [CrossRef]
- Cao, M.; Zheng, C.; Zhou, H.; Wang, X.; Chen, Z.; Zhang, L.; Cao, X.; Tian, Y.; Han, X.; Liu, H.; et al. Air pollution attenuated the benefits of physical activity on blood pressure: Evidence from a nationwide cross-sectional study. Ecotoxicol. Environ. Saf. 2023, 262, 115345. [Google Scholar] [CrossRef]
- Kim, S.R.; Choi, S.; Kim, K.; Chang, J.; Kim, S.M.; Cho, Y.; Oh, Y.H.; Lee, G.; Son, J.S.; Kim, K.H.; et al. Association of the combined effects of air pollution and changes in physical activity with cardiovascular disease in young adults. Eur. Heart J. 2021, 42, 2487–2497. [Google Scholar] [CrossRef] [PubMed]
- Pasqua, L.A.; Damasceno, M.V.; Cruz, R.; Matsuda, M.; Garcia Martins, M.; Lima-Silva, A.E.; Marquezini, M.; Saldiva, P.H.N.; Bertuzzi, R. Exercising in air pollution: The cleanest versus dirtiest cities challenge. Int. J. Environ. Res. Public Health 2018, 15, 1502. [Google Scholar] [CrossRef]
- Tainio, M.; de Nazelle, A.J.; Götschi, T.; Kahlmeier, S.; Rojas-Rueda, D.; Nieuwenhuijsen, M.J.; de Sá, T.H.; Kelly, P.; Woodcock, J. Can air pollution negate the health benefits of cycling and walking? Prev. Med. 2016, 87, 233–236. [Google Scholar] [CrossRef]
| Author and Publication Year | Study Design | Population | Mean Age (Years) |
|---|---|---|---|
| Sinharay, R., et al. 2018 [26] | Randomized crossover study | 135 participants | 61.8 |
| Matt, F., et al. 2016 [27] | Controlled crossover study | 30 adults | 36.0 |
| Kocot, K., Zejda, J.E. 2020 [28] | Controlled clinical trial | 30 young adults | 22.5 |
| Pagani, L.G., et al. 2020 [29] | Quasi-experimental study | 28 adults | 37.4 |
| Strak, M., et al. 2010 [30] | Quasi-experimental study | 12 adults | 30.0 |
| Kocot, K., et al. 2021 [31] | Quasi-experimental study | 71 young adults | 20.7 |
| Kesavachandran, C.N., et al. 2015 [32] | Cross-sectional analytical study | 378 adults | 30.6 ± 10.6 |
| Marmett, B., 2023 [33] | Cross-sectional study | 100 adult males | 25.5 ± 6.2 |
| Respiratory System Outcome | Authors | Exposure Time | Reported Air Pollutants | Main Results | Statistical Significance |
|---|---|---|---|---|---|
| Lung Function (FEV1, FVC, PEF) | Matt, F., et al. [27] | 15 min | NOx, NO, BC, UP, PM2.5, PM10, Coarse PM |
| p = 0.02 |
| Kocot, K., Zejda, J.E. [28] | 15 min | NOx, PM2.5, PM10, SO2 |
| OR: 1.03 (IC95%: 1.00–1.06) | |
| Strak, M., et al. [30] | 45–60 min | PM10, BC, PNC |
| p = 0.04 | |
| Kocot, K., et al. [31] | 45–60 min | PM10, PM2.5, SO2, NO2 |
| p = 0.02 | |
| Kesavachandran, C.N., et al. [32] | 45–60 min | PM10, PM2.5 |
| p = 0.001 | |
| Sinharay, R., et al. [26] | 1 h | BC, NO2, PM10, PM 2.5, UP |
| p < 0.05 | |
| Inflammatory Biomarkers | Pagani, L.G., et al. [29] | Not specified | PM2.5, PM10 |
| p = 0.025 |
| Marmett, B., et al. [33] | Not specified | O3, NO2 |
| p = 0.01 | |
| FeNO | Pagani, L.G., et al. [29] | Not specified | PM2.5, PM10 |
| p = 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortés González, S.L.; López Pereira, K.A. Air Pollution and Respiratory System Responses in Healthy Adults Engaging in Outdoor Physical Exercise in Urban Environments: A Scoping Review. Int. J. Environ. Res. Public Health 2025, 22, 1347. https://doi.org/10.3390/ijerph22091347
Cortés González SL, López Pereira KA. Air Pollution and Respiratory System Responses in Healthy Adults Engaging in Outdoor Physical Exercise in Urban Environments: A Scoping Review. International Journal of Environmental Research and Public Health. 2025; 22(9):1347. https://doi.org/10.3390/ijerph22091347
Chicago/Turabian StyleCortés González, Sergio Leonardo, and Katy Alexandra López Pereira. 2025. "Air Pollution and Respiratory System Responses in Healthy Adults Engaging in Outdoor Physical Exercise in Urban Environments: A Scoping Review" International Journal of Environmental Research and Public Health 22, no. 9: 1347. https://doi.org/10.3390/ijerph22091347
APA StyleCortés González, S. L., & López Pereira, K. A. (2025). Air Pollution and Respiratory System Responses in Healthy Adults Engaging in Outdoor Physical Exercise in Urban Environments: A Scoping Review. International Journal of Environmental Research and Public Health, 22(9), 1347. https://doi.org/10.3390/ijerph22091347






