Effect of a School-Based Physical Activity and Multi-Micronutrient Supplementation Intervention on Cognitive Function and Academic Achievement Among Schoolchildren in Tanzania: Secondary Outcome from the KaziAfya Cluster-Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants and Procedures
2.3. Interventions
2.3.1. Physical Activity
2.3.2. Multi-Micronutrient Supplementation
2.4. Sample Size Calculation
2.5. Data Assessment
2.6. Measures
2.7. Academic Achievement
2.8. Cognitive Function
2.9. Statistical Analysis
3. Results
3.1. Baseline Demographic and Anthropometric Characteristics of Schoolchildren
3.1.1 Cognitive Function
3.1.2 Academic Achievement
3.2. Exploratory Post Hoc Analyses
3.3. Mixed Multiple Linear Regression Analyses
3.3.1. Unadjusted Model
3.3.2. Adjusted Model
Cognitive Function
Academic Achievement
3.4. Summary of Key Results
4. Discussion
4.1. Effect of the MMNS Intervention
4.2. Effect of the PA + Placebo Intervention
4.3. Effect of the Combined PA and MMNS Intervention
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ADHD | Attention deficit hyperactivity disorder |
| BAZ | Body mass index-for-age Z-score |
| BIA | Bioelectrical impedance analysis |
| BMI | Body mass index |
| CI | Confidence interval |
| CONSORT | Consolidated Standards of Reporting Trials |
| COVID-19 | Coronavirus disease 2019 |
| DSM | DSM N.V. (Dutch multinational company that manufactures the MixMeTM supplement) |
| GEE | Generalized estimating equation |
| HAZ | Height-for-age Z-score |
| Hb | Hemoglobin |
| HHS | Household hunger scale |
| ISRCTN | International Standard Randomised Controlled Trial Number (registry) |
| LMICs | Low- and middle-income countries |
| MMNS | Multi-micronutrient supplementation |
| MRCC | Medical research coordinating committee |
| MVPA | Moderate-to-vigorous physical activity |
| NIMR | National Institute for Medical Research |
| PA | Physical activity |
| PA + MMNS | Combined physical activity and multi-micronutrient supplementation intervention |
| RCT | Randomized controlled trial |
| SD | Standard deviation |
| SES | Socioeconomic status |
| UNESCO | United Nations Educational, Scientific and Cultural Organization |
| WDDS | Women’s dietary diversity score |
| WHO | World Health Organization |
| zBMI | Age- and sex-standardized BMI Z-score |
References
- Feinstein, L.; Bynner, J. The importance of cognitive development in middle childhood for adulthood socioeconomic status, mental health, and problem behavior. Child Dev. 2004, 75, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Lee, K. Interventions shown to aid executive function development in children 4 to 12 years old. Science 2011, 333, 959–964. [Google Scholar] [CrossRef]
- Honja Kabero, T.; Bosha, T.; Feleke, F.W.; Haile Weldegebreal, D.; Stoecker, B. Nutritional status and its association with cognitive function among school aged children at Soddo Town and Soddo Zuriya District, southern Ethiopia: Institution based comparative study. Glob. Pediatr. Health 2021, 8, 2333794X211028198. [Google Scholar] [CrossRef]
- Donnelly, J.E.; Hillman, C.H.; Castelli, D.; Etnier, J.L.; Lee, S.; Tomporowski, P.; Lambourne, K.; Szabo-Reed, A.N. Physical activity, fitness, cognitive function, and academic achievement in children: A systematic review. Med. Sci. Sports Exerc. 2016, 48, 1197. [Google Scholar] [CrossRef]
- Gautam, N.; Dessie, G.; Rahman, M.M.; Khanam, R. Socioeconomic status and health behavior in children and adolescents: A systematic literature review. Front. Public Health 2023, 11, 1228632. [Google Scholar] [CrossRef]
- UNICEF; WHO. UNICEF-WHO-The World Bank: Joint Child Malnutrition Estimates (JME)—Levels and Trends–2023 Edition; World Health Organization: Geneva, Switzerland, 2023.
- Said, F.A.; Khamis, A.G.; Habib, A.; Yang, H.; He, Z.; Luo, X. Prevalence and determinants of anemia among children in Zanzibar, Tanzania: Analysis of cross-sectional population representative surveys. Children 2021, 8, 1091. [Google Scholar] [CrossRef]
- Minja, E.G.; Mrimi, E.C.; Mponzi, W.P.; Mollel, G.J.; Lang, C.; Beckmann, J.; Gerber, M.; Pühse, U.; Long, K.Z.; Masanja, H.; et al. Prevalence and determinants of undernutrition in schoolchildren in the Kilombero District, south-eastern Tanzania. Trop. Med. Infect. Dis. 2024, 9, 96. [Google Scholar] [CrossRef]
- Mohamed, I.; Kinung’hi, S.; Mwinzi, P.N.; Onkanga, I.O.; Andiego, K.; Muchiri, G.; Odiere, M.R.; Vennervald, B.J.; Olsen, A. Diet and hygiene practices influence morbidity in schoolchildren living in schistosomiasis endemic areas along Lake Victoria in Kenya and Tanzania—A cross-sectional study. PLoS Negl. Trop. Dis. 2018, 12, e0006373. [Google Scholar] [CrossRef]
- Walker, S.P.; Wachs, T.D.; Gardner, J.M.; Lozoff, B.; Wasserman, G.A.; Pollitt, E.; Carter, J.A. Child development: Risk factors for adverse outcomes in developing countries. Lancet 2007, 369, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Grantham-McGregor, S.; Cheung, Y.B.; Cueto, S.; Glewwe, P.; Richter, L.; Strupp, B. Developmental potential in the first 5 years for children in developing countries. Lancet 2007, 369, 60–70. [Google Scholar] [CrossRef]
- Victora, C.G.; Adair, L.; Fall, C.; Hallal, P.C.; Martorell, R.; Richter, L.; Sachdev, H.S. Maternal and child undernutrition: Consequences for adult health and human capital. Lancet 2008, 371, 340–357. [Google Scholar] [CrossRef]
- Drago, F.; Scharf, R.J.; Maphula, A.; Nyathi, E.; Mahopo, T.C.; Svensen, E.; Mduma, E.; Bessong, P.; Rogawski McQuade, E.T. Psychosocial and environmental determinants of child cognitive development in rural South Africa and Tanzania: Findings from the mal-ed cohort. BMC Public Health 2020, 20, 505. [Google Scholar] [CrossRef]
- Carson, V.; Lee, E.Y.; Hewitt, L.; Jennings, C.; Hunter, S.; Kuzik, N.; Stearns, J.A.; Unrau, S.P.; Poitras, V.J.; Gray, C.; et al. Systematic review of the relationships between physical activity and health indicators in the early years (0–4 years). BMC Public Health 2017, 17 (Suppl. 5), 854. [Google Scholar]
- Li, D.; Wang, D.; Zou, J.; Li, C.; Qian, H.; Yan, J.; He, Y. Effect of physical activity interventions on children’s academic performance: A systematic review and meta-analysis. Eur. J. Pediatr. 2023, 182, 3587–3601. [Google Scholar] [CrossRef]
- Singh, A.S.; Saliasi, E.; van den Berg, V.; Uijtdewilligen, L.; de Groot, R.H.M.; Jolles, J.; Andersen, L.B.; Bailey, R.; Chang, Y.K.; Diamond, A.; et al. Effects of physical activity interventions on cognitive and academic performance in children and adolescents: A novel combination of a systematic review and recommendations from an expert panel. Br. J. Sports Med. 2019, 53, 640–647. [Google Scholar] [CrossRef]
- Moreau, D.; Kirk, I.J.; Waldie, K.E. High-intensity training enhances executive function in children in a randomized, placebo-controlled trial. eLife 2017, 6, e25062. [Google Scholar] [CrossRef]
- Benzing, V.; Heinks, T.; Eggenberger, N.; Schmidt, M. Acute cognitively engaging exergame-based physical activity enhances executive functions in adolescents. PLoS ONE 2016, 11, e0167501. [Google Scholar] [CrossRef]
- Cocco, S.; Diaz, G.; Stancampiano, R.; Diana, A.; Carta, M.; Curreli, R.; Sarais, L.; Fadda, F. Vitamin A deficiency produces spatial learning and memory impairment in rats. Neuroscience 2002, 115, 475–482. [Google Scholar] [CrossRef]
- Al Mamun, M.A.; Ghani, R.B.A. The role of iron and zinc in cognitive development of children. Asian J. Med. Biol. Res. 2017, 3, 145–151. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Taneja, S. Zinc and cognitive development. Br. J. Nutr. 2001, 85, S139–S145. [Google Scholar] [CrossRef]
- Constant, E.; De Volder, A.; Ivanoiu, A.; Bol, A.; Labar, D.; Seghers, A.; Cosnard, G.; Melin, J.; Daumerie, C. Cerebral blood flow and glucose metabolism in hypothyroidism: A positron emission tomography study. J. Clin. Endocrinol. Metab. 2001, 86, 3864–3870. [Google Scholar] [CrossRef]
- Lam, L.F.; Lawlis, T.R. Feeding the brain–the effects of micronutrient interventions on cognitive performance among school-aged children: A systematic review of randomized controlled trials. Clin. Nutr. 2017, 36, 1007–1014. [Google Scholar] [CrossRef]
- Stevens, G.A.; Beal, T.; Mbuya, M.N.; Luo, H.; Neufeld, L.M.; Global Micronutrient Deficiencies Research Group. Micronutrient deficiencies among preschool-aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob. Health 2022, 10, e1590–e1599. [Google Scholar] [CrossRef]
- Passarelli, S.; Free, C.M.; Shepon, A.; Beal, T.; Batis, C.; Golden, C.D. Global estimation of dietary micronutrient inadequacies: A modelling analysis. Lancet Glob. Health 2024, 12, e1590–e1599. [Google Scholar] [CrossRef]
- Benton, D. The influence of dietary status on the cognitive performance of children. Mol. Nutr. Food Res. 2010, 54, 457–470. [Google Scholar] [CrossRef]
- Eilander, A.; Gera, T.; Sachdev, H.S.; Transler, C.; van der Knaap, H.C.; Kok, F.J.; Osendarp, S.J. Multiple micronutrient supplementation for improving cognitive performance in children: Systematic review of randomized controlled trials. Am. J. Clin. Nutr. 2010, 91, 115–130. [Google Scholar] [CrossRef]
- Khor, G.L.; Misra, S. Micronutrient interventions on cognitive performance of children aged 5–15 years in developing countries. Asia Pac. J. Clin. Nutr. 2012, 21, 476–486. [Google Scholar]
- Clark, C.A.; Pritchard, V.E.; Woodward, L.J. Preschool executive functioning abilities predict early mathematics achievement. Dev. Psychol. 2010, 46, 1176. [Google Scholar] [CrossRef]
- Engle, P.L.; Fernández, P.D. INCAP studies of malnutrition and cognitive behavior. Food Nutr. Bull. 2010, 31, 83–94. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, H.; Wang, D.; Sudfeld, C.R.; Zhao, A.; Xin, Y.; Chen, J.C.; Fawzi, W.W.; Xing, Y.; Li, Z. Effect of oral iron supplementation on cognitive function among children and adolescents in low-and middle-income countries: A systematic review and meta-analysis. Nutrients 2022, 14, 5332. [Google Scholar] [CrossRef]
- Gordon, R.C.; Rose, M.C.; Skeaff, S.A.; Gray, A.R.; Morgan, K.M.; Ruffman, T. Iodine supplementation improves cognition in mildly iodine-deficient children. Am. J. Clin. Nutr. 2009, 90, 1264–1271. [Google Scholar] [CrossRef]
- Baumgartner, J.; Smuts, C.M.; Malan, L.; Kvalsvig, J.; Van Stuijvenberg, M.E.; Hurrell, R.F.; Zimmermann, M.B. Effects of iron and n-3 fatty acid supplementation, alone and in combination, on cognition in school children: A randomized, double-blind, placebo-controlled intervention in South Africa. Am. J. Clin. Nutr. 2012, 96, 1327–1338. [Google Scholar] [CrossRef]
- Nga, T.T.; Winichagoon, P.; Dijkhuizen, M.A.; Khan, N.C.; Wasantwisut, E.; Wieringa, F.T. Decreased parasite load and improved cognitive outcomes caused by deworming and consumption of multi-micronutrient fortified biscuits in rural Vietnamese schoolchildren. Am. J. Trop. Med. Hyg. 2011, 85, 333–340. [Google Scholar] [CrossRef]
- Solon, F.S.; Sarol, J.N., Jr.; Bernardo, A.B.; Solon, J.A.A.; Mehansho, H.; Sanchez-Fermin, L.E.; Wambangco, L.S.; Juhlin, K.D. Effect of a multiple-micronutrient-fortified fruit powder beverage on the nutrition status, physical fitness, and cognitive performance of schoolchildren in the Philippines. Food Nutr. Bull. 2003, 24 (Suppl. 1), S129–S140. [Google Scholar] [CrossRef]
- Haskell, C.F.; Scholey, A.B.; Jackson, P.A.; Elliott, J.M.; Defeyter, M.A.; Greer, J.; Robertson, B.C.; Buchanan, T.; Tiplady, B.; Kennedy, D.O. Cognitive and mood effects in healthy children during 12 weeks’ supplementation with multi-vitamin/minerals. Br. J. Nutr. 2008, 100, 1086–1096. [Google Scholar] [CrossRef]
- Schoenthaler, S.J.; Bier, I.D.; Young, K.; Nichols, D.; Jansenns, S. The effect of vitamin-mineral supplementation on the intelligence of American schoolchildren: A randomized, double-blind placebo-controlled trial. J. Altern. Complement. Med. 2000, 6, 19–29. [Google Scholar] [CrossRef]
- Meli, A.M.; Ali, A.; Mhd Jalil, A.M.; Mohd Yusof, H.; Tan, M.M. Effects of physical activity and micronutrients on cognitive performance in children aged 6 to 11 years: A systematic review and meta-analysis of randomized controlled trials. Medicina 2021, 58, 57. [Google Scholar] [CrossRef]
- Langa, N.; Bhatta, T. The rural-urban divide in Tanzania: Residential context and socioeconomic inequalities in maternal health care utilization. PLoS ONE 2020, 15, e0241746. [Google Scholar] [CrossRef] [PubMed]
- Mosha, D.; Paulo, H.A.; Mwanyika-Sando, M.; Mboya, I.B.; Madzorera, I.; Leyna, G.H.; Msuya, S.E.; Bärnighausen, T.W.; Killewo, J.; Fawzi, W.W. Risk factors for overweight and obesity among women of reproductive age in Dar es Salaam, Tanzania. BMC Nutr. 2021, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.; Keats, E.C.; Rind, F.; Das, J.K.; Bhutta, Z.A. Micronutrient supplementation and fortification interventions on health and development outcomes among children under-five in low-and middle-income countries: A systematic review and meta-analysis. Nutrients 2020, 12, 289. [Google Scholar] [CrossRef]
- Roberts, M.; Tolar-Peterson, T.; Reynolds, A.; Wall, C.; Reeder, N.; Rico Mendez, G. The effects of nutritional interventions on the cognitive development of preschool-age children: A systematic review. Nutrients 2022, 14, 532. [Google Scholar] [CrossRef] [PubMed]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef]
- Mrema, J.D.; Elisaria, E.; Mwanri, A.W.; Nyaruhucha, C.M. Prevalence and determinants of undernutrition among 6- to 59-months-old children in lowland and highland areas in Kilosa District, Tanzania: A cross-sectional study. J. Nutr. Metab. 2021, 2021, 6627557. [Google Scholar] [CrossRef]
- Mrimi, E.C.; Palmeirim, M.S.; Minja, E.G.; Long, K.Z.; Keiser, J. Malnutrition, anemia, micronutrient deficiency and parasitic infections among schoolchildren in rural Tanzania. PLoS Negl. Trop. Dis. 2022, 16, e0010261. [Google Scholar] [CrossRef] [PubMed]
- Sando, D.; Sachin, S.; Moshi, G.; Sando, M.M.; Yussuf, M.; Mwakitalima, A.; Fawzi, W. School health and nutrition services for children and adolescents in Tanzania: A review of policies and programmes. Matern. Child Nutr. 2024, 21, e13544. [Google Scholar] [CrossRef] [PubMed]
- Gerber, M.; Ayekoé, S.A.; Beckmann, J.; Bonfoh, B.; Coulibaly, J.T.; Daouda, D.; du Randt, R.; Finda, L.; Gall, S.; Mollel, G.J.; et al. Effects of school-based physical activity and multi-micronutrient supplementation intervention on growth, health and well-being of schoolchildren in three African countries: The KaziAfya cluster randomised controlled trial protocol with a 2 × 2 factorial design. Trials 2020, 21, 22. [Google Scholar]
- Canas, J.A.; Lochrie, A.; McGowan, A.G.; Hossain, J.; Schettino, C.; Balagopal, P.B. Effects of mixed carotenoids on adipokines and abdominal adiposity in children: A pilot study. J. Clin. Endocrinol. Metab. 2017, 102, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Ip, P.; Ho, F.K.W.; Rao, N.; Sun, J.; Young, M.E.; Chow, C.B.; Tso, W.; Hon, K.L. Impact of nutritional supplements on cognitive development of children in developing countries: A meta-analysis. Sci. Rep. 2017, 7, 10611. [Google Scholar] [CrossRef]
- Ludyga, S.; Gerber, M.; Pühse, U.; Looser, V.N.; Kamijo, K. Systematic review and meta-analysis investigating moderators of long-term effects of exercise on cognition in healthy individuals. Nat. Hum. Behav. 2020, 4, 603–612. [Google Scholar] [CrossRef]
- Bell, M.L.; Kenward, M.G.; Fairclough, D.L.; Horton, N.J. Differential dropout and bias in randomised controlled trials: When it matters and when it may not. BMJ 2013, 346, e8668. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.C.J.; Swindale, A.; Deitchler, M. Household Hunger Scale: Indicator Definition and Measurement Guide; FANTA: Washington, DC, USA, 2011. [Google Scholar]
- Swindale, A.B.P. Household Dietary Diversity Score (hdds) for Measurement of Household Food Access: Indicator Guide (v.2); FANTA: Washington, DC, USA, 2006. [Google Scholar]
- Kennedy, G.; Ballard, T.; Dop, M. Guidelines for Measuring Household and Individual Dietary Diversity; Food and Agriculture Organization: Rome, Italy, 2011. [Google Scholar]
- Filmer, D.; Pritchett, L.H. Estimating wealth effects without expenditure data—Or tears: An application to educational enrollments in states of India. Demography 2001, 38, 115–132. [Google Scholar] [CrossRef] [PubMed]
- WHO. Nutritional Anaemias: Tools for Effective Prevention and Control; World Health Organization: Geneva, Switzerland, 2017.
- Minja, E.G.; Swai, J.K.; Mponzi, W.; Ngowo, H.; Okumu, F.; Gerber, M.; Pühse, U.; Long, K.Z.; Utzinger, J.; Lang, C. Dietary diversity among households living in Kilombero District, in Morogoro Region, south-eastern Tanzania. J. Agric. Food Res. 2021, 5, 100171. [Google Scholar] [CrossRef]
- Beckmann, J.; Nqweniso, S.; Ludyga, S.; du Randt, R.; Gresse, A.; Long, K.Z.; Nienaber, M.; Seelig, H.; Pühse, U.; Steinmann, P.; et al. Evaluation of a physical activity and multi-micronutrient intervention on cognitive and academic performance in South African primary schoolchildren. Nutrients 2022, 14, 2609. [Google Scholar] [CrossRef]
- Ogunlade, A.O.; Kruger, H.S.; Jerling, J.C.; Smuts, C.M.; Covic, N.; Hanekom, S.M.; Mamabolo, R.L.; Kvalsvig, J. Point-of-use micronutrient fortification: Lessons learned in implementing a preschool-based pilot trial in South Africa. Int. J. Food Sci. Nutr. 2011, 62, 1–16. [Google Scholar] [CrossRef]
- Black, M.M.; Fernandez-Rao, S.; Nair, K.M.; Balakrishna, N.; Tilton, N.; Radhakrishna, K.V.; Ravinder, P.; Harding, K.B.; Reinhart, G.; Yimgang, D.P.; et al. A randomized multiple micronutrient powder point-of-use fortification trial implemented in Indian preschools increases expressive language and reduces anemia and iron deficiency. J. Nutr. 2021, 151, 2029–2042. [Google Scholar] [CrossRef]
- Dye, M.W.; Bavelier, D. Differential development of visual attention skills in school-age children. Vis. Res. 2010, 50, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Weicker, J.; Villringer, A.; Thöne-Otto, A. Can impaired working memory functioning be improved by training? A meta-analysis with a special focus on brain injured patients. Neuropsychology 2016, 30, 190. [Google Scholar] [CrossRef]
- Sala, G.; Gobet, F. Working memory training in typically developing children: A meta-analysis of the available evidence. Dev. Psychol. 2017, 53, 671. [Google Scholar] [CrossRef]
- Gall, S.; Adams, L.; Joubert, N.; Ludyga, S.; Müller, I.; Nqweniso, S.; Pühse, U.; du Randt, R.; Seelig, H.; Smith, D.; et al. Effect of a 20-week physical activity intervention on selective attention and academic performance in children living in disadvantaged neighborhoods: A cluster randomized control trial. PLoS ONE 2018, 13, e0206908. [Google Scholar] [CrossRef]
- Costello, S.E.; Geiser, E.; Schneider, N. Nutrients for executive function development and related brain connectivity in school-aged children. Nutr. Rev. 2021, 79, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Kolovelonis, A.; Goudas, M. Exploring the effects of three different types of cognitively challenging physical activity games on students’ executive functions and situational interest in physical education. Cogent Educ. 2022, 9, 2148448. [Google Scholar] [CrossRef]
- Pesce, C.; Faigenbaum, A.D.; Goudas, M.; Tomporowski, P. Coupling our plough of thoughtful moving to the star of children’s right to play: From neuroscience to multisectoral promotion. In Physical Activity and Educational Achievement; Routledge: Oxfordshire, UK, 2017; pp. 247–274. [Google Scholar]
- Perlman, A.I.; Worobey, J.; Maillet, J.O.S.; Touger-Decker, R.; Hom, D.L.; Smith, J.K. Multivitamin/mineral supplementation does not affect standardized assessment of academic performance in elementary school children. J. Am. Diet. Assoc. 2010, 110, 1089–1093. [Google Scholar] [CrossRef] [PubMed]

| Measures | N | All Children (n = 559) | Boys (n = 233) | Girls (n = 326) | χ2/F Score | p-Value | Effect Size |
|---|---|---|---|---|---|---|---|
| M (95% CI) | M (95% CI) | M (95% CI) | |||||
| Age (years) | 570 | 9.8 (9.7, 9.9) | 10.1 (9.9, 10.3) | 9.5 (9.4, 9.7) | −4.29 | <0.01 | 0.58 (0.32, 0.84) |
| Height (cm) | 547 | 129.6 (128.8, 130.5) | 130.9 (129.6, 132.2) | 128.8 (127.7, 129.9) | −2.38 | 0.01 | 2.07 (0.37, 3.78) |
| Weight (kg) | 547 | 27.5 (27.0, 28.1) | 28.1 (27.3, 28.9) | 27.1 (26.4, 27.8) | −1.89 | 0.06 | 1.01 (−0.04, 2.06) |
| BMI (kg/m2) | 570 | 11.5 (9.6, 13.4) | 12.3 (9.6, 15.0) | 10.9 (8.2, 13.5) | −0.75 | 0.45 | 1.44 (−2.39, 5.30) |
| zBMI | 547 | −0.33 (−0.42, −0.24) | −0.34 (−0.47, −0.21) | −0.32 (−0.44, −0.21) | 0.24 | 0.81 | −0.02 (−0.19, 0.15) |
| Cognitive function | |||||||
| Accuracy (congruent stimuli) | 570 | 0.90 (0.89, 0.92) | 0.91 (0.89, 0.93) | 0.90 (0.88, 0.92) | −1.35 | 0.17 | 0.01 (−0.00, 0.03) |
| Accuracy (incongruent stimuli) | 570 | 0.85 (0.83, 0.87) | 0.86 (0.84, 0.89) | 0.83 (0.81, 0.86) | −1.64 | 0.09 | 0.03 (−0.00, 0.06) |
| Reaction time (congruent stimuli) | 570 | 1146 (1125, 1168) | 1110 (1077, 1142) | 1173 (1145, 1201) | 2.88 | 0.01 | −63.03 (−105.95, −20.11) |
| Reaction time (incongruent stimuli) | 570 | 1201 (1178, 1224) | 1173 (1137, 1209) | 1221 (1191, 1251) | 1.99 | 0.05 | −47.87 (−94.76, −0.99) |
| Academic achievement | |||||||
| End-of-year results | 555 | 59.8 (57.9, 61.7) | 58.6 (55.4, 61.9) | 60.6 (58.3, 62.9) | 0.98 | 0.32 | −1.98 (−5.83, 1.86) |
| Kiswahili language | 555 | 66.2 (64.0, 68.4) | 63.2 (59.4, 67.0) | 68.4 (65.7, 71.1) | 2.19 | <0.01 | −5.21 (−9.73, −0.68) |
| Mathematics | 555 | 53.4 (51.3, 55.5) | 54.1 (50.7, 57.5) | 52.9 (50.3, 55.5) | −0.57 | 0.56 | 1.23 (−2.94, 5.41) |
| Dietary | |||||||
| Dietary diversity (WDDS) | 532 | 2.92 (2.85, 2.98) | 2.96 (2.85, 3.08) | 2.89 (2.80, 2.97) | −1.03 | 0.30 | 0.07 (−0.06, 0.22) |
| Food insecurity (HHS) | 532 | 1.30 (1.23, 1.36) | 1.20 (1.11, 1.29) | 1.37 (1.27, 1.46) | 2.51 | 0.01 | 0.08 (−0.22, 0.40) |
| Nutrition | |||||||
| Hemoglobin level (g/dL) | 546 | 12.79 (12.70, 12.88) | 12.85 (12.71, 12.99) | 12.74 (12.62, 12.86) | −1.14 | 0.25 | 0.11 (−0.08, 0.30) |
| Stunting | 547 | 119 (22) | 53 (45) | 66 (55) | 0.25 | 0.61 | 0.12 (−0.28, 0.54) |
| Meeting MVPA recommendation | 506 | 464 (92) | 195 (42) | 269 (58) | 5.96 | 0.01 | 0.97 (0.22, 1.74) |
| SES | |||||||
| Low | 570 | 179 (32) | 80 (45) | 99 (55) | 2.08 | 0.35 | −0.25 (−0.62, 0.10) |
| Middle | 197 (35) | 85 (43) | 112 (57) | ||||
| High | 186 (33) | 70 (38) | 116 (62) | ||||
| Child Characteristics | Interventions | ||||||
|---|---|---|---|---|---|---|---|
| Placebo M (95% CI) | MMNS M (95% CI) | PA + MMNS M (95% CI) | PA + Placebo M (95% CI) | χ2/F Score | p-Value | Effect Size | |
| Female, n (%) | 75 (60.0%) | 95 (49.2%) | 86 (66.2%) | 71 (62.3%) | |||
| Male, n (%) | 50 (40.0%) | 98 (50.8%) | 44 (33.9%) | 43 (37.7%) | |||
| Stunting | 33 (26.4%) | 46 (23.5%) | 37 (27.8%) | 32 (27.6%) | 1.90 | 0.59 | 0.18 (−0.37, 0.74) |
| Age (years) | 11.3 (11.2, 11.4) | 11.7 (11.5, 11.8) | 12.4 (12.3, 12.5) | 11.8 (11.6, 11.9) | 10.68 | <0.01 | 1.06 (0.69, 1.44) |
| Height (cm) | 128.4 (125.1, 133.0) | 134.4 (131.0, 137.0) | 121.9 (116.0, 128.0) | 128.0 (124.0, 132.0) | 1.69 | 0.16 | −6.50 (−18.56, 5.56) |
| Weight (kg) | 28.1 (26.1, 30.2) | 31.9 (30.1, 33.6) | 25.9 (22.6, 29.1) | 28.9 (26.6, 31.3) | 1.21 | 0.30 | −2.23 (−9.21, 4.75) |
| BMI (kg/m2) | 17.2 (17.0, 17.4) | 17.6 (17.4, 17.8) | 18.2 (18.0, 18.5) | 17.9 (17.8, 18.1) | 3.23 | 0.02 | 1.02 (0.33, 1.71) |
| zBMI | −0.31 (−0.40, −0.21) | −0.18 (−0.27, −0.10) | −0.16 (−0.25, −0.07) | −0.03 (−0.11, 0.04) | 1.31 | 0.27 | 0.14 (−0.11, 0.40) |
| Cognitive function | |||||||
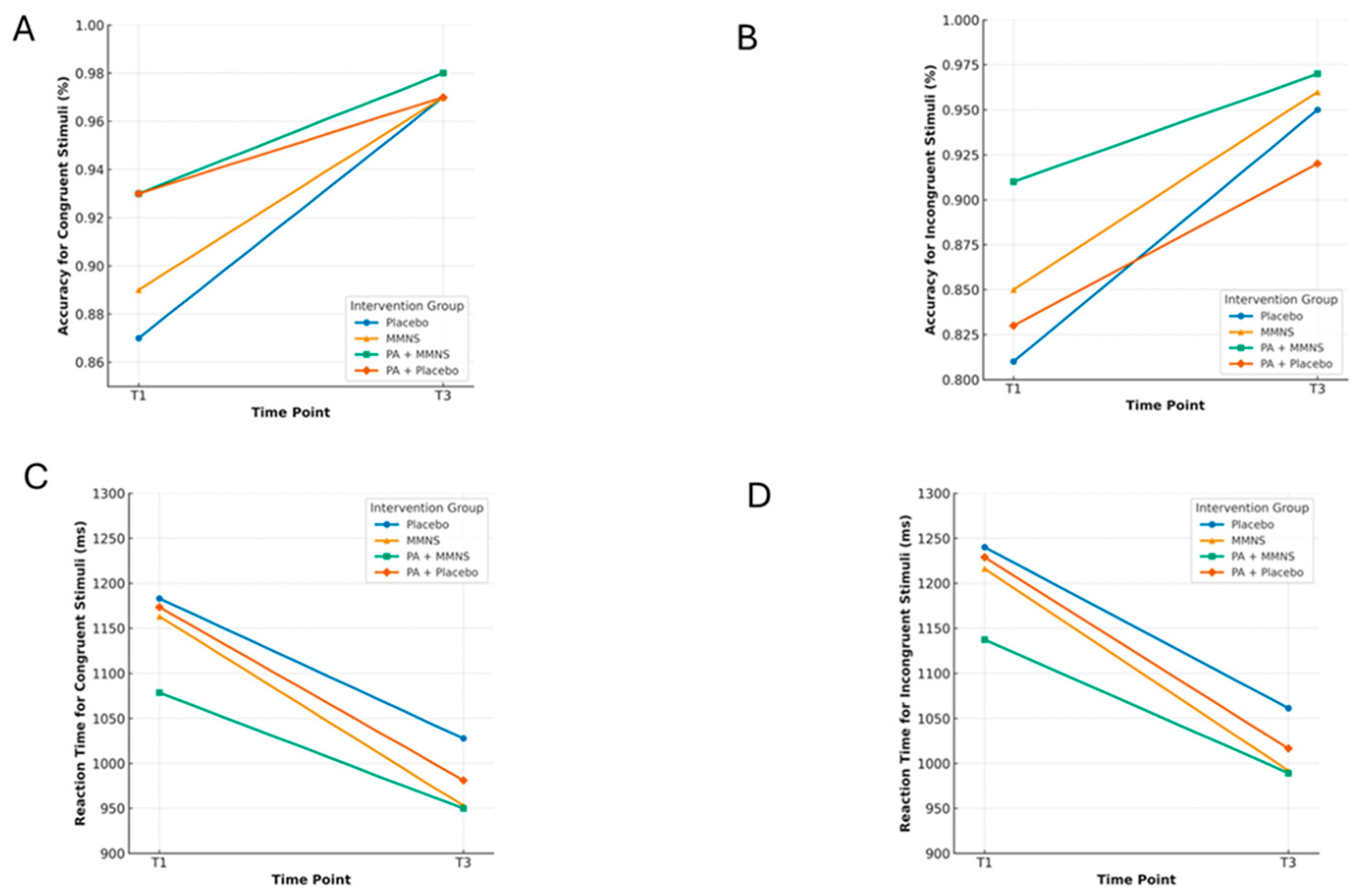
| Accuracy (congruent stimuli) | 0.97 (0.97, 0.98) | 0.97 (0.96, 0.97) | 0.98 (0.98, 0.99) | 0.97 (0.96, 0.97) | 1.70 | 0.16 | 0.00 (−0.00, 0.02) |
| Accuracy (incongruent stimuli) | 0.95 (0.95, 0.96) | 0.96 (0.95, 0.97) | 0.97 (0.97, 0.98) | 0.92 (0.91, 0.94) | 4.61 | <0.01 | 0.02 (−0.00, 0.04) |
| Reaction time (congruent stimuli) | 1028 (1008, 1048) | 953 (934, 972) | 950 (934, 966) | 981 (960, 1002) | 3.27 | 0.02 | −77.87 (−134.77, −20.96) |
| Reaction time (incongruent stimuli) | 1061 (104, 1082) | 992 (971, 1013) | 989 (971, 1007) | 1016 (995, 1038) | 2.46 | 0.06 | −71.95 (−132.68, −11.22) |
| Academic achievement | |||||||
| End-of-year results | 313.3 (307.0, 319.0) | 281.6 (275.0, 288.0) | 291.3 (285.0, 298.0) | 271.2 (263.0, 279.0) | 5.48 | <0.01 | −21.96 (−42.99, −0.93) |
| Kiswahili language | 48.9 (47.7, 50.0) | 58.0 (56.6, 59.4) | 49.0 (47.1, 49.9) | 47.6 (46.1, 49.1) | 13.25 | <0.01 | −0.37 (−4.64, 3.88) |
| Mathematics | 40.4 (38.9, 41.9) | 31.9 (30.4, 33.5) | 30.0 (29.2, 30.9) | 35.2 (33.7, 36.7) | 7.13 | <0.01 | −10.36 (−15.23, −5.49) |
| Explanatory Variables | Mixed Multiple Linear Regression | ||
|---|---|---|---|
| Adjusted | |||
| Estimate | SE | p-Value | |
| Accuracy at T3 (congruent stimuli) | |||
| MMNS | −0.00 | 0.00 | 0.49 |
| PA + placebo | −0.00 | 0.00 | 0.09 |
| PA + MMNS | 0.00 | 0.00 | 0.95 |
| Sex (0 = girls, 1 = boys) | 0.00 | 0.00 | 0.01 |
| Age (years) | −0.00 | 0.00 | 0.06 |
| zBMI | 0.00 | 0.00 | 0.73 |
| Stunting (0 = not stunted, 1 = stunted) | −0.01 | 0.00 | 0.12 |
| MVPA (0 = not met, 1 = met) | 0.00 | 0.00 | 0.76 |
| Low SES | −0.00 | 0.00 | 0.05 |
| Middle SES | −0.00 | 0.00 | 0.17 |
| Hemoglobin | 0.01 | 0.00 | <0.01 |
| Dietary diversity (WDDS) | −0.00 | 0.00 | 0.42 |
| Food security (HHS) | −0.00 | 0.00 | 0.68 |
| Baseline accuracy (congruent stimuli) | 0.00 | 0.00 | <0.01 |
| Accuracy at T3 (incongruent stimuli) | |||
| MMNS | −0.00 | 0.01 | 0.95 |
| PA + placebo | −0.02 | 0.01 | 0.08 |
| PA + MMNS | 0.00 | 0.01 | 0.49 |
| Sex | 0.00 | 0.00 | 0.46 |
| Age (years) | 0.00 | 0.00 | 0.01 |
| zBMI | −0.00 | 0.04 | 0.22 |
| Stunting (0 = not stunted, 1 = stunted) | −0.01 | 0.01 | 0.19 |
| MVPA (0 = not met, 1 = met) | −0.00 | 0.01 | 0.80 |
| Low SES | −0.01 | 0.01 | 0.08 |
| Middle SES | −0.01 | 0.01 | 0.05 |
| Hemoglobin | 0.00 | 0.01 | 0.57 |
| Dietary diversity (WDDS) | −0.00 | 0.00 | 0.25 |
| Food security (HHS) | 0.00 | 0.01 | 0.92 |
| Baseline accuracy (incongruent stimuli) | 0.20 | 0.02 | <0.01 |
| Reaction time at T3 (congruent stimuli) | |||
| MMNS | −14.17 | 31.74 | 0.65 |
| PA + placebo | −24.07 | 30.75 | 0.43 |
| PA + MMNS | 17.95 | 34.78 | 0.61 |
| Sex | −106.33 | 20.91 | <0.01 |
| Age (years) | −9.14 | 9.62 | 0.34 |
| zBMI | 0.89 | 9.52 | 0.92 |
| Stunting (0 = not stunted, 1 = stunted) | 13.95 | 23.80 | 0.55 |
| MVPA (0 = not met, 1 = met) | 32.07 | 37.09 | 0.38 |
| Low SES | 10.63 | 23.88 | 0.65 |
| Middle SES | −4.75 | 23.53 | 0.84 |
| Hemoglobin | −2.18 | 8.97 | 0.80 |
| Dietary diversity (WDDS) | −10.18 | 11.72 | 0.38 |
| Food security (HHS) | −13.19 | 12.81 | 0.30 |
| Baseline reaction time (congruent stimuli) | 0.23 | 0.04 | <0.01 |
| Reaction time at T3 (incongruent stimuli) | |||
| MMNS | −14.93 | 34.29 | 0.66 |
| PA + placebo | −17.00 | 33.17 | 0.60 |
| PA + MMNS | 11.31 | 37.48 | 0.76 |
| Sex | −117.81 | 22.34 | <0.01 |
| Age (years) | −5.08 | 10.43 | 0.63 |
| zBMI | 1.55 | 10.30 | 0.88 |
| Stunting (0 = not stunted, 1 = stunted) | 7.39 | 25.71 | 0.77 |
| MVPA (0 = not met, 1 = met) | 23.97 | 40.02 | 0.55 |
| Low SES | 18.49 | 25.81 | 0.47 |
| Middle SES | −1.38 | 25.45 | 0.95 |
| Hemoglobin | 1.15 | 9.66 | 0.90 |
| Dietary diversity (WDDS) | −16.41 | 12.69 | 0.19 |
| Food security (HHS) | −11.18 | 13.84 | 0.42 |
| Baseline reaction time (incongruent stimuli) | 0.21 | 0.03 | <0.01 |
| End-of-year results at T3 | |||
| MMNS | 8.75 | 1.02 | 0.39 |
| PA + placebo | −4.51 | 9.87 | <0.01 |
| PA + MMNS | 2.00 | 1.11 | 0.07 |
| Sex | 1.22 | 6.49 | 0.06 |
| Age (years) | −4.65 | 3.16 | 0.14 |
| zBMI | 7.86 | 3.02 | 0.79 |
| Stunting (0 = not stunted, 1 = stunted) | 8.08 | 7.59 | 0.28 |
| MVPA (0 = not meet, 1 = meet) | 1.02 | 1.13 | 0.36 |
| Low SES | −3.91 | 7.49 | 0.25 |
| Middle SES | −8.29 | 7.31 | 0.25 |
| Hemoglobin | −2.49 | 2.84 | 0.38 |
| Dietary diversity (WDDS) | 0.00 | 3.85 | 0.99 |
| Food security (HHS) | 1.20 | 4.03 | 0.76 |
| Baseline end-of-year results | 2.05 | 0.14 | <0.01 |
| Performance in Kiswahili at T3 | |||
| MMNS | 12.23 | 2.10 | <0.01 |
| PA + placebo | −3.88 | 2.02 | 0.05 |
| PA + MMNS | 7.00 | 2.24 | 0.01 |
| Sex | 0.76 | 1.34 | 0.56 |
| Age (years) | −0.74 | 0.63 | 0.23 |
| zBMI | 0.50 | 0.62 | 0.41 |
| Stunting (0 = not stunted, 1 = stunted) | −0.01 | 1.56 | 0.99 |
| MVPA (0 = not met, 1 = met) | 0.79 | 2.32 | 0.73 |
| Low SES | 0.37 | 1.55 | 0.80 |
| Middle SES | −2.28 | 1.50 | 0.12 |
| Hemoglobin | −1.05 | 0.58 | 0.07 |
| Dietary diversity (WDDS) | −0.16 | 0.79 | 0.83 |
| Food security (HHS) | −0.14 | 0.83 | 0.86 |
| Baseline performance in Kiswahili | 0.35 | 0.02 | <0.01 |
| Performance in mathematics at T3 | |||
| MMNS | −2.73 | 2.35 | 0.24 |
| PA + placebo | 0.90 | 2.10 | 0.66 |
| PA + MMNS | 0.63 | 2.84 | 0.82 |
| Sex | 2.73 | 1.33 | 0.04 |
| Age (years) | −1.39 | 0.65 | 0.03 |
| zBMI | −0.45 | 0.63 | 0.47 |
| Stunting (0 = not stunted, 1 = stunted) | −1.10 | 1.69 | 0.51 |
| MVPA (0 = not met, 1 = met) | 3.06 | 2.66 | 0.25 |
| Low SES | −0.59 | 1.59 | 0.71 |
| Middle SES | −0.53 | 1.54 | 0.73 |
| Hemoglobin | −0.01 | 0.62 | 0.98 |
| Dietary diversity (WDDS) | 0.66 | 0.81 | 0.41 |
| Food security (HHS) | −0.02 | 0.83 | 0.97 |
| Baseline performance in mathematics | 0.29 | 0.02 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minja, E.G.; Mrimi, E.C.; Mponzi, W.P.; Beckmann, J.; Finda, M.F.; Okumu, F.O.; Long, K.Z.; Lang, C.; Utzinger, J.; Gerber, M. Effect of a School-Based Physical Activity and Multi-Micronutrient Supplementation Intervention on Cognitive Function and Academic Achievement Among Schoolchildren in Tanzania: Secondary Outcome from the KaziAfya Cluster-Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2025, 22, 1335. https://doi.org/10.3390/ijerph22091335
Minja EG, Mrimi EC, Mponzi WP, Beckmann J, Finda MF, Okumu FO, Long KZ, Lang C, Utzinger J, Gerber M. Effect of a School-Based Physical Activity and Multi-Micronutrient Supplementation Intervention on Cognitive Function and Academic Achievement Among Schoolchildren in Tanzania: Secondary Outcome from the KaziAfya Cluster-Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2025; 22(9):1335. https://doi.org/10.3390/ijerph22091335
Chicago/Turabian StyleMinja, Elihaika G., Emmanuel C. Mrimi, Winfrida P. Mponzi, Johanna Beckmann, Marceline F. Finda, Fredros O. Okumu, Kurt Z. Long, Christin Lang, Jürg Utzinger, and Markus Gerber. 2025. "Effect of a School-Based Physical Activity and Multi-Micronutrient Supplementation Intervention on Cognitive Function and Academic Achievement Among Schoolchildren in Tanzania: Secondary Outcome from the KaziAfya Cluster-Randomized Controlled Trial" International Journal of Environmental Research and Public Health 22, no. 9: 1335. https://doi.org/10.3390/ijerph22091335
APA StyleMinja, E. G., Mrimi, E. C., Mponzi, W. P., Beckmann, J., Finda, M. F., Okumu, F. O., Long, K. Z., Lang, C., Utzinger, J., & Gerber, M. (2025). Effect of a School-Based Physical Activity and Multi-Micronutrient Supplementation Intervention on Cognitive Function and Academic Achievement Among Schoolchildren in Tanzania: Secondary Outcome from the KaziAfya Cluster-Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 22(9), 1335. https://doi.org/10.3390/ijerph22091335







