“If You Haven’t Slept a Lot (…) You Don’t Want to Go Out for a Run, You Don’t Want to Ride a Bike, You Just Kind of Sit and You Just (…) Do Nothing”—Perceptions of 24-Hour Movement Behaviours Among Adolescents Living with Type 1 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.4. Data Analysis
2.5. Researcher Reflexivity
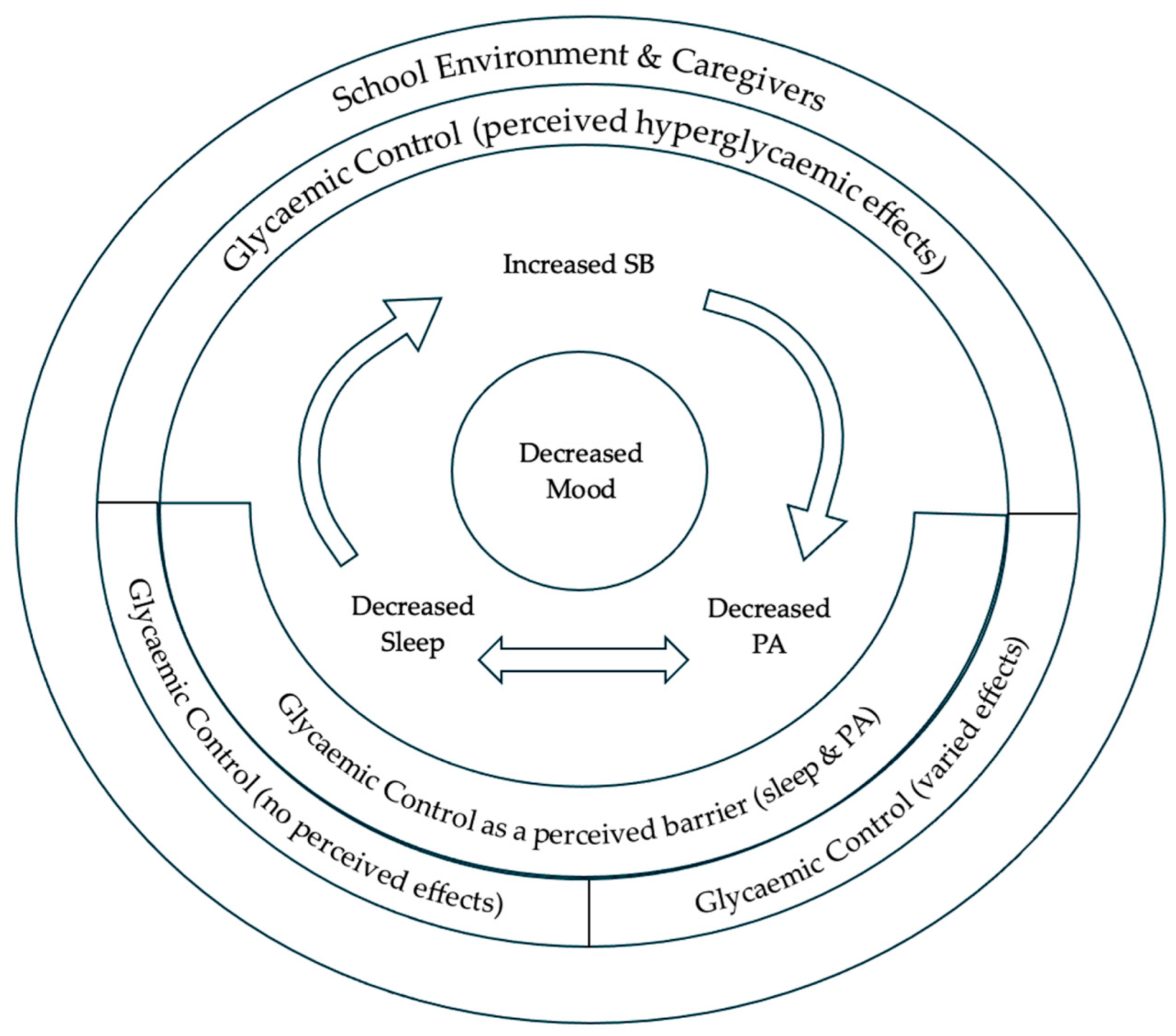
3. Results
3.1. Participant Demographics
3.2. Theme 1: Sleep and Physical Activity Understood and Valued Above Sedentary Behaviour
“Physical activity, well it depends how old you are, but I think it’s an hour a day or something like that on average and then seven to eight hours of sleep, honestly, I have no idea about sitting.”(P12, female, and 16 years of age).
“The only thing I really track is how many steps I’ve done and how much sleep I get.”(P6, male, and 14 years of age).
“I think I can get a bit obsessive with things that show you so exactly like an Apple Watch does. I noticed that I was doing that—oh it had stand hours on it! Sorry I just realised that for the sitting thing.”(P3, female, and 18 years of age).
3.3. Theme 2: Recognition of Movement Behaviours’ Interconnection
“I am literally sitting at my desk all day working and I don’t have time to do any kind of exercise. Then also that affects my sleep as well because I might be mentally tired, but I am not like physically tired because I have not done any physical activity which makes it a bit more difficult to get to sleep.”(P1, female, and 16 years of age).
“If you haven’t slept a lot then the next day you don’t want to be going out for loads of walks, you don’t want to go out for a run, you don’t want to ride a bike you just kind of sit and you just kind of do nothing.”(P11, female, and 17 years of age).
“If you have a more restful sleep and like a good sleep you will be more energetic so you will have more energy to do exercise.”(P10, male, and 16 years of age).
“If I do a physical activity then I would sit down after just because I’m tired.”(P6, male, and 14 years of age).
3.4. Theme 3: Movement Behaviours’ Interaction with Health Outcomes
3.4.1. Mood
“I think if I’m sat down for long periods of time or even if it were short periods of time broken up for just a long day it just makes you feel a bit… you know. Yeah, and then, that can kind of effect your mood.”(P12, female, and 16 years of age).
“You can’t just say ‘I’m going to sit down and do nothing’ you say, ‘I am going to go do exercise because it’s good for me’, it’s good for my physical and mental health.”(P14, male, and 13 years of age).
“If I have a day and I have been sitting around all day, I’ve not done any exercise and got no sleep I get really grumpy.”(P5, female, and 13 years of age).
3.4.2. Glycaemic Control
“I’ve forgotten which way around it is but anaerobic and aerobic exercises. One will make your blood sugars go up and one of them will make you go down straight away.”(P14, male, and 13 years of age).
“If you’re sitting, you might need to get up because if you’ve been sitting for too long your blood might go a little bit high.”(P4, male, and 14 years of age).
“I think the thing with sleep is it doesn’t affect my blood sugars so the issue in sleep is if something happened that has made my blood sugar go low whilst I’m asleep.”(P1, female, and 16 years of age).
3.4.3. Glycaemic Control as a Barrier to Movement Behaviours
“I am sleeping with an actual machine attached to me. It’s more difficult, I think. I know I’ve had sleep issues since before I was diagnosed but notable ones since I was diagnosed.”(P3, female, and 18 years of age).
“When I was on my way home from school I was like ‘oh it would be really nice to do a workout today’ but I don’t think I will be able to because I don’t think I will be able to get my blood sugar to go up for long enough. That makes me feel annoyed because it’s inconvenient. If that was someone without diabetes that wouldn’t be a problem.”(P1, female, and 16 years of age).
3.5. Theme 4: Movement Behaviours Within the Environmental Context of the Adolescent
3.5.1. School
“Physical activity, I probably should be doing more of it to be honest because I only really do it in school and maybe a bit at home.”(P15, female, and 12 years of age).
“I usually go to my bed at like half nine or ten now because I’m in a school routine but when I’m not it’s kind of all over the place and my sleep pattern is just so all over the place. It kind of gets ruined and I feel icky.”(P9, female, and 16 years of age).
“So, at school I will find that it’s kind of a decent environment because I will be sitting down for a couple of lessons for a few hours and then I go out with my friends for a walk which is a mile or so then come back. So, I find they balance each other fairly nicely.”(P11, female, and 17 years of age).
“I think just the social pressure of knowing that if my blood sugars were good and I went and did some form of physical activity, if I had a hypo in the middle of a gym hall that was embarrassing for me.”(P3, female, and 18 years of age).
“Sitting, I guess I do it a lot because I’ve got a lot of homework and then studying.”(P2, female, and 13 years of age).
“So, say I hadn’t a decent nights sleep especially if my alarm had been going off or I didn’t go to sleep till late and sometimes I have to wake up early for school. I will be tired and then I might be quite moody and then because I’m tired my levels might go high which then stresses me out because I will be at school, and I don’t like injecting in public. So, then I might get more stressed, and it might make me quite anxious.”(P11, female, and 17 years of age).
3.5.2. Caregivers
“When I was eleven, I would probably get more sleep because I would go to bed earlier, and my parents would expect me to go to bed earlier. But then as I get older, I go to sleep whenever I want, and you have potentially less sleep and also end up just getting less sleep to get up early to go to school and work.”(P1, female, 16 years of age).
“I’m a very deep sleeper so, if I’ve got a high I just sleep like this *imitates sleeping* and my dad just like comes in and says ‘[NAME]’ and I’m like half-awake. So, he just types it into the pump anyway, so it is not an issue.”(P14, male, 13 years of age).
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PA | Physical Activity |
| MVPA | Moderate-to-Vigorous Physical Activity |
| SB | Sedentary Behaviour |
| 24-h MB | 24-Hour Movement Behaviour |
| T1D | Type 1 Diabetes |
References
- American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44 (Suppl. S1), S180–S199. [Google Scholar] [CrossRef] [PubMed]
- Ogle, G.D.; James, S.; Dabelea, D.; Pihoker, C.; Svennson, J.; Maniam, J.; Klatman, E.L.; Patterson, C.C. Global estimates of incidence of type 1 diabetes in children and adolescents: Results from the International Diabetes Federation Atlas. Diabetes Res. Clin. Pract. 2022, 183, 109083. [Google Scholar] [CrossRef] [PubMed]
- Gregory, J.W.; Cameron, F.J.; Joshi, K.; Eiswirth, M.; Garrett, C.; Garvey, K.; Agarwal, S.; Codner, E. ISPAD clinical practice consensus guidelines 2022: Diabetes in adolescence. Pediatr. Diabetes 2022, 23, 857. [Google Scholar] [CrossRef]
- Tilden, D.R.; Noser, A.E.; Jaser, S.S. Sedentary Behavior and Physical Activity Associated with Psychosocial Outcomes in Adolescents with Type 1 Diabetes. Pediatr. Diabetes 2023, 2023, 1395466. [Google Scholar] [CrossRef]
- Huerta-Uribe, N.; Ramirez-Velez, R.; Izquierdo, M.; Garcia-Hermoso, A. Association between physical activity, sedentary behavior and physical fitness and glycated hemoglobin in youth with type 1 diabetes: A systematic review and meta-analysis. Sports Med. 2023, 53, 111–123. [Google Scholar] [CrossRef]
- Adolfsson, P.; Taplin, C.E.; Zaharieva, D.P.; Pemberton, J.; Davis, E.A.; Riddell, M.C.; McGavock, J.; Moser, O.; Szadkowska, A.; Lopez, P. ISPAD Clinical Practice Consensus Guidelines 2022: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2022, 23, 1341–1372. [Google Scholar] [CrossRef]
- Patel, N.J.; Savin, K.L.; Kahanda, S.N.; Malow, B.A.; Williams, L.A.; Lochbihler, G.; Jaser, S.S. Sleep habits in adolescents with type 1 diabetes: Variability in sleep duration linked with glycemic control. Pediatr. Diabetes 2018, 19, 1100–1106. [Google Scholar] [CrossRef]
- Barone, M.T.; Wey, D.; Schorr, F.; Franco, D.R.; Carra, M.K.; Lorenzi Filho, G.; Menna-Barreto, L. Sleep and glycemic control in type 1 diabetes. Arch. Endocrinol. Metab. 2015, 59, 71–78. [Google Scholar] [CrossRef]
- Carreon, S.A.; Cao, V.T.; Anderson, B.J.; Thompson, D.I.; Marrero, D.G.; Hilliard, M.E. ‘I don’t sleep through the night’: Qualitative study of sleep in type 1 diabetes. Diabet. Med. 2022, 39, e14763. [Google Scholar] [CrossRef] [PubMed]
- Patience, M.; Janssen, X.; Kirk, A.; McCrory, S.; Russell, E.; Hodgson, W.; Crawford, M. 24-hour movement behaviours (physical activity, sedentary behaviour and sleep) association with glycaemic control and psychosocial outcomes in adolescents with type 1 diabetes: A systematic review of quantitative and qualitative studies. Int. J. Environ. Res. Public Health 2023, 20, 4363. [Google Scholar] [CrossRef]
- Pedišić, Z.; Dumuid, D.; Olds, T.S. Integrating Sleep, Sedentary Behaviour and Physical Activity Research in the Emerging Field of Time-Use Epidemiology:Definitions, Concepts, Statistical Methods, Theoretical Framework, and Future Directions. Kinesiology 2017, 49, 252–269. [Google Scholar]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Recommended Amount of Sleep for a Healthy Adult: A Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society. J. Clin. Sleep. Med. 2015, 11, 591–592. [Google Scholar] [CrossRef]
- Rollo, S.; Antsygina, O.; Tremblay, M.S. The whole day matters: Understanding 24-hour movement guideline adherence and relationships with health indicators across the lifespan. J. Sport Health Sci. 2020, 9, 493–510. [Google Scholar] [CrossRef]
- Fairclough, S.J.; Clifford, L.; Brown, D.; Tyler, R. Characteristics of 24-hour movement behaviours and their associations with mental health in children and adolescents. J. Act. Sedent. Sleep Behav. 2023, 2, 11. [Google Scholar] [CrossRef]
- Tremblay, M.S.; Carson, V.; Chaput, J.P.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Faulkner, G.; Gray, C.E.; Gruber, R.; Janson, K.; et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016, 41 (Suppl. S3), S311–S327. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Dash, K.; Goyder, E.C.; Quirk, H. A qualitative synthesis of the perceived factors that affect participation in physical activity among children and adolescents with type 1 diabetes. Diabet. Med. 2020, 37, 934–944. [Google Scholar] [CrossRef]
- Fried, L.; Chetty, T.; Cross, D.; Breen, L.; Davis, E.; Roby, H.; Jackiewicz, T.; Nicholas, J.; Jones, T. The challenges of being physically active: A qualitative study of young people with type 1 diabetes and their parents. Can. J. Diabetes 2021, 45, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bergner, E.M.; Williams, R.; Hamburger, E.R.; Lyttle, M.; Davis, A.C.; Malow, B.; Simmons, J.H.; Lybarger, C.; Capin, R.; Jaser, S.S. Sleep in Teens With Type 1 Diabetes: Perspectives From Adolescents and Their Caregivers. Diabetes Educ. 2018, 44, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Denzin, N.K.; Lincoln, Y.S. The Sage Handbook of Qualitative Research; Sage: Thousand Oaks, CA, USA, 2011. [Google Scholar]
- Creswell, J.W.; Poth, C.N. Qualitative Inquiry and Research Design: Choosing Among Five Approaches; Sage Publications: Thousand Oaks, CA, USA, 2016. [Google Scholar]
- Grewal, R.; Gupta, S.; Hamilton, R. Marketing Insights from Multimedia Data: Text, Image, Audio, and Video; SAGE Publications Sage: Los Angeles, CA, USA, 2021; pp. 1025–1033. [Google Scholar]
- Varni, J.W.; Delamater, A.M.; Hood, K.K.; Raymond, J.K.; Chang, N.T.; Driscoll, K.A.; Wong, J.C.; Yi-Frazier, J.P.; Grishman, E.K.; Faith, M.A. PedsQL 3.2 diabetes module for children, adolescents, and young adults: Reliability and validity in type 1 diabetes. Diabetes Care 2018, 41, 2064–2071. [Google Scholar] [CrossRef]
- Gray, L.M.; Wong-Wylie, G.; Rempel, G.R.; Cook, K. Expanding qualitative research interviewing strategies: Zoom video communications. Qual. Rep. 2020, 25, 1292–1301. [Google Scholar] [CrossRef]
- Mepieza, R.Y. The Power of Ice Breaker Activity: Examining the Impact of Icebreakers on Student Participation and Engagement in the Classroom. Eur. J. Learn. Hist. Soc. Sci. 2024, 1, 22–36. [Google Scholar]
- Merriam, S.B.; Tisdell, E.J. Qualitative Research: A Guide to Design and Implementation; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Strauss, A.; Corbin, J. Basics of Qualitative Research; Sage: Thousand Oaks, CA, USA, 1998. [Google Scholar]
- Hennink, M.; Kaiser, B.N. Sample sizes for saturation in qualitative research: A systematic review of empirical tests. Soc. Sci. Med. 2022, 292, 114523. [Google Scholar] [CrossRef]
- McMullin, C. Transcription and qualitative methods: Implications for third sector research. Volunt. Int. J. Volunt. Nonprofit Organ. 2023, 34, 140–153. [Google Scholar] [CrossRef]
- Oliver, D.G.; Serovich, J.M.; Mason, T.L. Constraints and opportunities with interview transcription: Towards reflection in qualitative research. Soc. Forces 2005, 84, 1273–1289. [Google Scholar] [CrossRef]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006, 3, 77–101. [Google Scholar] [CrossRef]
- Braund, H.; Turnnidge, J.; Cofie, N.; Kuforiji, O.; Greco, S.; Hastings-Truelove, A.; Hill, S.; Dalgarno, N. Six ways to get a grip on developing reflexivity statements. Can. Med. Educ. J. 2024, 15, 146–149. [Google Scholar] [CrossRef]
- Carson, V.; Hunter, S.; Kuzik, N.; Gray, C.E.; Poitras, V.J.; Chaput, J.-P.; Saunders, T.J.; Katzmarzyk, P.T.; Okely, A.D.; Connor Gorber, S.; et al. Systematic review of sedentary behaviour and health indicators in school-aged children and youth: An update. Appl. Physiol. Nutr. Metab. 2016, 41 (Suppl. S3), S240–S265. [Google Scholar] [CrossRef]
- Sampasa-Kanyinga, H.; Lien, A.; Hamilton, H.A.; Chaput, J.-P. The Canadian 24-hour movement guidelines and self-rated physical and mental health among adolescents. Can. J. Public Health 2022, 113, 312–321. [Google Scholar] [CrossRef]
- Müller, T.; Klein-Flügge, M.C.; Manohar, S.G.; Husain, M.; Apps, M.A. Neural and computational mechanisms of momentary fatigue and persistence in effort-based choice. Nat. Commun. 2021, 12, 4593. [Google Scholar] [CrossRef] [PubMed]
- Perez, K.M.; Hamburger, E.R.; Lyttle, M.; Williams, R.; Bergner, E.; Kahanda, S.; Cobry, E.; Jaser, S.S. Sleep in Type 1 Diabetes: Implications for Glycemic Control and Diabetes Management. Curr. Diabetes Rep. 2018, 18, 5. [Google Scholar] [CrossRef]
- Macaulay, G.C.; Boucher, S.E.; Yogarajah, A.; Galland, B.C.; Wheeler, B.J. Sleep and night-time caregiving in parents of children and adolescents with type 1 diabetes mellitus–a qualitative study. Behav. Sleep Med. 2020, 18, 622–636. [Google Scholar] [CrossRef]
- Livny, R.; Said, W.; Shilo, S.; Bar-Yoseph, R.; Gal, S.; Oren, M.; Levy, M.; Weiss, R.; Shehadeh, N.; Zuckerman-Levin, N. Identifying sources of support and barriers to physical activity in pediatric type 1 diabetes. Pediatr. Diabetes 2020, 21, 128–134. [Google Scholar] [CrossRef]
- Rodrigo-Sanjoaquín, J.; Bois, J.E.; Aibar Solana, A.; Lhuisset, L.; Corral-Abós, A.; Zaragoza Casterad, J. Are school-based interventions promoting 24-hour movement guidelines among children? A scoping review. Health Educ. J. 2023, 82, 444–460. [Google Scholar] [CrossRef]
- Donnelly, S.; Buchan, D.S.; McLellan, G.; Roberts, R.; Arthur, R. Exploring the feasibility of a cluster pilot randomised control trial to improve children’s 24-hour movement behaviours and dietary intake: Happy homework. J. Sports Sci. 2023, 41, 1787–1800. [Google Scholar] [CrossRef]
- Parrish, A.-M.; Okely, A.D.; Salmon, J.; Trost, S.; Hammersley, M.; Murdoch, A. Making ‘being less sedentary feel normal’–investigating ways to reduce adolescent sedentary behaviour at school: A qualitative study. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 85. [Google Scholar] [CrossRef]
- MacMillan, F.; Kirk, A.; Mutrie, N.; Moola, F.; Robertson, K. Supporting participation in physical education at school in youth with type 1 diabetes. Eur. Phys. Educ. Rev. 2014, 21, 3–30. [Google Scholar] [CrossRef]
- Duncan, S.; Stewart, T.; Mackay, L.; Neville, J.; Narayanan, A.; Walker, C.; Berry, S.; Morton, S. Wear-Time Compliance with a Dual-Accelerometer System for Capturing 24-h Behavioural Profiles in Children and Adults. Int. J. Environ. Res. Public Heal. 2018, 15, 1296. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, S.J.; Noonan, R.; Rowlands, A.V.; VAN Hees, V.; Knowles, Z.; Boddy, L.M. Wear Compliance and Activity in Children Wearing Wrist- and Hip-Mounted Accelerometers. Med. Sci. Sports Exerc. 2016, 48, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Fairclough, S.J.; Rowlands, A.V.; Cruz, B.d.P.; Crotti, M.; Foweather, L.; Graves, L.E.F.; Hurter, L.; Jones, O.; MacDonald, M.; McCann, D.A.; et al. Reference values for wrist-worn accelerometer physical activity metrics in England children and adolescents. Int. J. Behav. Nutr. Phys. Act. 2023, 20, 1–14. [Google Scholar] [CrossRef]
- Hildebrand, M.; VAN Hees, V.T.; Hansen, B.H.; Ekelund, U. Age Group Comparability of Raw Accelerometer Output from Wrist- and Hip-Worn Monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Hildebrand, M.; Hanson, B.H.; van Hees, V.T.; Ekelund, U. Evaluation of raw acceleration sedentary thrsholds in children and adults. Scand. J. Med. Sci. Sports 2017, 27, 1814–1823. [Google Scholar] [CrossRef]
- Rosenberger, M.E.; Fulton, J.E.; Buman, M.P.; Troiano, R.P.; Grandner, M.A.; Buchner, D.M.; Haskell, W.L. The 24-Hour Activity Cycle: A New Paradigm for Physical Activity. Med. Sci. Sports Exerc. 2019, 51, 454–464. [Google Scholar] [CrossRef] [PubMed]
- van Hees, V.T.; Sabia, S.; Anderson, K.N.; Denton, S.J.; Oliver, J.; Catt, M.; Abell, J.G.; Kivimäki, M.; Trenell, M.I.; Singh-Manoux, A.; et al. A Novel, Open Access Method to Assess Sleep Duration Using a Wrist-Worn Accelerometer. PLoS ONE 2015, 10, e0142533. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, V.T.; Gorzelniak, L.; Dean León, E.C.; Eder, M.; Pias, M.; Taherian, S.; Ekelund, U.; Renström, F.; Franks, P.W.; Horsch, A.; et al. Separating Movement and Gravity Components in an Acceleration Signal and Implications for the Assessment of Human Daily Physical Activity. PLoS ONE 2013, 8, e61691. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Full Sample (n = 15) |
|---|---|
| Interview Length (Minutes) | 23 ± 6.5 |
| Age | |
| Younger Adolescent (11–15 Years) | 8 (53.3) |
| Older Adolescent (16–18 Years) | 7 (46.7) |
| Sex | |
| Female | 9 (60) |
| Male | 6 (40) |
| Ethnicity | |
| White | 15 (100) |
| Insulin Delivery | |
| Pen | 5 (33.3) |
| Pump | 10 (66.7) |
| Blood Glucose Measurement Method | |
| CGM | 12 (80) |
| Finger-Prick | 3 (20) |
| Age (Years) | 14.6 ± 2.0 |
| Diabetes Duration (Years) | 3.7 ± 3.1 |
| HbA1c (%) | 7.4 ± 1.0 |
| Paediatric Quality of Life Total Score | 62.7 ± 14.3 |
| Sleep (Hours·per Day) a | 8.1 ± 0.7 |
| SED (Hours·per Day) a | 9.8 ± 1.7 |
| MVPA (Min·per Day) a | 28.1 ± 24 |
| Theme | Subtheme | Definition |
|---|---|---|
| Theme 1: Sleep and PA understood and valued above SB | Adolescent understands and values sleep and PA more than SB | |
| Theme 2: Recognition of movement behaviours’ interconnection | Adolescent perceptions on how sleep, PA, and SB might interact and impact one another | |
| Theme 3: Movement behaviours interaction with health outcomes | Mood | Adolescent perceptions on how sleep, PA, and SB interact with their mood |
| Glycaemic control | Adolescent mixed perceptions on how sleep, PA, and SB interact with their glycaemic control | |
| Glycaemic control as a barrier to movement behaviours | Adolescent perceptions of glycaemic control as a barrier to movement behaviours | |
| Theme 4: Movement behaviours within the environmental context of the adolescent | School | Adolescent perceptions of school and how it affects their sleep, PA, and SB participation and understanding |
| Caregivers | Adolescent perceptions of their caregivers’ role in their sleep, PA, and SB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patience, M.; Kirk, A.; Janssen, X.; Sanders, J.; Crawford, M. “If You Haven’t Slept a Lot (…) You Don’t Want to Go Out for a Run, You Don’t Want to Ride a Bike, You Just Kind of Sit and You Just (…) Do Nothing”—Perceptions of 24-Hour Movement Behaviours Among Adolescents Living with Type 1 Diabetes. Int. J. Environ. Res. Public Health 2025, 22, 1295. https://doi.org/10.3390/ijerph22081295
Patience M, Kirk A, Janssen X, Sanders J, Crawford M. “If You Haven’t Slept a Lot (…) You Don’t Want to Go Out for a Run, You Don’t Want to Ride a Bike, You Just Kind of Sit and You Just (…) Do Nothing”—Perceptions of 24-Hour Movement Behaviours Among Adolescents Living with Type 1 Diabetes. International Journal of Environmental Research and Public Health. 2025; 22(8):1295. https://doi.org/10.3390/ijerph22081295
Chicago/Turabian StylePatience, Mhairi, Alison Kirk, Xanne Janssen, James Sanders, and Megan Crawford. 2025. "“If You Haven’t Slept a Lot (…) You Don’t Want to Go Out for a Run, You Don’t Want to Ride a Bike, You Just Kind of Sit and You Just (…) Do Nothing”—Perceptions of 24-Hour Movement Behaviours Among Adolescents Living with Type 1 Diabetes" International Journal of Environmental Research and Public Health 22, no. 8: 1295. https://doi.org/10.3390/ijerph22081295
APA StylePatience, M., Kirk, A., Janssen, X., Sanders, J., & Crawford, M. (2025). “If You Haven’t Slept a Lot (…) You Don’t Want to Go Out for a Run, You Don’t Want to Ride a Bike, You Just Kind of Sit and You Just (…) Do Nothing”—Perceptions of 24-Hour Movement Behaviours Among Adolescents Living with Type 1 Diabetes. International Journal of Environmental Research and Public Health, 22(8), 1295. https://doi.org/10.3390/ijerph22081295









