Using Functional Near-Infrared Spectroscopy to Elucidate Neurophysiological Mechanism of Action of Equine-Assisted Services: Proof-of-Concept Study
Abstract
1. Introduction
2. Methods
2.1. Recruitment
2.2. Participants and Procedures
2.3. Measures
2.3.1. Neurological Measurements
2.3.2. Psychological Assessments
2.4. Statistical Analysis
2.4.1. Psychological Assessments Analysis
2.4.2. fNIRS Data Analysis
3. Results
3.1. Subjects
3.2. Psychometric Instrument Results
3.3. Safety Results
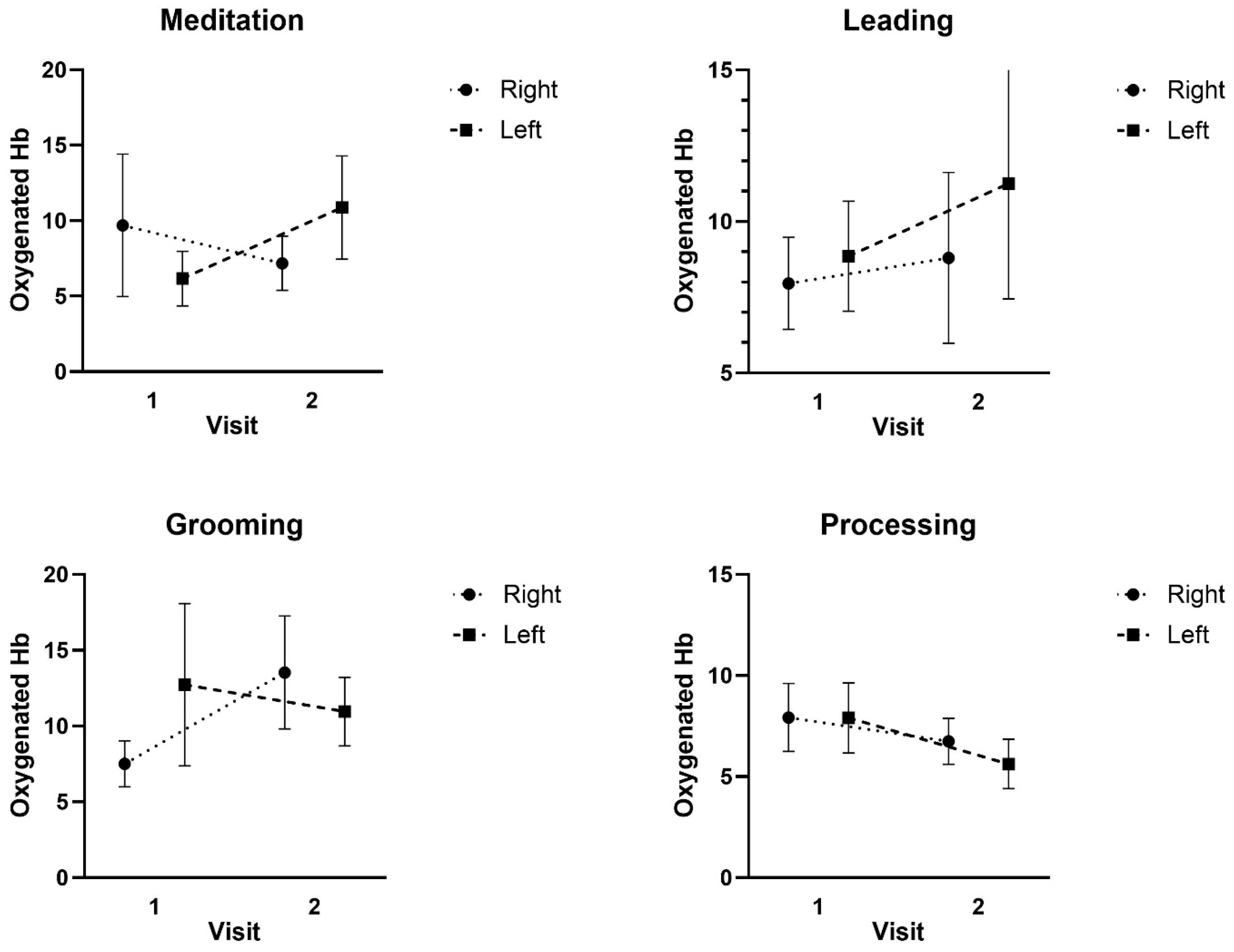
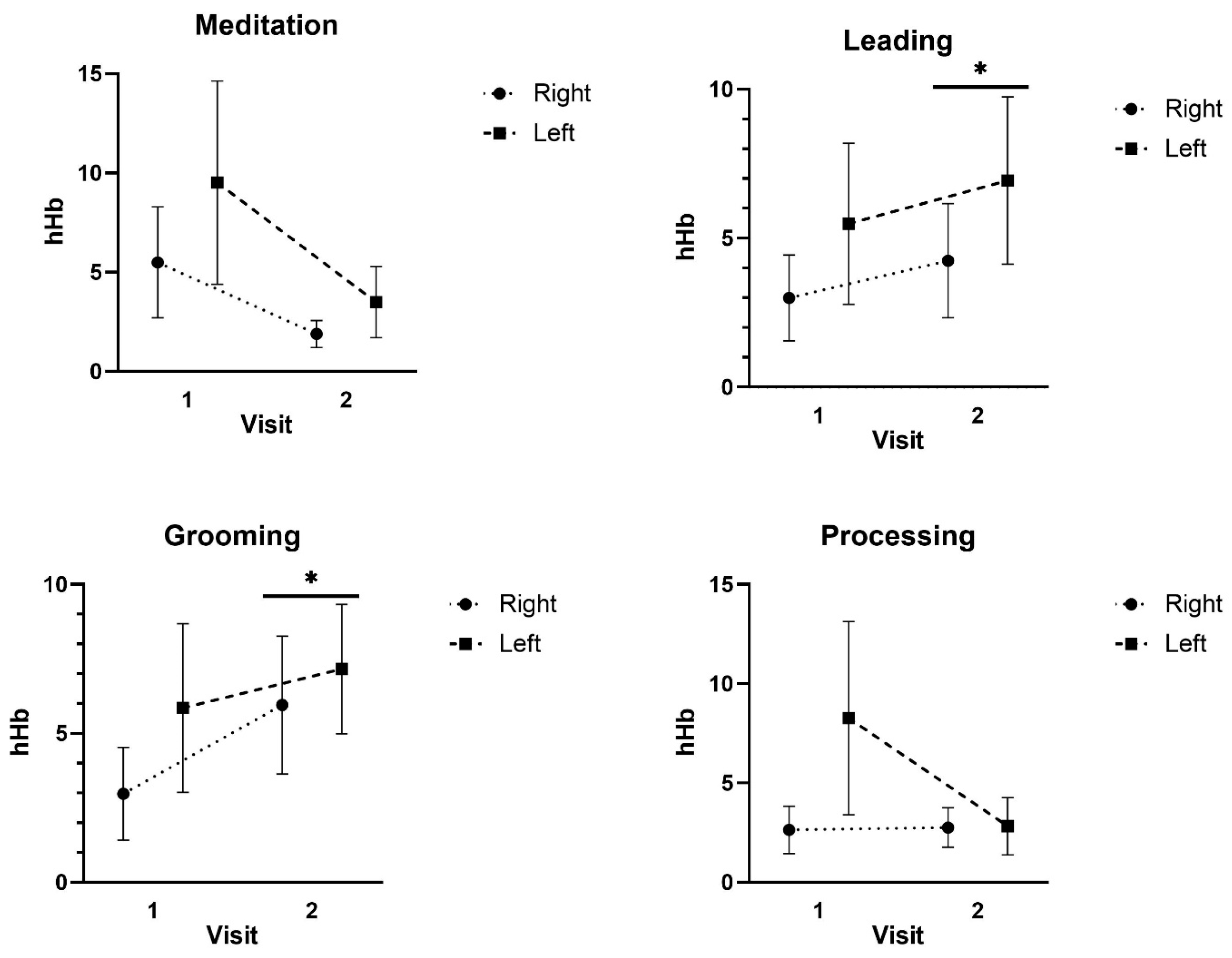
3.4. fNIRS Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goldstein, R.B.; Smith, S.M.; Chou, S.P.; Saha, T.D.; Jung, J.; Zhang, H.; Pickering, R.P.; Ruan, W.J.; Huang, B.; Grant, B.F. The epidemiology of DSM-5 posttraumatic stress disorder in the United States: Results from the national epidemiologic survey on alcohol and related conditions-III. Soc. Psychiatry Psychiatr. Epidemiol. 2016, 51, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). American Psychiatric Pub. 2013. Available online: https://psycnet.apa.org/record/2013-14907-000 (accessed on 6 January 2025).
- Kessler, R.C.; Wang, P.S. The descriptive epidemiology of commonly occurring mental disorders in the United States. Ann. Rev. Public Health 2008, 29, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.J.; Hamblen, J.L.; Holtzheimer, P.; Kelly, U.; Norman, S.B.; Riggs, D.; Schnurr, P.P.; Wiechers, I. A clinician’s guide to the 2023 VA/DOD clinical practice guideline for management of posttraumatic stress disorder and acute stress disorder. J. Traum. Stress 2024, 37, 19–34. [Google Scholar] [CrossRef]
- Vella, E.J.; Milligan, B.; Bennett, J.L. Participation in outdoor recreation program predicts improved psychosocial well-being among veterans with post-traumatic stress disorder: A pilot study. Mil. Med. 2013, 178, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Sylvia, L.; West, E.; Blackburn, A.M.; Gupta, C.; Bui, E.; Mahoney, T.; Duncan, G.; Wright, E.C.; Lejeune, S.; Spencer, T.J. Acceptability of an adjunct equine-assisted activities and therapies program for veterans with posttraumatic stress disorder and/or traumatic brain injury. J. Integr. Med. 2020, 18, 169–173. [Google Scholar] [CrossRef]
- Steenkamp, M.M.; Litz, B.T.; Hoge, C.W.; Marmar, C.R. Psychotherapy for military-related PTSD: A review of randomized clinical trials. JAMA 2015, 314, 489–500. [Google Scholar] [CrossRef]
- Kehle-Forbes, S.M.; Chen, S.; Polusny, M.A.; Lynch, K.G.; Koffel, E.; Ingram, E.; Foa, E.B.; Van Horn, D.H.A.; Drapkin, M.L.; Yusko, D.A.; et al. A randomized controlled trial evaluating integrated versus phased application of evidence-based psychotherapies for military veterans with comorbid PTSD and substance use disorders. Drug Alcohol Depen. 2019, 205, 107647. [Google Scholar] [CrossRef]
- Wood, W.; Alm, K.; Benjamin, J.; Thomas, L.; Anderson, D.; Pohl, L.; Kane, M. Optimal terminology for services in the United States that incorporate horses to benefit people: A consensus document. J. Alt. Compl. Med. 2021, 27, 88–95. [Google Scholar] [CrossRef]
- Provan, M.; Ahmed, Z.; Stevens, A.R.; Sardeli, A.V. Are equine-assisted services beneficial for military veterans with post-traumatic stress disorder? A systematic review and meta-analysis. BMC Psychiatry 2024, 24, 544. [Google Scholar] [CrossRef]
- Bachi, K. Application of Attachment Theory to Equine-Facilitated Psychotherapy. J. Contemp. Psychother. 2013, 43, 187–196. [Google Scholar] [CrossRef]
- Meola, C.C. Addressing the needs of the millennial workforce through equine assisted learning. J. Manag. Dev. 2016, 35, 294–303. [Google Scholar] [CrossRef]
- Rentko, V.T.; Warner, A.E.; Timlege, E.; Richman, E. Equine-assisted learning—An experiential, facilitated learning model for development of professional skills and resiliency in veterinary students. J. Vet. Med. Edu. 2023, 50, 413–420. [Google Scholar] [CrossRef]
- Lanning, B.A.; Wilson, A.L.; Krenek, N.; Beaujean, A.A. Using therapeutic riding as an intervention for combat veterans: An international classification of functioning, disability, and health (ICF) approach. Occup. Ther. Ment. Health 2017, 33, 259–278. [Google Scholar] [CrossRef]
- Shelef, A.; Brafman, D.; Rosing, T.; Weizman, A.; Stryjer, R.; Barak, Y. Equine assisted therapy for patients with post traumatic stress disorder: A case series study. Mil. Med. 2019, 184, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R. Potential mechanisms of action and outcomes of equine-assisted services for veterans with a history of trauma: A narrative review of literature. Int. J. Environ. Res. Public Health 2023, 20, 6377. [Google Scholar] [CrossRef]
- Kinney, A.R.; Eakman, A.M.; Lassell, R.; Wood, W. Equine-assisted interventions for veterans with service-related health conditions: A systematic mapping review. Mil. Med. Res. 2019, 6, 28. [Google Scholar] [CrossRef]
- Marchand, W.R.; Andersen, S.J.; Smith, J.E.; Hoopes, K.H.; Carlson, J.K. Equine-assisted activities and therapies for veterans with posttraumatic stress disorder: Current state, challenges and future directions. Chronic Stress 2021, 5, 2470547021991556. [Google Scholar] [CrossRef]
- García-Gómez, A.; Guerrero-Barona, E.; García-Peña, I.; Rodríguez-Jiménez, M.; Moreno-Manso, J.M. Equine-assisted therapeutic activities and their influence on the heart rate variability: A systematic review. Compl. Ther. Clin. Pract. 2020, 39, 101167. [Google Scholar] [CrossRef] [PubMed]
- McDuffee, L.A.; Montelpare, W.J.; LeBlanc, C. Psychophysiological effects of equine-facilitated psychotherapy on veterans with PTSD and their horse partners. J. Mil. Veteran Fam. Health 2024, 10, 135–147. [Google Scholar] [CrossRef]
- Zhu, X.; Suarez-Jimenez, B.; Zilcha-Mano, S.; Lazarov, A.; Arnon, S.; Lowell, A.L.; Bergman, M.; Ryba, M.; Hamilton, A.J.; Hamilton, J.F.; et al. Neural changes following equine-assisted therapy for posttraumatic stress disorder: A longitudinal multimodal imaging study. Hum. Brain Mapp. 2021, 42, 1930–1939. [Google Scholar] [CrossRef]
- Ben-Zion, Z.; Korem, N.; Fine, N.B.; Katz, S.; Siddhanta, M.; Funaro, M.C.; Duek, O.; Spiller, T.R.; Danböck, S.K.; Levy, I.; et al. Structural neuroimaging of hippocampus and amygdala subregions in posttraumatic stress disorder: A scoping review. Biol. Psychiat. Glob. Open Sci. 2024, 4, 120–134. [Google Scholar] [CrossRef] [PubMed]
- Balters, S.; Li, R.; Espil, F.M.; Piccirilli, A.; Liu, N.; Gundran, A.; Carrion, V.G.; Weems, C.F.; Cohen, J.A.; Reiss, A.L. Functional near-infrared spectroscopy brain imaging predicts symptom severity in youth exposed to traumatic stress. J. Psychiat. Res. 2021, 144, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Gramlich, M.A.; Neer, S.M.; Beidel, D.C.; Bohil, C.J.; Bowers, C.A. A functional near-infrared spectroscopy study of trauma-related auditory and olfactory cues: Posttraumatic stress disorder or combat experience? J. Trauma Stress 2017, 30, 656–665. [Google Scholar] [CrossRef]
- Kalanadhabhatta, M.; Roy, S.; Grant, T.; Salekin, A.; Rahman, T.; Bergen-Cico, D. Detecting PTSD using neural and physiological signals: Recommendations from a pilot study. In Proceedings of the 2023 11th International Conference on Affective Computing and Intelligent Interaction (ACII), Cambridge, MA, USA, 10–13 September 2023; pp. 1–8. [Google Scholar] [CrossRef]
- Cazzell, M.; Li, L.; Lin, Z.J.; Patel, S.J.; Liu, H. Comparison of neural correlates of risk decision making between genders: An exploratory fNIRS study of the Balloon Analogue Risk Task (BART). Neuroimage 2012, 62, 1896–1911. [Google Scholar] [CrossRef]
- Saleem, S.; Hussain, M.M.; Majeed, S.M.; Khan, M.A. Gender differences of heart rate variability in healthy volunteers. J. Pak. Med. Assoc. 2012, 62, 422–425. [Google Scholar]
- Verkuil, B.; Brosschot, J.F.; Marques, A.H.; Kampschroer, K.; Sternberg, E.M.; Thayer, J.F. Gender differences in the impact of daily sadness on 24-h heart rate variability. Psychophysiology 2015, 52, 1682–1688. [Google Scholar] [CrossRef]
- Helpman, L.; Suarez-Jimenez, B.; Lazarov, A.; Monk, C.; Neria, Y. Sex differences in trauma-related psychopathology: A critical review of neuroimaging literature (2014–2017). Curr. Psychiatry Rep. 2017, 19, 104. [Google Scholar] [CrossRef]
- Marchand, W.R.; Lackner, R.; Hartquist, A.; Finnell, L.; Nazarenko, E. Evaluation of a mindfulness and self-compassion-based psychotherapy incorporating horses for veterans who have experienced trauma. Compl. Ther. Med. 2023, 72, 102914. [Google Scholar] [CrossRef]
- Professional Association of Therapeutic Horsemanship International. Available online: https://www.pathintl.org/ (accessed on 6 December 2024).
- Holtzer, R.; Mahoney, J.R.; Izzetoglu, M.; Izzetoglu, K.; Onaral, B.; Verghese, J. fNIRS study of walking and walking while talking in young and old individuals. J. Gerontol. Biol. Sci. Med. Sci. 2011, 66, 879–887. [Google Scholar] [CrossRef]
- Suzuki, M.; Miyai, I.; Ono, T.; Kubota, K. Activities in the frontal cortex and gait performance are modulated by preparation. An fNIRS study. Neuroimage 2008, 39, 600–607. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Dan, I.; Tsuzuki, D.; Kato, M.; Okamoto, M.; Kyutoku, Y.; Soya, H. Acute moderate exercise elicits increased dorsolateral prefrontal activation and improves cognitive performance with Stroop test. Neuroimage 2010, 50, 1702–1710. [Google Scholar] [CrossRef]
- Bond, F.W.; Hayes, S.C.; Baer, R.A.; Carpenter, K.M.; Guenole, N.; Orcutt, H.K.; Waltz, T.; Zettle, R.D. Preliminary psychometric properties of the Acceptance and Action Questionnaire-II: A revised measure of psychological inflexibility and experiential avoidance. Behav. Therapy 2011, 42, 676–688. [Google Scholar] [CrossRef]
- Marteau, T.M.; Bekker, H. The development of a six-item short-form of the State Scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br. J. Clin. Psychol. 1992, 31, 301–306. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of Positive and Negative Affect: The PANAS Scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Yoo, J.H.; Oh, Y.; Jang, B.; Song, J.; Kim, J.; Kim, S.; Lee, J.; Shin, H.Y.; Kwon, J.Y.; Kim, Y.H.; et al. The effects of equine-assisted activities and therapy on resting-state brain function in attention-deficit/hyperactivity disorder: A pilot study. Clin. Psychopharmacol. Neurosci. 2016, 14, 357–364. [Google Scholar] [CrossRef]
- Carius, D.; Herold, F.; Clauss, M.; Kaminski, E.; Wagemann, F.; Sterl, C.; Ragert, P. Increased cortical activity in novices compared to experts during table tennis: A whole-brain fNIRS study using threshold-free cluster enhancement analysis. Brain Topogr. 2023, 36, 500–516. [Google Scholar] [CrossRef]
- Tempest, G.D.; Reiss, A.L. The utility of functional near-infrared spectroscopy for measuring cortical activity during cycling exercise. Med. Sci. Sports Exerc. 2019, 51, 979–987. [Google Scholar] [CrossRef]
- Jones, J.L. Horse Brain, Human Brain: The Neuroscience of Horsemanship; Trafalgar Square Books: North Pomfret, VT, USA, 2020. [Google Scholar]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Gunaydin, L.A.; Kreitzer, A.C. Cortico–basal ganglia circuit function in psychiatric disease. Ann. Rev. Physiol. 2016, 78, 327–350. [Google Scholar] [CrossRef]
- Marchand, W.R.; Joubert, K.; Smith, J.; Nazarenko, E.; Klinger, W.; Sheppard, S.; Hoopes, K.H. A pilot observational study of implementing an equine-a ssisted services program within a VA medical center residential substance use disorder treatment program. Mil. Med. 2023, 188, e2175–e2180. [Google Scholar] [CrossRef]
- Monroe, M.; Whitworth, J.D.; Wharton, T.; Turner, J. Effects of an equine-assisted therapy program for military veterans with self-reported PTSD. Soc. Anim. 2019, 29, 577–590. [Google Scholar] [CrossRef]
- Romaniuk, M.; Evans, J.; Kidd, C. Evaluation of an equine-assisted therapy program for veterans who identify as 'wounded, injured or ill' and their partners. PLoS ONE 2018, 13, e0203943. [Google Scholar] [CrossRef]
- Gehrke, E.K.; Noquez, A.E.; Ranke, P.L.; Myers, M.P. Measuring the psychophysiological changes in combat veterans participating in an equine therapy program. J. Mil. Veteran Fam. Health 2018, 4, 60–69. [Google Scholar] [CrossRef]
- Gehrke, E.K.; Tontz, P.; Bhawal, R.; Schiltz, P.; Mendez, S.; Myers, M.P. A mixed-method analysis of an equine complementary therapy program to heal combat veterans. J. Compl. Alter. Healthc. 2018, 8, 555739. [Google Scholar] [CrossRef]
- Fisher, P.W.; Lazarov, A.; Lowell, A.; Arnon, S.; Turner, B.; Bergman, M.; Ryba, M.; Such, S.; Marohasy, C.; Zhu, X.; et al. Equine-assisted therapy for posttraumatic stress disorder among military veterans: An open trial. J. Clin. Psychiatry 2021, 82, 36449. [Google Scholar] [CrossRef]

| Activity | Facilitator(s) | Details | Role of Equine | Duration |
|---|---|---|---|---|
| (A) Equine grooming | ES | Instruction by the ES on how to properly groom a horse and subsequent practice by participant. | Receives grooming | Ten minutes |
| (B) Equine groundwork | ES | Instruction by the ES on groundwork and subsequent practice by participant. | Participates in activities | Ten minutes |
| (C) Mindfulness meditation | MH (ES participating in meditation) | MH provides a brief description of mindfulness and then leads a breath & body focused mindfulness meditation | Near activity but not directly involved | Ten minutes |
| (D) Processing/discussion | MH (ES participating in discussion) | MH facilitates discussion about experimental activities | Near activity but not directly involved | Ten minutes |
| Characteristic | n/M | %/SD |
|---|---|---|
| Age | 40.5 | 7.5 |
| Male gender | 15 | 100% |
| Race | ||
| White | 12 | 80% |
| Black | 1 | 0.7% |
| Asian | 2 | 1.3% |
| Military branch | ||
| Army | 9 | 60% |
| Navy | 1 | 0.7% |
| Marine Corps | 3 | 20% |
| Air Force | 2 | 1.3% |
| Combat deployments | ||
| Yes | 13 | 87% |
| No | 2 | 13% |
| Military-related disability | 15 | 100% |
| Psychiatric diagnoses | ||
| PTSD | 15 | 100% |
| Depressive disorder | 5 | 33% |
| Anxiety disorder | 3 | 20% |
| Pain disorder | 9 | 60% |
| Number of medical diagnoses | 4.7 | 2.6 |
| T1 | T2 | ||||
|---|---|---|---|---|---|
| Assessment | M (SD) | M (SD) | T (df) | p | 95% CI |
| STAI-Day 1 | 14.13 (3.23) | 8.67 (2.71) | −7.24 (14) | <0.001 | [−7.086, −3.847] |
| STAI-Day 2 | 13.71 (3.68) | 9.14 (2.98) | −0.8955 (13) | <0.001 | [−5.674, −3.469] |
| AAQ-II Day 1 | 37.73 (8.11) | 34.07 (8.32) | −3.214 (14) | 0.003 | [−6.114, −1.220] |
| AAQ-II Day 2 | 36.71 (10.37) | 32.64 (8.82) | −3.337 (13) | 0.003 | [−6.707, −1.436] |
| PANAS Positive-Day 1 | 30.93 (6.90) | 36.20 (6.38) | 4.861 (13) | <0.001 | [2.857, 7.428] |
| PANAS Positive Day 2 | 27.79 (6.37) | 34.46 (6.46) | 3.692 (12) | 0.001 | [2.775, 10.764] |
| PANAS Negative * Day 1 | Mdn = 14 | Mdn = 12 | Z = −3.20 | 0.001 | [−7.500, −1.000] |
| PANAS Negative * Day 2 | Mdn = 14.5 | Mdn = 12 | Z = −3.06 | 0.002 | [−7.000, −1.500] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanning, B.A.; Smith, C.M.; Ugale, C.; Nazarenko, E.; Marchand, W.R. Using Functional Near-Infrared Spectroscopy to Elucidate Neurophysiological Mechanism of Action of Equine-Assisted Services: Proof-of-Concept Study. Int. J. Environ. Res. Public Health 2025, 22, 1294. https://doi.org/10.3390/ijerph22081294
Lanning BA, Smith CM, Ugale C, Nazarenko E, Marchand WR. Using Functional Near-Infrared Spectroscopy to Elucidate Neurophysiological Mechanism of Action of Equine-Assisted Services: Proof-of-Concept Study. International Journal of Environmental Research and Public Health. 2025; 22(8):1294. https://doi.org/10.3390/ijerph22081294
Chicago/Turabian StyleLanning, Beth A., Cory M. Smith, Cierra Ugale, Elena Nazarenko, and William R. Marchand. 2025. "Using Functional Near-Infrared Spectroscopy to Elucidate Neurophysiological Mechanism of Action of Equine-Assisted Services: Proof-of-Concept Study" International Journal of Environmental Research and Public Health 22, no. 8: 1294. https://doi.org/10.3390/ijerph22081294
APA StyleLanning, B. A., Smith, C. M., Ugale, C., Nazarenko, E., & Marchand, W. R. (2025). Using Functional Near-Infrared Spectroscopy to Elucidate Neurophysiological Mechanism of Action of Equine-Assisted Services: Proof-of-Concept Study. International Journal of Environmental Research and Public Health, 22(8), 1294. https://doi.org/10.3390/ijerph22081294





