Emerging Contaminants in Source and Finished Drinking Waters Across Minnesota (U.S.) and Potential Health Implications
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Selection
2.1.1. Surface Water Network Facilities
2.1.2. Groundwater Network Facilities
2.2. Sample Collection and Analysis
2.3. Data Preparation and Analysis
2.4. Human-Health Risk Screening
3. Results and Discussion
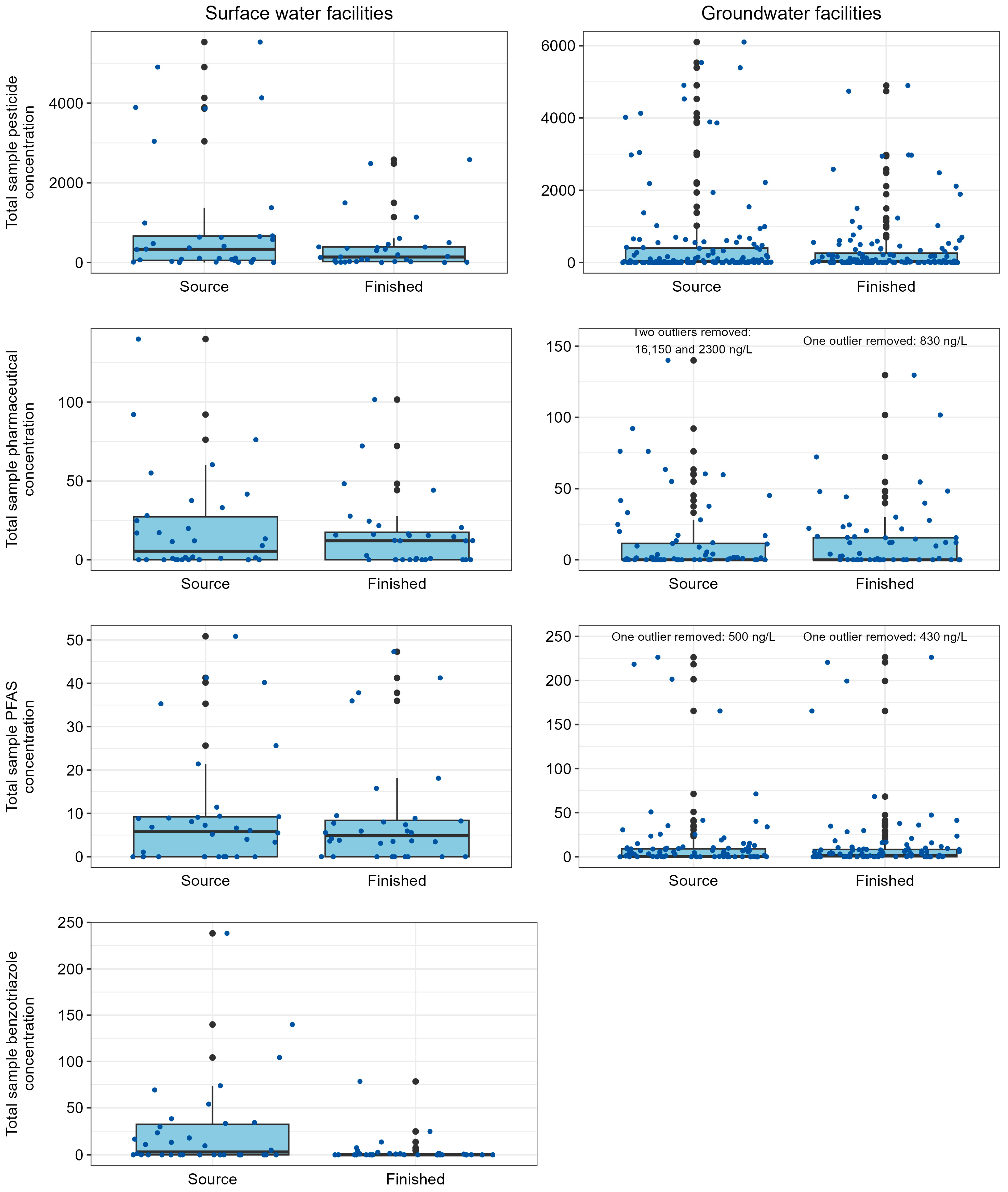
3.1. Contaminant Presence in Source and Finished Drinking Waters
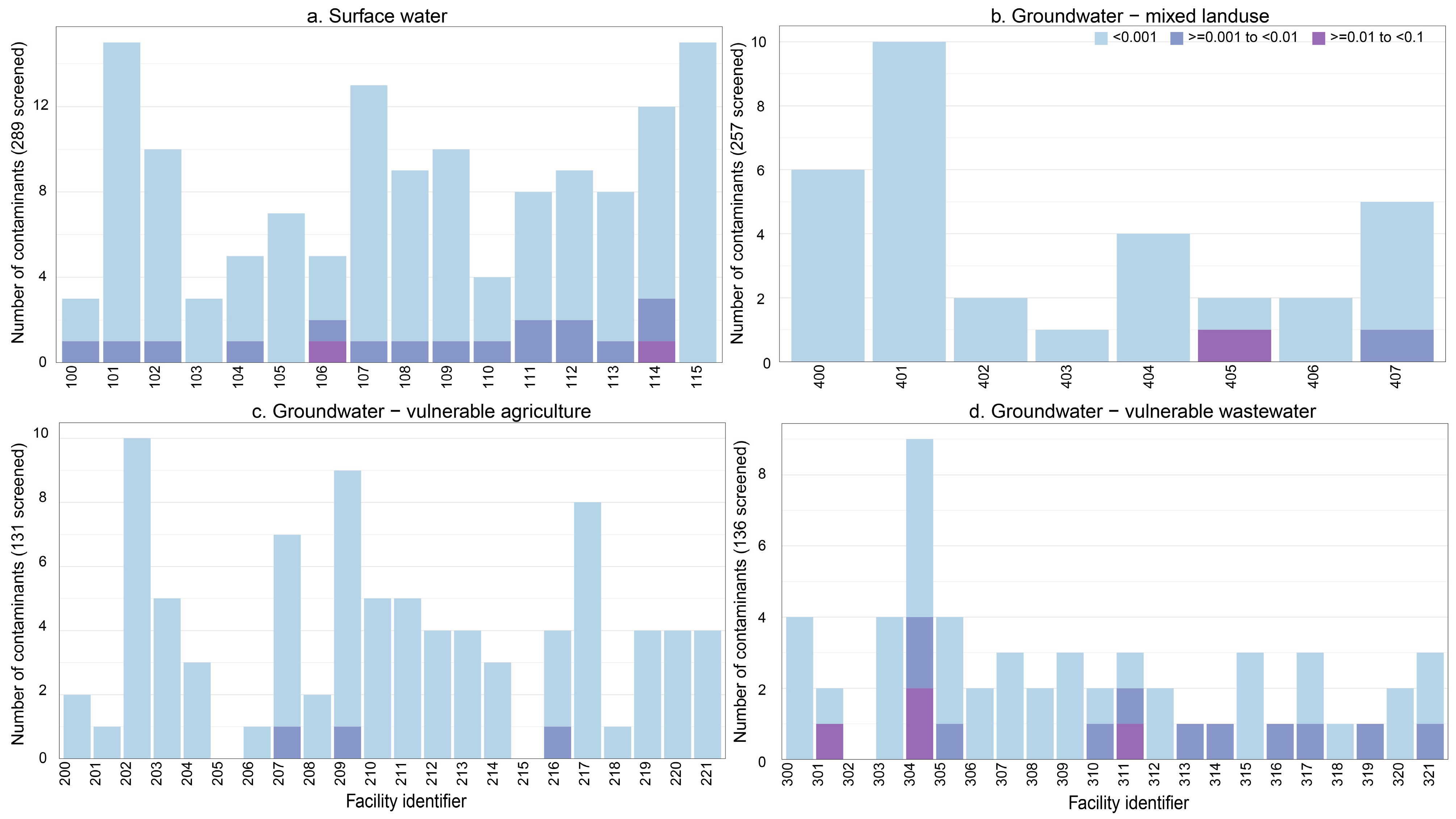
3.1.1. Surface Water Network
3.1.2. Groundwater Sourced Facilities Likely to Be Influenced by Agricultural Activities on the Landscape
Vulnerable Groundwater Wells Likely to Be Influenced by Agricultural Activities on the Landscape
Non-Vulnerable Groundwater Wells Likely to Be Influenced by Agricultural Activities on the Landscape
3.1.3. Groundwater Sourced Facilities Likely to Be Influenced by Wastewater Inputs to the Environment
Vulnerable Groundwater Wells Likely to Be Influenced by Wastewater Inputs to the Environment
Non-Vulnerable Groundwater Wells Likely to Be Influenced by Wastewater Inputs to the Environment
3.1.4. Groundwater Sourced Facilities Likely to Be Influenced by Both Agricultural Activities on the Landscape and Wastewater Inputs to the Environment
3.2. Comparison of Contaminants in Source Waters
3.3. Human-Health Screening
3.3.1. Single Chemical Human-Health Risk Screening
3.3.2. Chemical Mixture Human-Health Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bunting, S.; Lapworth, D.; Crane, E.; Grima-Olmedo, J.; Koroša, A.; Kuczyńska, A.; Mali, N.; Rosenqvist, L.; van Vliet, M.; Togola, A.; et al. Emerging organic compounds in European groundwater. Environ. Pollut. 2021, 269, 115945. [Google Scholar] [CrossRef]
- Reberski, J.L.; Terzić, J.; Maurice, L.D.; Lapworth, D.J. Emerging organic contaminants in karst groundwater: A global level assessment. J. Hydrol. 2022, 604, 127242. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Duttagupta, S.; Mukherjee, A. Emerging organic contaminants in global community drinking water sources and supply: A review of occurrence, processes and remediation. J. Environ. Chem. Eng. 2022, 10, 107560. [Google Scholar] [CrossRef]
- Pereira, L.C.; de Souza, A.O.; Bernardes, M.F.F.; Pazin, M.; Tasso, M.J.; Pereira, P.H.; Dorta, D.J. A perspective on the potential risks of emerging contaminants to human and environmental health. Environ. Sci. Pollut. Res. 2015, 22, 13800–13823. [Google Scholar] [CrossRef] [PubMed]
- United States Congress. Safe Drinking Water Act; United States Congress: Washington, DC, USA, 1974.
- Rosenblum, J.S.; Liethen, A.; Miller-Robbie, L. Prioritization and risk ranking of regulated and unregulated chemicals in US drinking water. Environ. Sci. Technol. 2024, 58, 6878–6889. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Villanueva, C.M.; Beene, D.; Cradock, A.L.; Donat-Vargas, C.; Lewis, J.; Martinez-Morata, I.; Minovi, D.; Nigra, A.E.; Olson, E.D.; et al. US drinking water quality; exposure risk profiles for seven legacy and emerging contaminants. J. Expo. Sci. Env. Epid. 2023, 34, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.M.; Journey, C.A.; Romanok, K.M.; Barber, L.B.; Buxton, H.T.; Foreman, W.T.; Furlong, E.T.; Glassmeyer, S.T.; Hladik, M.L.; Iwanowicz, L.R.; et al. Expanded target-chemical analysis reveals extensive mixed-organic-contaminant exposure in U.S. Streams. Environ. Sci. Technol. 2017, 51, 4792–4802. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Batt, A.L.; Benson, R.; Boone, J.S.; Conerly, O.; Donohue, M.J.; King, D.N.; Kostich, M.S.; et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Sci. Total Environ. 2017, 581–582, 909–922. [Google Scholar] [CrossRef]
- Singh, P.K.; Kumar, U.; Kumar, I.; Dwivedi, A.; Singh, P.; Mishra, S.; Seth, C.S.; Sharma, R.K. Critical review on toxic contaminants in surface water ecosystem: Sources, monitoring, and its impact on human health. Environ. Sci. Pollut. R. 2024, 31, 56428–56462. [Google Scholar] [CrossRef]
- Bai, X.; Lutz, A.; Carroll, R.; Keteles, K.; Dahlin, K.; Murphy, M.; Nguyen, D. Occurrence, distribution, and seasonality of emerging contaminants in urban watersheds. Chemosphere 2018, 200, 133–142. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Lichtfouse, E.; Liu, G.; Balaram, V.; Ribeiro, A.R.L.; Lu, Z.; Stock, F.; Carmona, E.; Teixeira, M.R.; Picos-Corrales, L.A.; et al. Worldwide cases of water pollution by emerging contaminants: A review. Environ. Chem. Lett. 2022, 20, 2311–2338. [Google Scholar] [CrossRef]
- Tran, N.H.; Li, J.; Hu, J.; Ong, S.L. Occurrence and suitability of pharmacetucials and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ. Sci. Pollut. R. 2014, 21, 4727–4740. [Google Scholar] [CrossRef] [PubMed]
- Masoner, J.R.; Kolpin, D.W.; Cozzarelli, I.M.; Barber, L.B.; Burden, D.S.; Foreman, W.T.; Forshay, K.J.; Furlong, E.T.; Groves, J.F.; Hladik, M.L.; et al. Urban stormwater: An overlooked pathway of extensive mixed contaminants to surface and groundwaters in the Unites States. Environ. Sci. Technol. 2019, 53, 10070–10081. [Google Scholar] [CrossRef]
- Santos, V.S.; Anjos, J.S.X.; de Medeiros, J.F.; Montagner, C.C. Impact of agricultural runoff and domestic sewage discharge on the spatial-temporal occurrence of emering contaminants in an urban stream in São Paulo, Brazil. Environ. Monit. Assess. 2022, 194, 637. [Google Scholar] [CrossRef]
- Masoner, J.R.; Kolpin, D.W.; Furlong, E.T.; Cozzarelli, I.M.; Gray, J.L. Landfill leachate as a mirror of today’s disposable society: Pharmaceuticals and other contaminants of emerging concern in final leachate from landfills in the conterminous United States. Environ. Toxicol. Chem. 2016, 35, 906–918. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Toor, G.S.; Wilson, P.C.; Williams, C.F. Micropollutants in groundwater from septic systems: Transformations, transport mechanisms, and human health risk assessment. Water Res. 2017, 123, 258–267. [Google Scholar] [CrossRef]
- Pinasseau, L.; Wiest, J.; Volatier, L.; Mermillod-Blondin, F.; Vulliet, E. Emerging polar pollutants in groundwater: Potential impact of urban stormwater infiltration practices. Environ. Pollut. 2020, 266, 115387. [Google Scholar] [CrossRef] [PubMed]
- Sjerps, R.M.A.; Kooij, P.J.F.; van Loon, A.; Van Wezel, A.P. Occurrence of pesticides in Dutch drinking water sources. Chemosphere 2019, 235, 510–518. [Google Scholar] [CrossRef]
- Smalling, K.L.; Bradley, P.M.; Romanok, K.M.; Elliott, S.M.; de Lambert, J.; Focazio, M.J.; Gordon, S.E.; Gray, J.L.; Kanagy, L.K.; Hladik, M.L.; et al. Exposures and potential health implications of contaminant mixtures in linked source water, finished drinking water, and tapwater from public-supply drinking water systems in Minneapolis/St. Paul area, USA. Environ. Sci. Water Res. Technol. 2023, 9, 1813–1828. [Google Scholar] [CrossRef]
- Bexfield, L.M.; Toccalino, P.L.; Belitz, K.; Foreman, W.T.; Furlong, E.T. Hormones and pharmaceuticals in groundwater used as a source of drinking water across the United States. Environ. Sci. Technol. 2019, 53, 2950–2960. [Google Scholar] [CrossRef]
- Riva, F.; Castiglioni, S.; Fattore, E.; Manenti, A.; Davoli, E.; Zuccato, E. Monitoring emerging contaminants in the drinking water of Milan and assessment of the human risk. Int. J. Hyg. Environ. Health 2018, 221, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Glassmeyer, S.T.; Burns, E.E.; Focazio, M.J.; Furlong, E.T.; Gribble, M.O.; Jahne, M.A.; Keely, S.P.; Kennicutt, A.R.; Kolpin, D.W.; Kakaley, E.K.M.; et al. Water, water everywhere, but every drop unique: Challenges in the science to understand the role of contaminants of emerging concern in the management of drinking water supplies. GeoHealth 2023, 7, e2022GH000716. [Google Scholar] [CrossRef]
- Zhang, S.; Gitungo, S.; Dyksen, J.E.; Raczko, R.F.; Axe, L. Indicator compounds representative of contaminants of emerging concern (CECs) found in the water cycle in the United States. Int. J. Environ. Res. Public Health 2021, 18, 1288. [Google Scholar] [CrossRef] [PubMed]
- Baken, K.A.; Sjerps, R.M.; Schriks, M.; van Wezel, A.P. Toxicological risk assessment and prioritization of drinking water relevant contaminants of emerging concern. Environ. Int. 2018, 118, 293–303. [Google Scholar] [CrossRef]
- Montagner, C.; Sodré, F.; Acayaba, R.; Vidal, C.; Campestrini, I.; Locatelli, M.; Pescara, I.; Albuquerque, A.; Umbuzeiro, G.; Jardim, W. Ten years-snapshot of the occurrence of emerging contaminants in drinking, surface and ground waters and wastewaters from São Paulo State, Brazil. J. Braz. Chem. Soc. 2018, 30, 614–632. [Google Scholar] [CrossRef]
- Johnson, H.; Schaefer, B.; Timm, D.; Rindal, D.; Schliep, A. 2015 Reconnaissance Study of Pesticide Compounds in Community Public Water Supply Wells; Minndesota Department of Agriculture & Minnesota Department of Health: St. Paul, MI, USA, 2016. Available online: https://www.mda.state.mn.us/sites/default/files/inline-files/2015PesticideReconReport_0.pdf (accessed on 17 March 2025).
- Minnesota Department of Health (MDH). Wellhead Protection Vulnerability Fact Sheet; Minnesota Department of Health: St Paul, MN, USA, 2018. Available online: https://www.health.state.mn.us/communities/environment/water/docs/swp/vulnerability.pdf (accessed on 2 June 2025).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 15 June 2024).
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.; Wagner, H. Vegan: Community Ecology Package. 2024. Available online: https://CRAN.R-project.org/package=vegan (accessed on 15 June 2024). [CrossRef]
- Julian, P.; Helsel, D. NADA2: Data Analysis for Censored Environmental Data; R Package: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=NADA2 (accessed on 15 June 2024). [CrossRef]
- DeCicco, L.; Corsi, S.; Villeneuve, D.; Blackwell, B.; Ankley, G.T. toxEval: Exploring Biological Relevance of Environmental Chemistry Observations. 2024. Available online: https://CRAN.R-project.org/package=toxEval (accessed on 1 December 2024). [CrossRef]
- Blackwell, B.R.; Ankley, G.T.; Corsi, S.R.; DeCicco, L.A.; Houck, K.A.; Judson, R.S.; Li, S.; Martin, M.T.; Murphy, E.; Schroeder, A.L.; et al. An ‘EAR’ on Environmental Surveillance and Monitoring: A Case Study on the Use of Exposure–Activity Ratios (EARs) to Prioritize Sites, Chemicals, and Bioactivities of Concern in Great Lakes Waters. Environ. Sci. Technol. 2017, 51, 8713–8724. [Google Scholar] [CrossRef] [PubMed]
- Corsi, S.R.; De Cicco, L.A.; Villeneuve, D.L.; Blackwell, B.R.; Fay, K.A.; Ankley, G.T.; Baldwin, A.K. Prioritizing chemicals of ecological concern in Great Lakes tributaries using high-throughput screening data and adverse outcome pathways. Sci. Total Environ. 2019, 686, 995–1009. [Google Scholar] [CrossRef]
- Gilliom, R.J. Pesticides in the Nation’s Streams and Ground Water, 1992–2001: The Quality of Our Nation’s Waters; Revised 15 February 2007; U.S. Geological Survey: Reston, VA, USA, 2006.
- Minnesota Department of Agriculture, Pesticides in Minnesota Lakes; Minnesota Department of Agriculture: St. Paul, MI, USA, 2019. Available online: https://wrl.mnpals.net/islandora/object/WRLrepository:3462 (accessed on 3 March 2025).
- Minnesota Department of Agriculture. 2023 Water Quality Monitoring Report. 2024. Available online: https://wrl.mnpals.net/node/4249 (accessed on 4 March 2025).
- Ryberg, K.R.; Sonte, W.W.; Baker, N.T. Causal factors for pesticide trends in streams of the United States: Atrazine and deethylatrazine. J. Environ. Qual. 2020, 49, 152–162. [Google Scholar] [CrossRef]
- Caldas, S.; Arias, J.; Rombaldi, C.; Mello, L.; Cerqueira, M.; Martins, A.; Primel, E. Occurrence of pesticides and PPCPs in surface and drinking water in southern Brazil: Data on 4-Year monitoring. J. Braz. Chem. Soc. 2018, 30, 71–80. [Google Scholar] [CrossRef]
- Fingler, S.; Mendaš, G.; Dvoršćak, M.; Stipičević, S.; Vasilić, Ž.; Drevenkar, V. Herbicide micropollutants in surface, ground and drinking waters within and near the area of Zagreb, Croatia. Environ. Sci. Pollut. Res. 2017, 24, 11017–11030. [Google Scholar] [CrossRef]
- Montiel-León, J.M.; Duy, S.V.; Munoz, G.; Bouchard, M.F.; Amyot, M.; Sauvé, S. Quality survey and spatiotemporal variations of atrazine and desethylatrazine in drinking water in Quebec, Canada. Sci. Total Environ. 2019, 671, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- Sadia, M.; Nollen, I.; Helmus, R.; ter Laak, T.L.; Béen, F.; Praetorius, A.; van Wezel, A.P. Occurrence, fate, and related health risks of PFAS in raw and produced drinking water. Environ. Sci. Technol. 2023, 57, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Breitmeyer, S.E.; Williams, A.M.; Duris, J.W.; Eicholtz, L.W.; Shull, D.R.; Wertz, T.A.; Woodward, E.E. Per- and polyfluorinated alkyl substances (PFAS) in Pennsylvania surface waters: A statewide assessment, associated sources, and land-use relations. Sci. Total Environ. 2023, 888, 164161. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Bradley, P.M.; Argos, M.; Kolpin, D.W.; Meppelink, S.M.; Romanok, K.M.; Smalling, K.L.; Focazio, M.J.; Allen, J.M.; Dietze, J.E.; Devito, M.J.; et al. Mixed organic and inorganic tapwater exposures and potential effects in greater Chicago area, USA. Sci. Total Environ. 2020, 719, 137236. [Google Scholar] [CrossRef]
- Bradley, P.M.; LeBlanc, D.R.; Romanok, K.M.; Smalling, K.L.; Focazio, M.J.; Cardon, M.C.; Clark, J.M.; Conley, J.M.; Evans, N.; Givens, C.E.; et al. Public and private tapwater: Comparative analysis of contaminant exposure and potential risk, Cape Cod, Massachusetts, USA. Environ. Int. 2021, 152, 106487. [Google Scholar] [CrossRef]
- Furlong, E.T.; Batt, A.L.; Glassmeyer, S.T.; Noriega, M.C.; Kolpin, D.W.; Mash, H.; Schenck, K.M. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Total. Environ. 2017, 579, 1629–1642. [Google Scholar] [CrossRef]
- Ambrosio-Albuquerque, E.P.; Cusioli, L.F.; Bergamasco, R.; Gigliolli, A.A.S.; Lupepsa, L.; Paupitz, B.R.; Barbieri, P.A.; Borin-Carvalho, L.A.; Portela-Castro, A.L.d.B. Metformin environmental exposure: A systematic review. Environ. Toxicol. Pharmacol. 2021, 83, 103588. [Google Scholar] [CrossRef]
- Zheng, Y.; Shao, Y.; Zhang, Y.; Liu, Z.; Zhao, Z.; Xu, R.; Ding, J.; Li, W.; Wang, B.; Zhang, H. Metformin as an emerging pollutant in the aquatic environment: Occurrence, analysis, and toxicity. Toxics 2024, 12, 483. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Sun, H.; Zhou, Q. Widespread occurrence of benzotriazoles and benzothiazoles in tap water: Influencing factors and contribution to human exposure. Environ. Sci. Technol. 2016, 50, 2709–2717. [Google Scholar] [CrossRef]
- Wang, W.; Park, S.; Choi, B.-G.; Oh, J.-E. Occurrence and removal of benzotriazole and benzothiazole in drinking water treatment plants. Environ. Pollut. 2023, 316, 120563. [Google Scholar] [CrossRef] [PubMed]
- Bexfield, L.M.; Belitz, K.; Lindsey, B.D.; Toccalino, P.L.; Nowell, L.H. Pesticides and pesticide degradates in groundwater used for public supply across the United States: Occurrence and human-health context. Environ. Sci. Technol. 2021, 55, 362–372. [Google Scholar] [CrossRef]
- Xiao, F.; Hanson, R.A.; Golovko, S.A.; Golovko, M.Y.; Arnold, W.A. PFOA and PFOS are generated from zwitterionic and cationic precursor compounds during water disinfection with chlorine or ozone. Environ. Sci. Technol. Lett. 2018, 5, 382–388. [Google Scholar] [CrossRef]
- Majee, P.; Reddy, P.H.P. Sorption behaviour of atrazine on agricutlural soils of different characteristics: Equilibrium and kinetics studies. Clean Technol. Environ. Policy 2023, 25, 3407–3417. [Google Scholar] [CrossRef]
- Nguyen, T.; Nsiah, G.A.; Crowder, E.; Garland, S.; Williams, C.F.; Conroy-Ben, O. Precited endocrine disrupting activity of unregulated drinking water contaminants. Environ. Sci. Technol. Water 2024, 4, 1000–1013. [Google Scholar] [CrossRef]
- Bruce, G.M.; Pleus, R.C.; Snyder, S.A. Toxicological relevance of pharmaceuticals in drinking water. Environ. Sci. Technol. 2010, 44, 5619–5626. [Google Scholar] [CrossRef]
- Webb, S.; Ternes, T.; Gibert, M.; Olejniczak, K. Indirect human exposure to pharmaceuticals via drinking water. Toxicol. Lett. 2003, 142, 157–167. [Google Scholar] [CrossRef]
- Wee, S.Y.; Haron, D.E.M.; Aris, A.Z.; Yusoff, F.M.; Praveena, S.M. Active pharmaceutical ingredients in Malaysian drinking water: Consumption, exposure, and human health risk. Environ. Geochem. Health 2020, 42, 3247–3261. [Google Scholar] [CrossRef]
- Ternes, T.A.; Meisenheimer, M.; McDowell, D.; Sacher, F.; Brauch, H.-J.; Haist-Gulde, B.; Preuss, G.; Wilme, U.; Zulei-Seibert, N. Removal of pharmaceuticals during drinking water treatment. Environ. Sci. Technol. 2002, 36, 3855–3863. [Google Scholar] [CrossRef]
- Benotti, M.J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, N.; Zhang, B.; Jin, L.; Li, M.; Hu, M.; Zhang, X.; Wei, S.; Yu, H. Occurrence of organophosphate flame retardants in drinking water from China. Water Res. 2014, 54, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Kaboré, H.A.; Duy, S.V.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef]
- Ericson, I.; Domingo, J.L.; Nadal, M.; Bigas, E.; Llebaria, X.; van Bavel, B.; Lindström, G. Levels of perfluorinated chemicals in municipal drinking water from Catalonia, Spain: Public health implications. Arch. Environ. Contam. Toxicol. 2009, 57, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Houtman, C.J.; Kroesbergen, J.; Lekkerkerker-Teunissen, K.; van der Hoek, J.P. Human health risk assessment of the mixture of pharmaceuticals in Dutch drinking water and its sources based on frequent monitoring data. Sci. Total Environ. 2014, 496, 54–62. [Google Scholar] [CrossRef]
- Bradley, P.M.; Romanok, K.M.; Smalling, K.L.; Gordon, S.E.; Huffman, B.J.; Friedman, K.P.; Villeneuve, D.L.; Blackwell, B.R.; Fitzpatrick, S.C.; Focazio, M.J.; et al. Private, public, and bottled drinking water: Shared contaminant-mixture exposures and effects challenge. Environ. Int. 2025, 195, 109220. [Google Scholar] [CrossRef]



| Contaminant Group | Number of Contaminants Analyzed a | Surface Water (SW) | Groundwater Agriculture (GW-AG) | Groundwater Wastewater (GW-WW) | Groundwater Agriculture + Wastewater (GW-AG/WW) |
|---|---|---|---|---|---|
| Wastewater indicators b | 49 | x | x | x | |
| Pharmaceuticals and personal care products | 165 | x | x c | x | |
| Per- and polyfluoroalkyl substances (PFAS) | 40 | x | x | x | x |
| Benzotriazoles/ benzothiazoles | 10 | x | x d | ||
| Illicit drugs | 4 | x | |||
| Alkylphenols and alkylphenol ethoxylates | 5 | x | |||
| Hormones e | 16 | x | |||
| Pesticides and pesticide degradates | 224 | x | x f | x f | |
| Total number of contaminants | 513 | 513 | 256 | 272 | 480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elliott, S.M.; Krall, A.L.; de Lambert, J.R.; Gilchrist, M.D.; Robertson, S.W. Emerging Contaminants in Source and Finished Drinking Waters Across Minnesota (U.S.) and Potential Health Implications. Int. J. Environ. Res. Public Health 2025, 22, 976. https://doi.org/10.3390/ijerph22070976
Elliott SM, Krall AL, de Lambert JR, Gilchrist MD, Robertson SW. Emerging Contaminants in Source and Finished Drinking Waters Across Minnesota (U.S.) and Potential Health Implications. International Journal of Environmental Research and Public Health. 2025; 22(7):976. https://doi.org/10.3390/ijerph22070976
Chicago/Turabian StyleElliott, Sarah M., Aliesha L. Krall, Jane R. de Lambert, Maya D. Gilchrist, and Stephen W. Robertson. 2025. "Emerging Contaminants in Source and Finished Drinking Waters Across Minnesota (U.S.) and Potential Health Implications" International Journal of Environmental Research and Public Health 22, no. 7: 976. https://doi.org/10.3390/ijerph22070976
APA StyleElliott, S. M., Krall, A. L., de Lambert, J. R., Gilchrist, M. D., & Robertson, S. W. (2025). Emerging Contaminants in Source and Finished Drinking Waters Across Minnesota (U.S.) and Potential Health Implications. International Journal of Environmental Research and Public Health, 22(7), 976. https://doi.org/10.3390/ijerph22070976






