Mobile and Web Apps for Weight Management in Overweight and Obese Adults: An Updated Umbrella Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
- Population: Adults (≥18 years) diagnosed with overweight or obesity (BMI ≥ 25).
- Intervention: Use of mobile or web-based applications specifically designed as strategies for weight loss.
- Comparator: Conventional treatment for overweight or obesity, including diet, exercise, behavioral therapy, pharmacological interventions, or minimal/no intervention, excluding digital technologies. Pharmacological interventions were considered an acceptable component of conventional treatment for comparator groups, as information about their use tends to be underreported or insufficiently detailed in systematic reviews. This a priori criterion was set to prevent the exclusion of potentially relevant reviews.
- Outcomes: At least one of the following: reduction in BMI, waist circumference, body fat percentage, or overall weight loss.
- Study design: Systematic reviews with meta-analyses, available in full text, assessing the effectiveness of mobile applications and web-based interventions compared to conventional treatments in adults with overweight or obesity. Systematic reviews including the general population were eligible if they performed separate analyses for adults and children. Exclusion criteria comprised clinical trials, abstracts, opinion pieces, narrative (non-systematic) reviews, and scoping reviews. No restrictions were applied regarding language or publication year at any stage of the search or screening process. However, all systematic reviews included in this umbrella review were published in English, and no potentially eligible reviews in other languages were retrieved. This a priori approach aligns with best practices for umbrella reviews and maximizes the comprehensiveness and temporal scope of the evidence, which is crucial in the rapidly evolving field of digital health.
2.2. Information Sources and Search Strategy
2.3. Study Selection and Data Extraction
2.4. Methodological Quality Assessment
2.5. Assessment of Risk of Bias Across Studies
2.6. Assessment of Overlap Among Primary Studies
2.7. Quantitative Synthesis and Subgroup Definitions
- Effect size corresponding to the longest follow-up period was selected whenever available;
- If multiple effect sizes were available at the same follow-up, preference was given to estimates based on intention-to-treat (ITT) analysis;
- In case of further ties, the result with the largest sample size was chosen.
3. Results
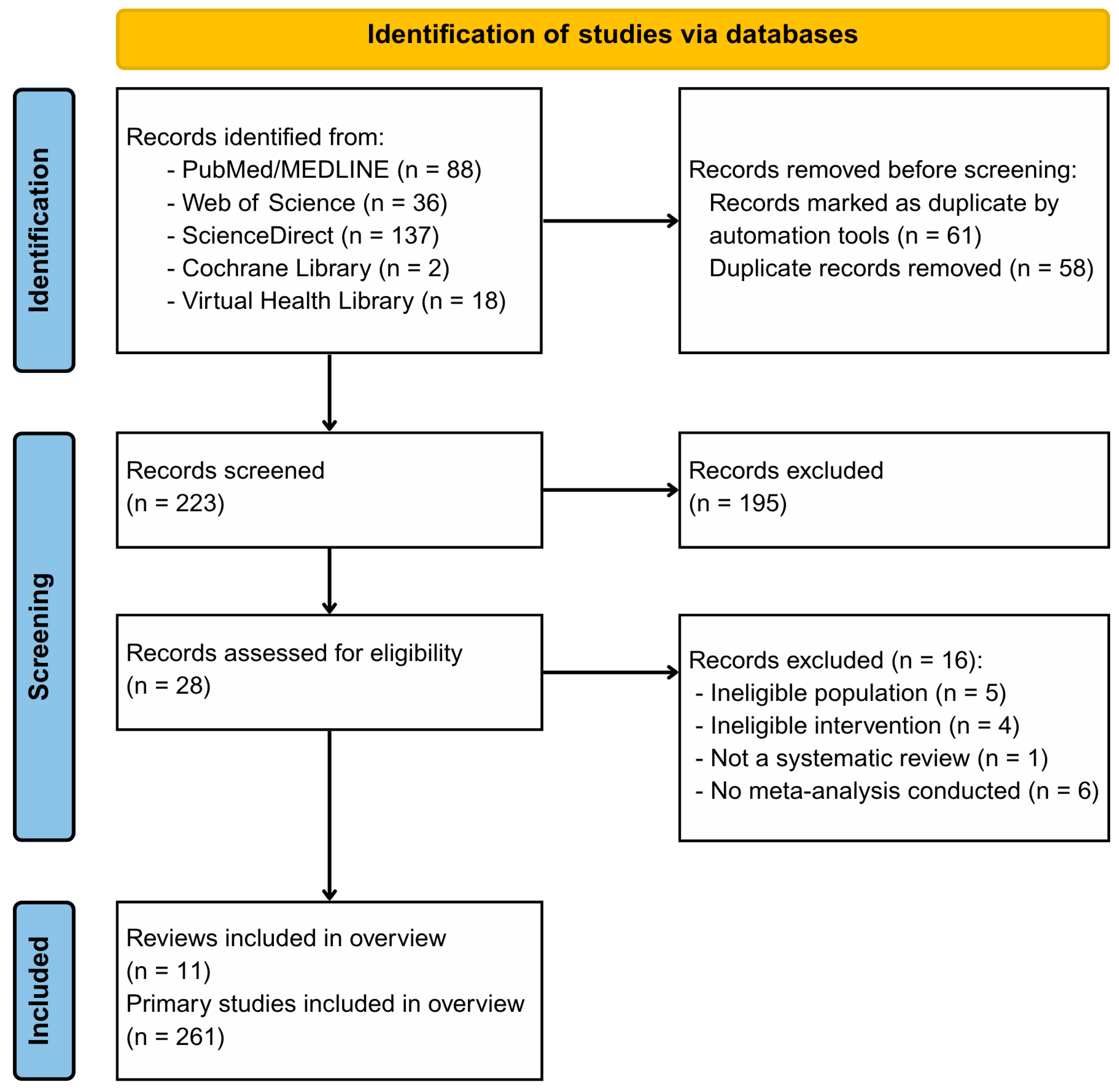
3.1. Study Selection
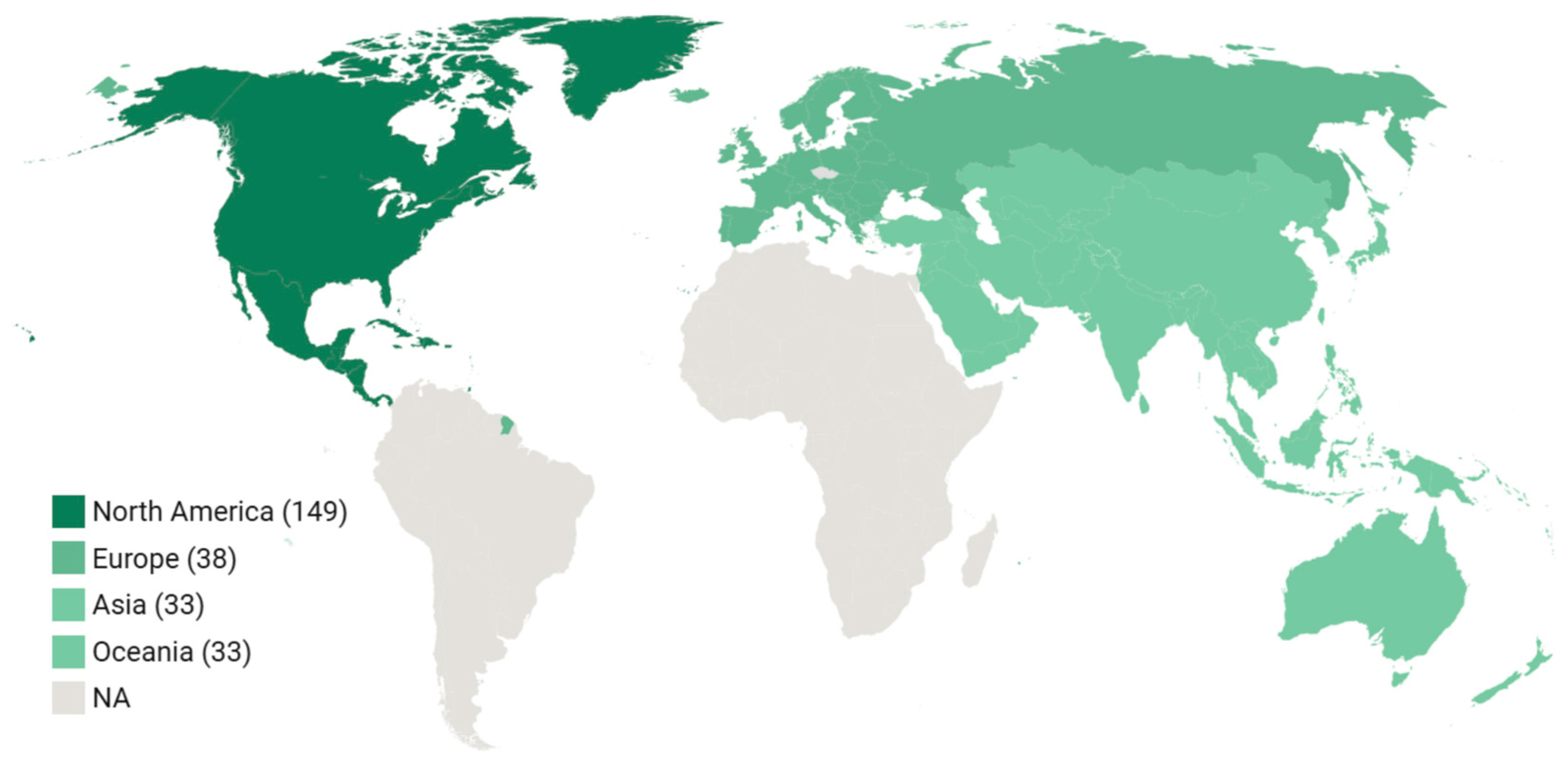
3.2. Study Characteristics
3.3. Overlap Among Primary Studies
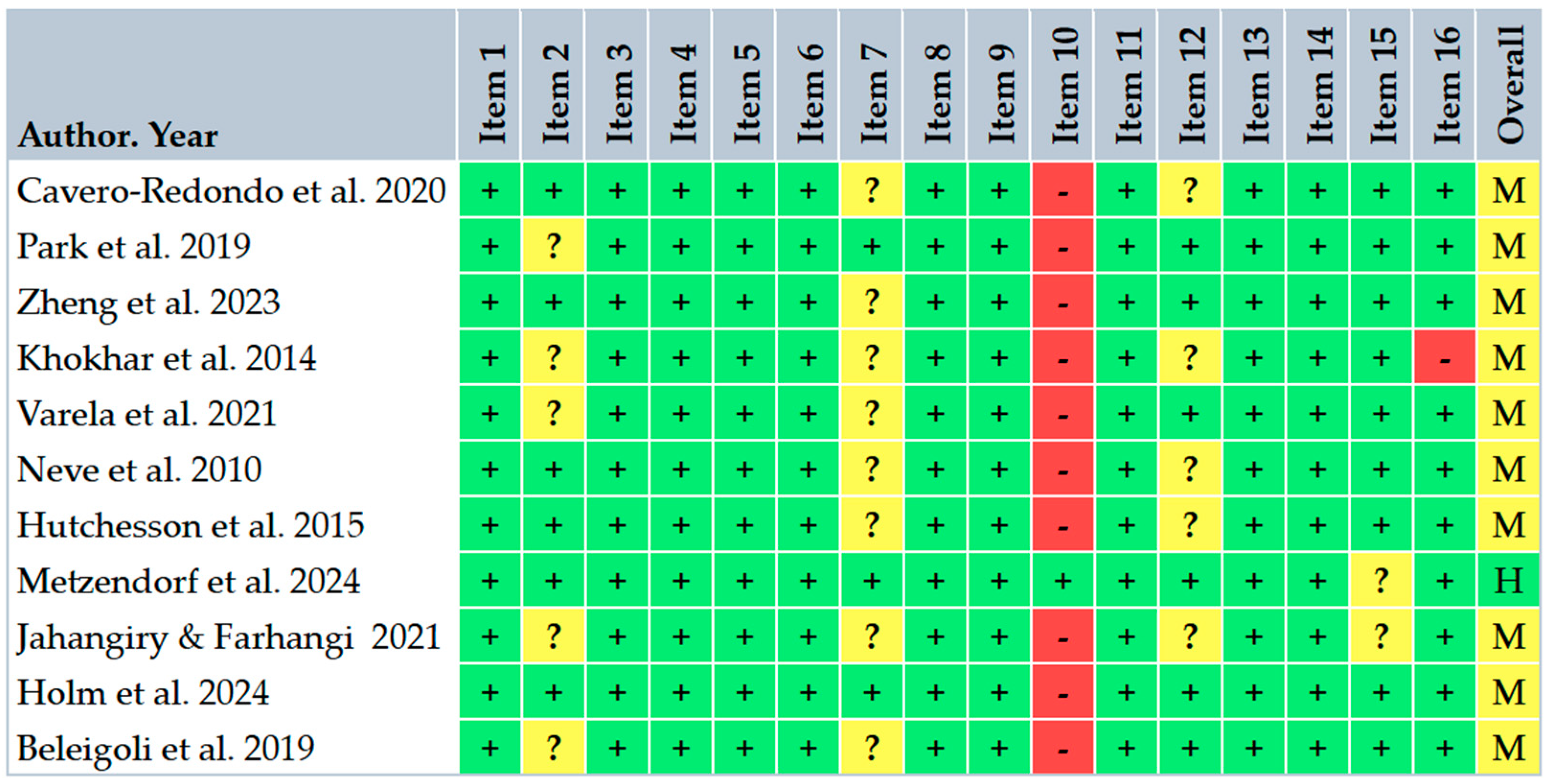
3.4. Risk of Bias Within Studies
3.5. Risk of Bias Across Studies
3.6. Representative Effect Sizes from Individual Studies
3.7. Moderators
3.8. Quantitative Synthesis and Subgroup Meta-Analyses
3.8.1. Mobile App Interventions
3.8.2. Web-Based Interventions with Human Contact
3.8.3. Web-Based Interventions Without Human Contact
3.8.4. Weight Outcome
3.8.5. BMI Outcome
3.8.6. Waist Circumference Outcome
3.8.7. Short-Term eHealth Interventions (≤6 Months)
3.8.8. Long-Term eHealth Interventions (≥12 Months)
3.9. Qualitative Synthesis
4. Discussion
4.1. Comparison with Previous Umbrella Reviews
4.2. Context and Need for Research in Diverse Settings
4.3. Factors Influencing Digital Intervention Outcomes
4.4. Implications for Clinical Practice and Public Health Policy
4.5. Equity and Accessibility Considerations
4.6. The Emerging Role of Artificial Intelligence in Digital Weight Management
4.7. Methodological Considerations and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Artificial Intelligence |
| AMSTAR 2 | A MeaSurement Tool to Assess Systematic Reviews (version 2) |
| BIB | BibTeX file format |
| BMI | Body Mass Index |
| CCA | Corrected Covered Area |
| CI | Confidence Interval |
| COVID-19 | Coronavirus Disease 2019 |
| DALYs | Disability-Adjusted Life Years |
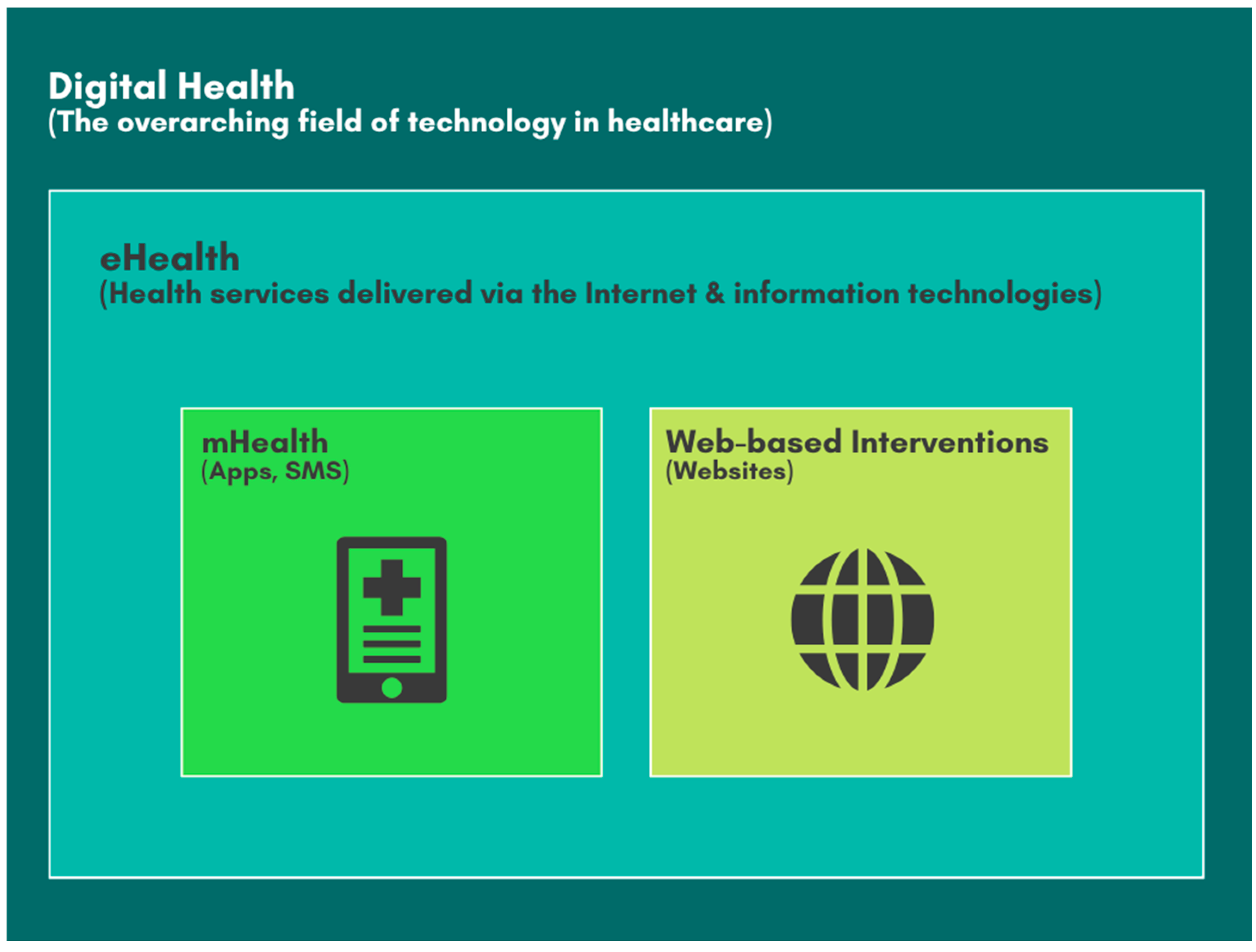
| eHealth | Electronic Health |
| GDP | Gross Domestic Product |
| GRADE | Grading of Recommendations, Assessment, Development and Evaluation |
| IMB | Information-Motivation-Behavioral Skills |
| ITT | Intention-To-Treat |
| JBI-MAStARI | Joanna Briggs Institute Meta-Analysis of Statistics Assessment and Review Instrument |
| MD | Mean Difference |
| mHealth | Mobile Health |
| NBIB | Native BibTeX format |
| PDA | Personal Digital Assistant |
| PHC | Primary Healthcare |
| PICOS | Population, Intervention, Comparator, Outcome, Study design |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| R | Programming language and statistical computing environment |
| RoB | Risk of Bias |
| ROBINS-I | Risk Of Bias In Non-Randomized Studies-of Interventions |
| RR | Risk Ratio |
| SMD | Standardized Mean Difference |
| SUS | Sistema Único de Saúde |
References
- World Health Organization. WHO Discussion Paper on Obesity; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- World Obesity Federation. Obesity Atlas. 2024. Available online: https://data.worldobesity.org/publications/WOF-Obesity-Atlas-v7.pdf (accessed on 24 May 2024).
- Tchang, B.G.; Saunders, K.H.; Igel, L.I.; Best, C.; Aronne, L.J. Pharmacologic treatment of overweight and obesity in adults. Emerg. Top. Life Sci. 2021, 5, 337–356. [Google Scholar]
- Liu, C.; Zhang, Z.; Wang, B.; Meng, T.; Li, C.; Zhang, X. Global health impacts of high BMI: A 30-year analysis of trends and disparities across regions and demographics. Diabetes Res. Clin. Pract. 2024, 217, 111883. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.P.; Machado, I.E.; Fogal, A.S.; de Sousa, C.A.C.; Malta, D.C. Burden of disease and direct costs to the health system attributable to high body mass index in Brazil. Public Health 2024, 233, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Massoomi, M.R.; Handberg, E.M. Increasing and evolving role of smart devices in modern medicine. Eur. Cardiol. Rev. 2019, 14, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Sherrington, A.; Newham, J.J.; Bell, R.; Adamson, A.; McColl, E.; Araujo-Soares, V. Systematic review and meta-analysis of internet-delivered interventions providing personalized feedback for weight loss in overweight and obese adults. Obes. Rev. 2016, 17, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Park, N.Y.; Jang, S. Effects of mHealth practice patterns on improving metabolic syndrome using the information–motivation–behavioral skills model. Nutrients 2024, 16, 2099. [Google Scholar] [CrossRef] [PubMed]
- Neve, M.; Morgan, P.J.; Jones, P.R.; Collins, C.E. Effectiveness of web-based interventions in achieving weight loss and weight loss maintenance in overweight and obese adults: A systematic review with meta-analysis. Obes. Rev. 2010, 11, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Hwang, J.; Choi, Y.K. Effect of mobile health on obese adults: A systematic review and meta-analysis. Healthc. Inform. Res. 2019, 25, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Clipper, B. The influence of the COVID-19 pandemic on technology adoption in health care. Nurse Lead. 2020, 18, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Senbekov, M.; Saliev, T.; Bukeyeva, Z.; Almabayeva, A.; Zhanaliyeva, M.; Aitenova, N.; Toishibekov, Y.; Fakhradiyev, I. The recent progress and applications of digital technologies in healthcare: A review. Int. J. Telemed. Appl. 2020, 2020, 8830200. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Zhang, M.; Qian, Y.; Yang, H.; Xu, X.; Wu, S.; Zhang, Y.; Deng, Y.; Wang, X. Exploring the Influence of Event Criticality, Disruption, and Novelty on mHealth App Use and Empowerment: Cross-sectional Study in China During COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 834. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-J.; Zhou, Y.; Ma, H.-M.; Wang, A.-Q.; Tang, X.-Y.; Pei, R.-Y.; Piao, M.-H. Effects of Mobile Applications on Weight-Related, Behavior and Metabolic Outcomes for Adults with Overweight and Obesity: A Systematic Review and Meta-Analysis. In Innovation in Applied Nursing Informatics; Strudwick, G., Hardiker, N.R., Rees, G., Cook, R., Lee, Y.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2024; pp. 386–391. [Google Scholar]
- Cavero-Redondo, I.; Martinez-Vizcaino, V.; Fernandez-Rodriguez, R.; Saz-Lara, A.; Pascual-Morena, C.; Álvarez-Bueno, C. Effect of behavioral weight management interventions using lifestyle mHealth self-monitoring on weight loss: A systematic review and meta-analysis. Nutrients 2020, 12, 1977. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Edney, S.M.; Goh, C.H.; Tai, B.C.; Mair, J.L.; Castro, O.; Salamanca-Sanabria, A.; Kowatsch, T.; van Dam, R.M.; Müller-Riemenschneider, F. Effectiveness of holistic mobile health interventions on diet, and physical, and mental health outcomes: A systematic review and meta-analysis. eClinicalMedicine 2023, 63, 102309. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, B.; Jones, J.; Ronksley, P.E.; Armstrong, M.J.; Caird, J.; Rabi, D. Effectiveness of mobile electronic devices in weight loss among overweight and obese populations: A systematic review and meta-analysis. BMC Obes. 2014, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Varela, C.; Oda-Montecinos, C.; Andrés, A.; Saldaña, C. Effectiveness of web-based feedback interventions for people with overweight and obesity: Systematic review and network meta-analysis of randomized controlled trials. J. Eat. Disord. 2021, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Hutchesson, M.J.; Rollo, M.E.; Krukowski, R.; Ells, L.; Harvey, J.; Morgan, P.J.; Callister, R.; Plotnikoff, R.; Collins, C.E. eHealth interventions for the prevention and treatment of overweight and obesity in adults: A systematic review with meta-analysis. Obes. Rev. 2015, 16, 376–392. [Google Scholar] [CrossRef] [PubMed]
- Metzendorf, M.I.; Wieland, L.S.; Richter, B. Mobile health (m-health) smartphone interventions for adolescents and adults with overweight or obesity. Cochrane Database Syst. Rev. 2024, 2, CD013591. [Google Scholar] [CrossRef] [PubMed]
- Jahangiry, L.; Farhangi, M.A. Obesity paradigm and web-based weight loss programs: An updated systematic review and meta-analysis of randomized controlled trials. J. Health Popul. Nutr. 2021, 40, 16. [Google Scholar] [CrossRef] [PubMed]
- Holm, T.F.; Udsen, F.W.; Giese, I.E.; Færch, K.; Jensen, M.H.; von Scholten, B.J.; Hangaard, S. The effectiveness of digital health lifestyle interventions on weight loss in people with prediabetes: A systematic review, meta-analysis, and meta-regression. J. Diabetes Sci. Technol. 2024, 18, 539–554. [Google Scholar]
- Beleigoli, A.M.; Andrade, A.Q.; Cançado, A.G.; Paulo, M.N.; Diniz, M.D.; Ribeiro, A.L. Web-based digital health interventions for weight loss and lifestyle habit changes in overweight and obese adults: Systematic review and meta-analysis. J. Med. Internet Res. 2019, 21, e9609. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.P.A. Integration of evidence from multiple meta-analyses: A primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ 2009, 181, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Aromataris, E.; Fernandez, R. Umbrella Reviews. In JBI Manual for Evidence Synthesis; Aromataris, E., Munn, Z., Eds.; Joanna Briggs Institute: Adelaide, Australia, 2023; Available online: https://jbi-global-wiki.refined.site/space/MANUAL/46873621/9.3.8+Synthesis+of+findings (accessed on 24 May 2024).
- Sorgente, A.; Pietrabissa, G.; Manzoni, G.M.; Re, F.; Simpson, S.; Perona, S.; Rossi, A.; Cattivelli, R.; Innamorati, M.; Jackson, J.B.; et al. Web-based interventions for weight loss or weight loss maintenance in overweight and obese people: A systematic review of systematic reviews. J. Med. Internet Res. 2017, 19, e229. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Verma, A.; Marupuru, S.; Rana, P.; Sharma, S.; Gupta, R.; Kamal, R.; Madaan, R.; Gupta, A.; Arora, R. Efficacy of eHealth, mHealth, and Digital Health Interventions on Physical Activity, Sedentary Behavior, and Weight Loss: An Umbrella Review and Meta-Analysis of Meta-Analyses. npj Digit. Med. 2024, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- König, L.M.; Western, M.J.; Denton, A.H.; Krukowski, R.A. Umbrella review of social inequality in digital interventions targeting physical activity, diet, and weight outcomes. NPJ Digit. Med. 2025, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Zhang, G.; Liu, D. The Impact of Using mHealth Apps on Improving Public Health Satisfaction during the COVID-19 Pandemic: A Digital Content Value Chain Perspective. Healthcare 2022, 10, 479. [Google Scholar] [CrossRef] [PubMed]
- Mahou, X.; Barral, B.; Fernández, Á.; Bouzas-Lorenzo, R.; Cernadas, A. eHealth and mHealth Development in Spain: Promise or Reality? Int. J. Environ. Res. Public Health 2021, 18, 13055. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K. Digital Health Equity. In Digital Health; Lin, S., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 9. [Google Scholar]
- Oliveira, T.F.V.; Dias, P.C.; Tavares, M.C.G.C.F.; Leite, I.C.; Jaime, P.C. Fortalecimento das ações de cuidado às pessoas com obesidade no contexto da pandemia de COVID-19: O caso do Brasil. Cien. Saude Colet. 2023, 28, 3673–3685. [Google Scholar] [CrossRef]
- Taylor, K.; Indulkar, T.; Thompson, B.; Pinkard, C.; Barron, E.; Frost, T.; Jayawardane, P.; Davies, N.; Bakhai, C.; Forouhi, N.G.; et al. Early outcomes of referrals to the English National Health Service Digital Weight Management Programme. Obesity 2024, 32, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Lozada-Tequeanes, A.L.; Théodore, F.L.; Kim-Herrera, E.; García-Guerra, A.; Quezada-Sánchez, A.D.; Alvarado-Casas, R.; Bonvecchio, A. Effectiveness and Implementation of a Text Messaging mHealth Intervention to Prevent Childhood Obesity in Mexico in the COVID-19 Context: Mixed Methods Study. JMIR mHealth uHealth 2024, 12, e55509. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.S.J.; Chew, N.W.; Loong, S.S.E.; Lim, S.L.; Tam, W.S.W.; Chin, Y.H.; Chao, A.M.; Dimitriadis, G.K.; Gao, Y.; So, J.B.Y.; et al. Effectiveness of an artificial intelligence–assisted app for improving eating behaviors: Mixed methods evaluation. J. Med. Internet Res. 2024, 26, e46036. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.S.J. The Use of Artificial Intelligence-Based Conversational Agents (Chatbots) for Weight Loss: Scoping Review and Practical Recommendations. JMIR Med. Inform. 2022, 10, e32578. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huhulea, E.N.; Abraham, E.; Bienenstock, R.; Aifuwa, E.; Hirani, R.; Schulhof, A.; Tiwari, R.K.; Etienne, M. The role of artificial intelligence in obesity risk prediction and management: Approaches, insights, and recommendations. Medicina 2025, 61, 358. [Google Scholar] [CrossRef] [PubMed]
- Weeks, W.B. Using artificial intelligence to advance public health. Int. J. Public Health 2023, 68, 1606716. [Google Scholar] [CrossRef] [PubMed]
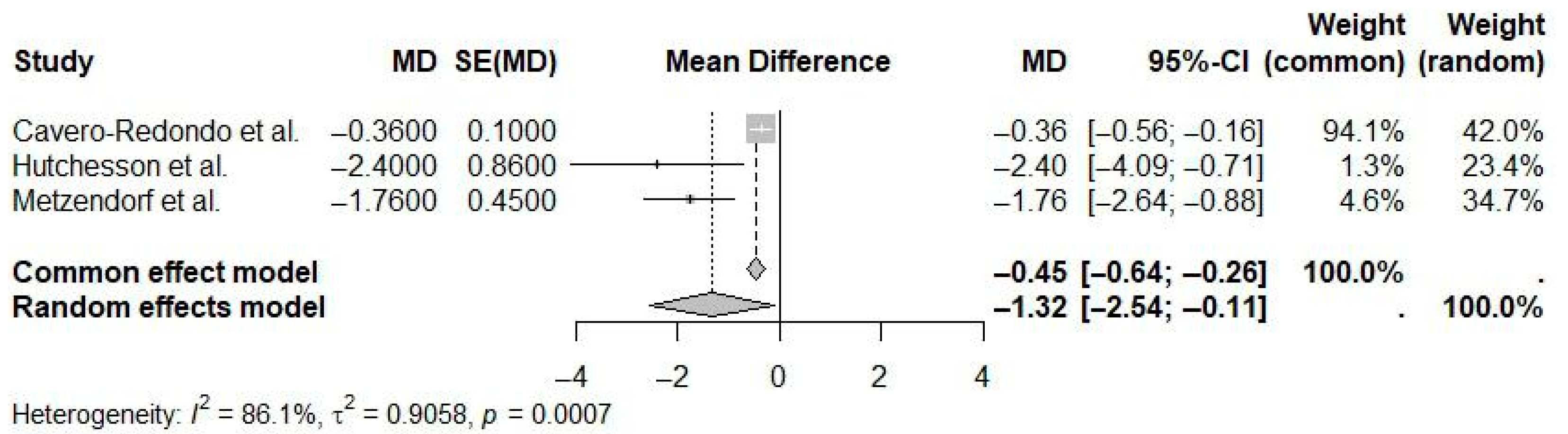
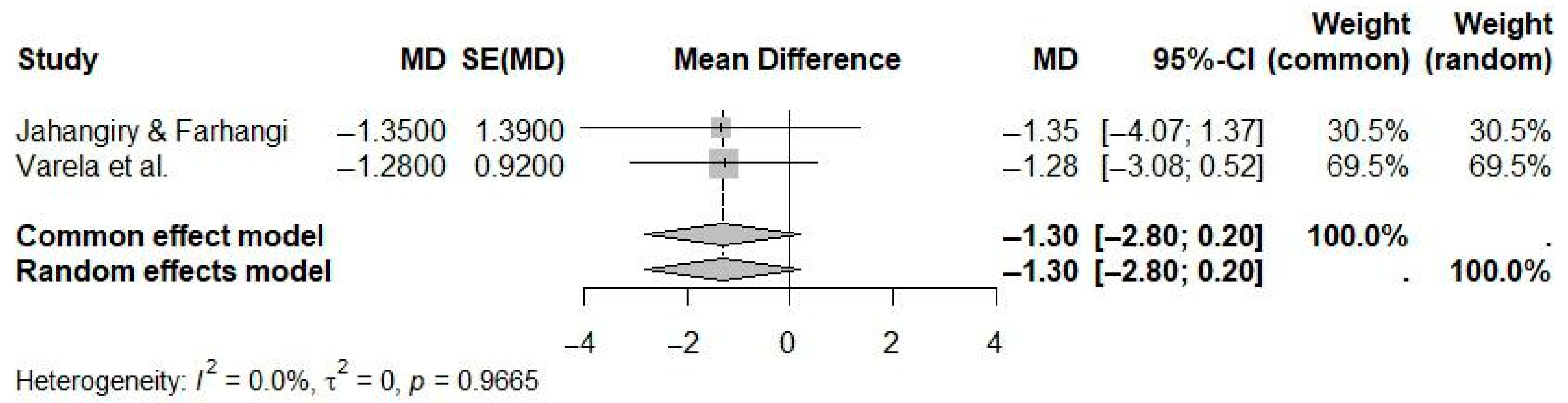
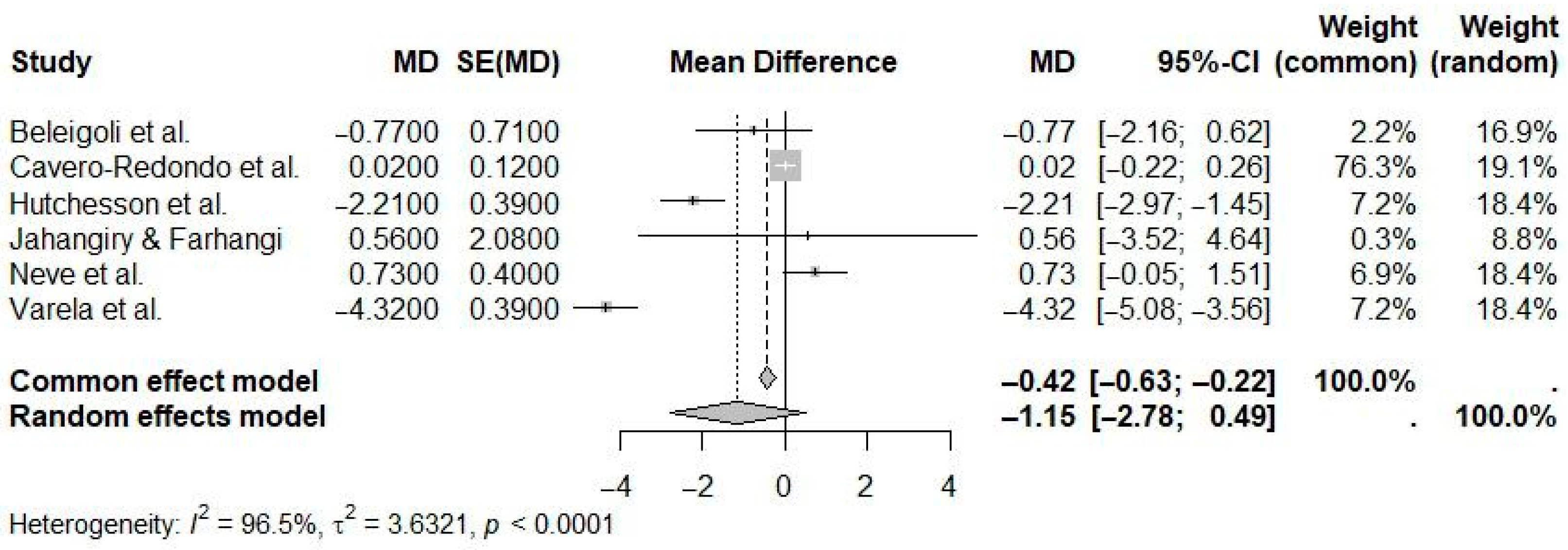
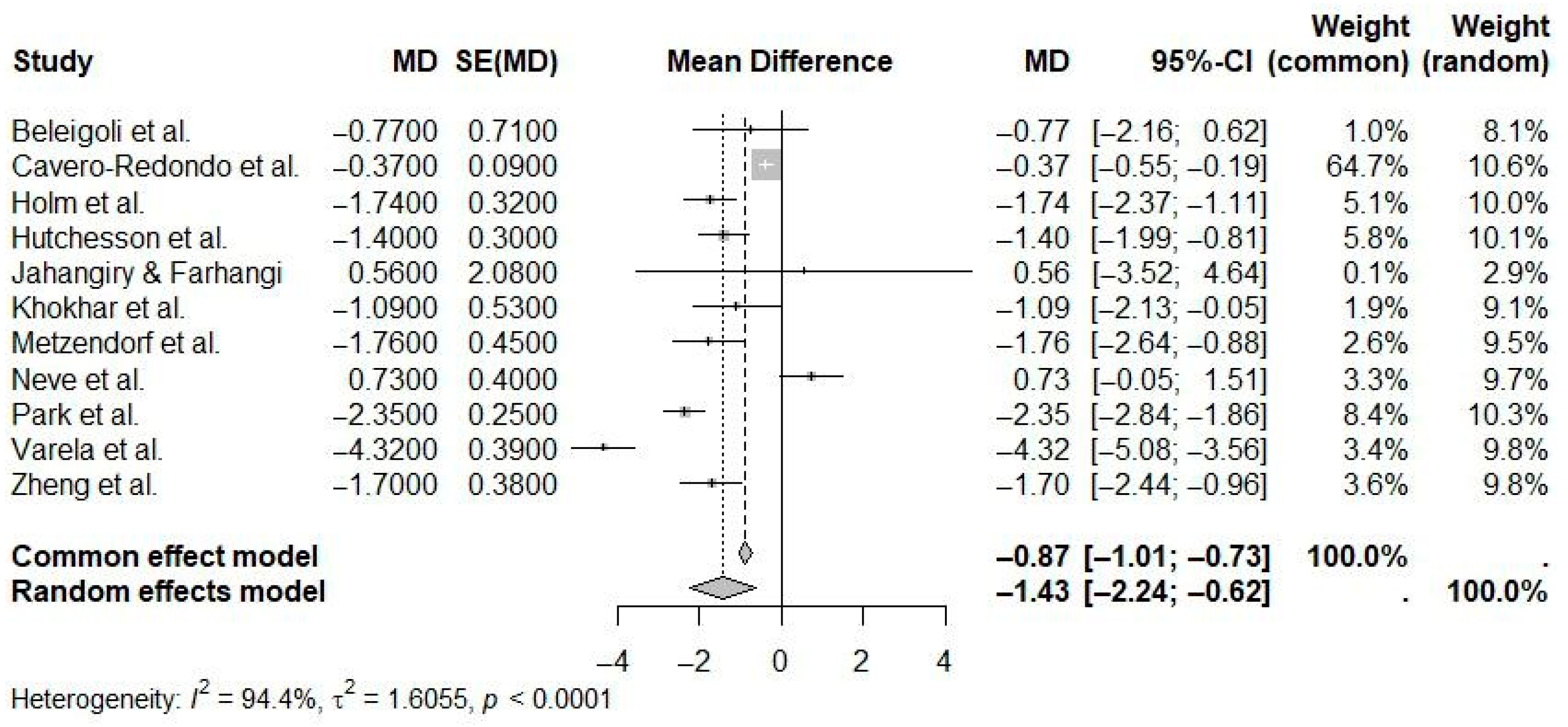
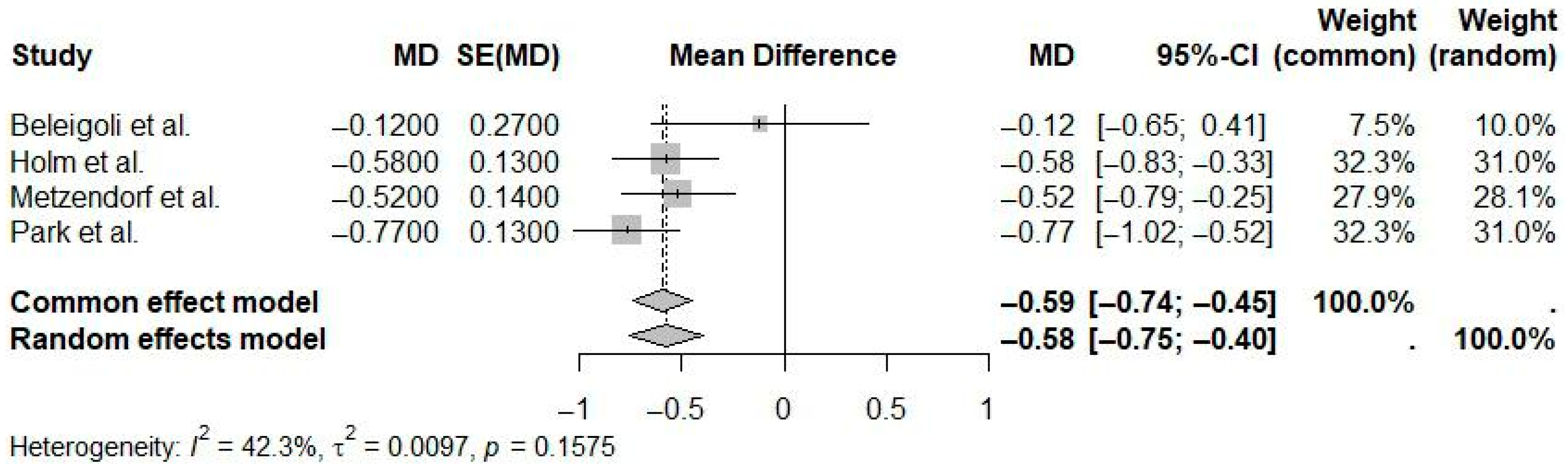
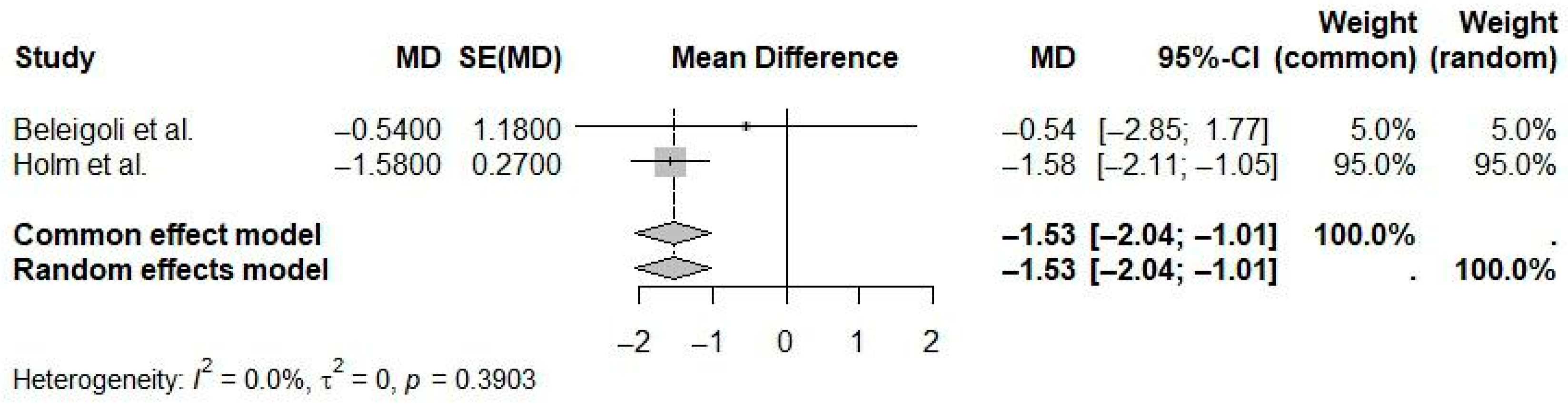
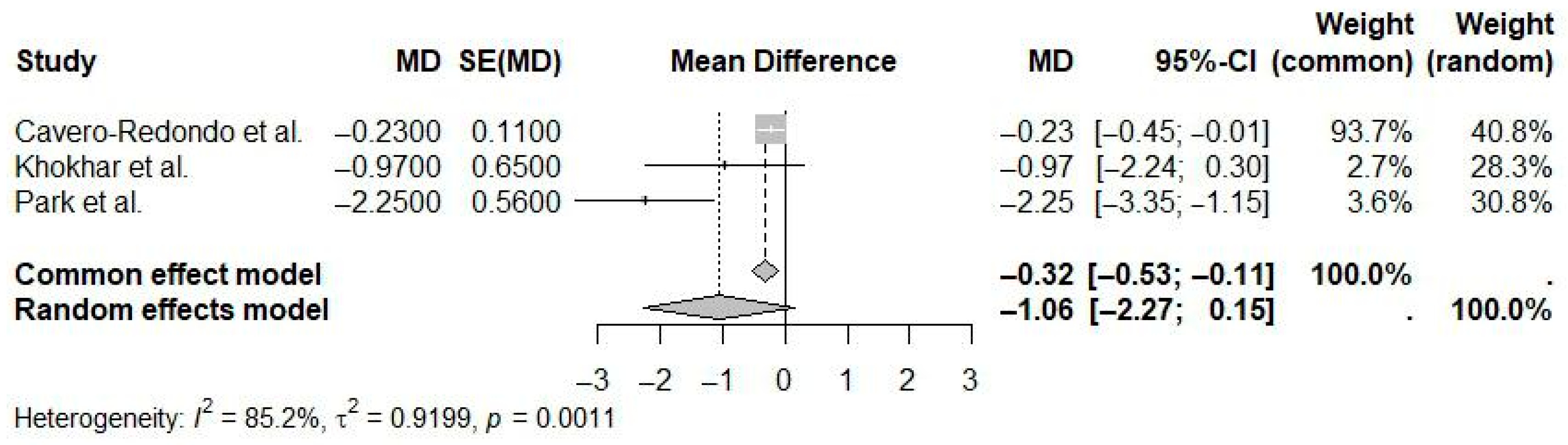
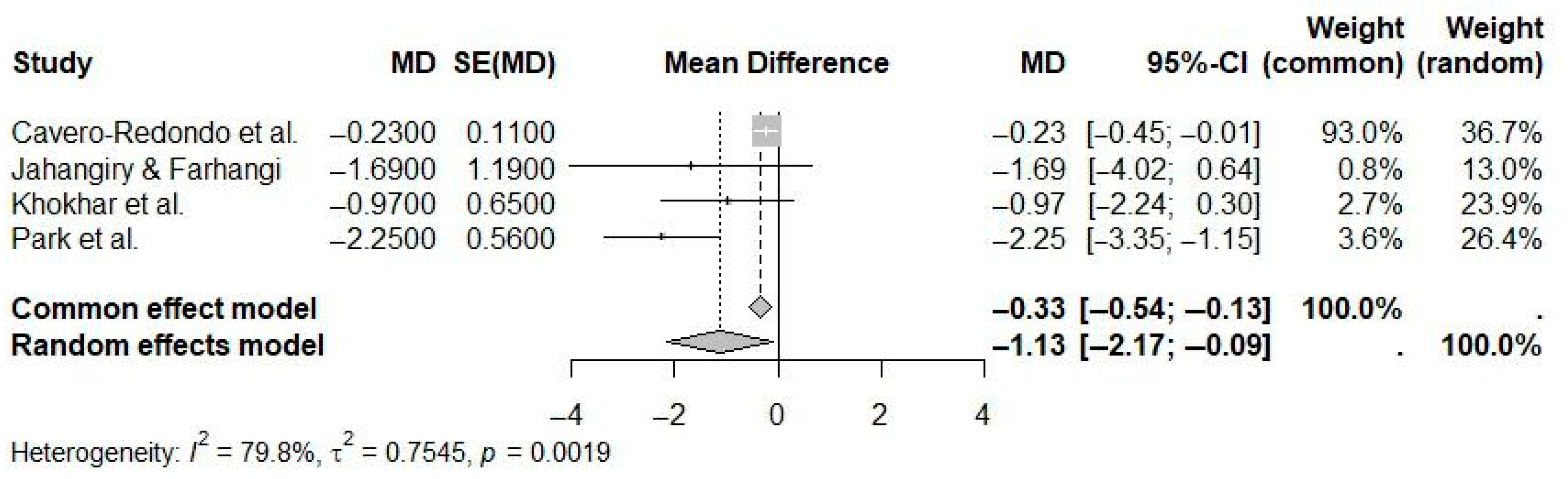
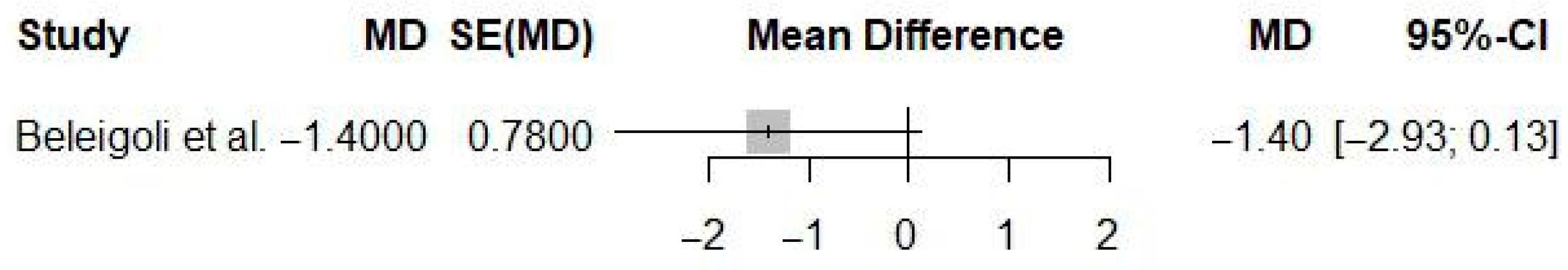
| Reference | N Studies | Countries/Regions of Studies (N) | Pub. Years | Population (C, I) | Age (Years) | Sex (M:F) | Intervention(s) | Comparison(s) | Outcome(s) | Main Effect Size |
|---|---|---|---|---|---|---|---|---|---|---|
| [18] | 20 | US (12), UK (2), AU (3), NZ (1), SK (1), FI (1) | 2007–2019 | 2196 (1064, 1132) | 20.5–59.8 (range) | NR | eHealth interventions (Smartphone, PDA, Web) | Conventional care Paper records Wait-list | Body weight reduction (kg) Intervention adherence | Weight: MD = −1.78 (−2.70 to −0.85) Adherence: RR = 0.78 (0.63 to 0.96) |
| [10] | 20 | US (15), UK (2), AU (2), CN (1) | 2011–2016 | 2318 (1162, 1156) | 18–70 (range) | NR | Mobile health (mHealth) interventions (SMS and/or Mobile Apps) | Conventional care No intervention | Body weight reduction (kg) BMI (kg/m2) | Weight: WMD = −2.35 (−2.84 to −1.87) BMI: WMD = −0.77 (−1.01 to −0.52) |
| [19] | 34 | US (12), AU (6), NZ (2), FI (2), JP (2), SG (2), TH (1), IR (1), DO (1), IS (1), NL (1), KR (1), SE (1), RO (1) | 2013–2023 | 5379 (2161, 3403) | 39 (mean; SD 12.5) | 1360:2340 * | eHealth interventions (Mobile Apps, SMS, Web, Wearables) | Wait-list Standard care Sham control No intervention | Body weight reduction (kg) Perceived stress reduction Diet quality Self-reported physical activity | Weight: MD = −1.70 (−2.45 to −0.95) Stress: SMD = −0.32 (−0.52 to −0.12) |
| [20] | 6 | US (4), FI (1), UK (1) | 2008–2013 | 632 (312, 320) | 38–53 (range of means) | 73:559 | Mobile health (mHealth) interventions (PDA, SMS, Mobile Apps) | Paper records Printed materials No intervention | Body weight reduction (kg) Waist circumference (cm) | Weight (PDA): WMD = −1.09 (−2.12 to −0.05) Weight (mHealth): WMD = −1.78 (−2.92 to −0.63) |
| [21] | 15 | AU (8), US (7) | 2001–2017 | 2630 (882, 1748) | 44.8 (mean; SD 0.9) | 1173:1253 | Web-based behavioral interventions | Face-to-face modalities Self-help, Wait-list | Body weight reduction (kg) Weight maintenance (follow-up) Dropout rates | Weight (vs WL): MD = −4.32 (−5.08 to −3.57) Weight (vs Self-help): MD = −4.31 (−5.22 to −3.41) |
| [9] | 18 | US (17), UK (1) | 2001–2008 | 5700 (2570; 3130) | 32–51 (range of means) | NR † | Web-based behavioral interventions | Minimal intervention Wait-list Printed materials Face-to-face programs | Body weight reduction (kg) Weight maintenance (kg) | Weight (vs Min. Interv.): SMD = 0.73 (not sig.) Weight (vs Edu-only): WMD = 2.24 (1.27 to 3.21) |
| [22] | 84 | North America (63), Europe/UK (10), Oceania (8), Asia (2), Multisite (1) | 2001–2014 | 24,010 (3707, 3906) | 35–65 (range of means) | NR ‡ | eHealth interventions (Web, Email, Mobile Apps, SMS) | Conventional care Minimal intervention No intervention Other eHealth Face-to-face modalities | Body weight reduction BMI Body fat (%) Waist circumference (cm) | Weight (vs NI): MD = −2.70 (−3.33 to −2.08) Weight (vs Min. Interv.): MD = −1.40 (−1.98 to −0.82) |
| [23] | 18 | US (6), UK (3), ES (3), JP (2), BE (1), DE (1), NZ (1), CH (1) | 2013–2023 | 2703 (NR) | 18–60 (range of means) § | NR | Mobile health (mHealth) interventions (Mobile Apps) | Conventional care Printed materials Wait-list Minimal intervention | Body weight reduction (kg) BMI (kg/m2) Waist circumference (cm) | Weight: MD = −1.40 (−2.13 to −0.67) BMI: MD = −0.39 (−0.63 to −0.15) WC: MD = −1.32 (−2.21 to −0.43) |
| [24] | 7 | US (4), AU (1), DE (1), SK (1) | 2009–2014 | 779 (371, 408) | 41.4–57.6 (range of means) | NR | Web-based behavioral interventions | Conventional care No intervention | Body weight reduction (kg) | Weight: WMD = 0.56 (−3.47 to 4.59) (not sig.) |
| [25] | 28 | US (7), SA (4), UK (4), IN (3), CN (3), SG (2), JP (2), KR (1), CA (1), TH (1), BD (1), AU (1), PK (1), LK (1) | 1999–2024 | 14,398 (NR) | 39–66 (range of means) | NR | eHealth interventions (Mobile Apps, SMS, Web) | Conventional care Printed materials | Body weight reduction (kg) BMI (kg/m2) Waist circumference (cm) HbA1c (%) Other metabolic outcomes | Weight: MD = −1.74 (−2.37 to −1.11) BMI: MD = −0.58 (−0.83 to −0.32) WC: MD = −1.58 (−2.11 to −1.04) |
| [26] | 11 | NR | 2007–2018 | 1662 (821, 841) | 18–65 (range) | NR | Web-based behavioral interventions | Face-to-face interventions Wait-list control | Body weight reduction (kg) BMI (kg/m2) Waist circumference (cm) Body fat (%) Diet quality Physical activity | Weight (<6 m): MD = −2.13 (−2.71 to −1.55) Weight (Overall): MD = −0.77 (not sig.) |
| Reference | Population | Intervention | Comparison Group | Outcome | Effect Size | 95% CI | p Value | I2 (%) | Publication Bias | Strength of Evidence | GRADE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [18] | Adults with overweight/obesity | mHealth lifestyle self-monitoring | Usual care, paper record, wait-list | Weight loss (kg) | −0.37 (Cohen’s d); −1.78 kg | −0.54 to −0.19 (d); −2.70 to −0.85 (kg) | <0.001 | 84.6 | Funnel plot and Egger’s test: bias detected (p = 0.016) | Moderate | NR |
| Adults with overweight/obesity | mHealth lifestyle self-monitoring | Usual care, paper record, wait-list | Adherence (risk of dropout) | RR = 0.78 | 0.63 to 0.96 | 0.049 | 37.8 | Funnel plot and Egger’s test: bias detected (p = 0.008) | Moderate | NR | |
| [10] | Adults with overweight/obesity | Mobile health (mHealth) interventions | Usual care/education | Weight loss (kg) | −2.35 kg | −2.84 to −1.87 | <0.001 | 94 | Mild bias; funnel plot, no formal test | NR | NR |
| Adults with overweight/obesity | Mobile health (mHealth) interventions | Usual care/education | BMI change (kg/m2) | −0.77 kg/m2 | −1.01 to −0.52 | <0.001 | 95 | NR | NR | NR | |
| [19] | Adults at risk of NCDs, including overweight/obese | Holistic mHealth interventions | Usual care, education, wait-list, or sham control | Weight change (kg) | −1.70 kg | −2.45 to −0.95 | <0.001 | 89 | Egger’s test: no bias (p = 0.95) | Small to moderate (main text) | NR |
| Adults at risk of NCDs, including overweight/obese | Holistic mHealth interventions | Usual care, education, wait-list, or sham control | Perceived stress (SMD) | −0.32 (SMD, Hedges’ g) | −0.52 to −0.12 | 0.49 | 14.5 | Not assessed (not enough studies) | Small (main text) | NR | |
| Adults at risk of NCDs, including overweight/obese | Holistic mHealth interventions | Usual care, education, wait-list, or sham control | Diet quality (SMD) | 0.21 (SMD, Hedges’ g) | −0.15 to 0.56 | 0.25 | 62.3 | Not assessed (not enough studies) | Not significant | NR | |
| Adults at risk of NCDs, including overweight/obese | Holistic mHealth interventions | Usual care, education, wait-list, or sham control | MVPA (SMD) | 0.21 (SMD, Hedges’ g) | −0.25 to 0.67 | 0.37 | 74.3 | Not assessed (not enough studies) | Not significant | NR | |
| [20] | Adults with overweight/obesity | Mobile health (mHealth) interventions | Usual care or paper-based diary | Weight loss (kg) | −1.09 kg (WMD) | −2.12 to −0.05 | 0.04 | 49.6 | No bias: Begg’s test and funnel plot | NR | NR |
| [21] | Adults with overweight/obesity | Intensive Contact Web-based interventions | Wait-list | Weight loss (kg) | −1.86 (MD, kg) | −3.61 to −0.12 | 0.04 | 80.2 | Funnel plot: possible bias, not formal | NR | NR |
| [9] | Adults with overweight/obesity | Web-based interventions | Control/minimal intervention | Weight loss (kg) | −0.73 (MD, kg) | −0.06 to 1.51 | 0.07 | 84.4 | Funnel plot: possible bias, not formal test | NR | NR |
| [22] | Adults with overweight/obesity | eHealth interventions (web, mobile, etc.) | No intervention control | Weight loss (kg) | −2.70 (MD, kg) | −3.33 to −2.08 | <0.00001 | 49 | Funnel plot: no bias detected | NR | NR |
| Adults with overweight/obesity | eHealth interventions (web, mobile, etc.) | Minimal intervention (e.g., written self-help) | Weight loss (kg) | −1.40 (MD, kg) | −1.98 to −0.82 | <0.0001 | 72 | Funnel plot: no bias detected | NR | NR | |
| Adults with overweight/obesity | eHealth with extra features/technologies | Standard eHealth interventions | Weight loss (kg) | 1.46 (MD, kg) | 0.80 to 2.13 | <0.001 | 60 | Funnel plot: no bias detected | NR | NR | |
| Adults with overweight/obesity | eHealth (mainly web/email) | Control/minimal intervention | Weight change (kg) | −0.27 (MD, kg) | −0.96 to 0.42 | 0.44 | 0 | NR | NR | NR | |
| [23] | General population with overweight/obesity | Mobile app-based mHealth | Minimal intervention, no intervention, or usual care | Weight change (kg) | −2.10 (MD, kg) | −2.79 to −1.41 | <0.00001 | 75 | NR | Moderate | Moderate |
| [24] | Adults with overweight/obesity | Web-based digital health interventions | Non-web-based control | Weight loss (kg) | 0.56 (WMD, kg) | −3.47 to 4.59 | 0.789 | 74.8 | No bias: Begg’s p = 0.67; Egger’s p = 0.78 | NR | NR |
| [25] | Adults with prediabetes (majority overweight/obese) | Digital health lifestyle interventions | Non-digital control | Weight change (kg) | −1.74 (MD, kg) | −2.37 to −1.11 | <0.01 | 84.4 | Funnel plot and Egger’s test: no bias (p = 0.23) | Moderate | Moderate |
| Adults with prediabetes (majority overweight/obese) | Digital health lifestyle interventions | Non-digital control | BMI (kg/m2) | −0.58 (MD) | −0.83 to −0.32 | <0.01 | 78 | NR | Low | Low | |
| Adults with prediabetes (majority overweight/obese) | Digital health lifestyle interventions | Non-digital control | Waist circumference (cm) | −1.58 (MD) | −2.11 to −1.04 | <0.01 | 62 | NR | Low | Low | |
| Adults with prediabetes (majority overweight/obese) | Digital health lifestyle interventions | Non-digital control | HbA1c (%) | −0.07 (MD) | −0.09 to −0.05 | <0.01 | 0 | NR | Moderate | Moderate | |
| Adults with prediabetes (majority overweight/obese) | Digital health lifestyle interventions | Non-digital control | More than 5% weight loss (log odds) | 1.49 (log odds) | 1.20 to 1.78 | <0.01 | NR | NR | Moderate | Moderate | |
| [26] | Adults with overweight/obesity | Web-based digital health interventions | Offline or face-to-face interventions, wait list | Weight change (kg) | −0.77 (MD, kg) | −2.16 to 0.62 | 0.28 | 94 | Not a major issue (GRADE, not formal test) | Moderate | Moderate |
| Adults with overweight/obesity | Web-based digital health interventions | Offline or face-to-face interventions, wait list | BMI change (kg/m2) | −0.12 (MD) | −0.64 to 0.41 | 0.65 | NR | Not a major issue (GRADE, not formal test) | Moderate | Moderate | |
| Adults with overweight/obesity | Web-based digital health interventions | Offline or face-to-face interventions, wait list | Waist circumference (cm) | −0.54 (MD, cm) | −5.17 to 4.10 | NR | NR | NR | NR | NR | |
| Adults with overweight/obesity | Web-based digital health interventions | Offline or face-to-face interventions, wait list | Body fat (%) | −1.40 (%) | −2.93 to 0.13 | NR | NR | NR | NR | NR |
| Meta-Analysis (Year) | Moderators Tested | Main Findings/Results | Significant Moderators |
|---|---|---|---|
| [18] | Type of intervention (mHealth: smartphone, PDA, web-based) Type of control group (usual care, paper record, wait-list) Duration of intervention (≤3, 6, ≥12 months) Participant characteristics (age, baseline weight, BMI, waist circumference) | mHealth self-monitoring interventions produced moderate weight loss vs. controls (SMD = −0.37; 95% CI: −0.54 to −0.19; I2 = 84.6%). Greatest effect for smartphone interventions and when usual care was control. Short-term interventions (≤3 m) more effective. Higher adherence vs. paper records. No significant moderation by age, baseline weight, or BMI. Publication bias present. | Intervention type (smartphone) Comparator (usual care) Intervention duration (≤3 months) |
| [10] | Duration of intervention (3–4, 6, 9, ≥12 months) Participant characteristics (mean age, baseline BMI, type of obesity) Study factor (year of publication) | mHealth interventions in adults resulted in significant weight loss vs. controls (WMD = −2.35 kg; 95% CI: −2.84 to −1.87; I2 = 94%). Effect greater at 6–9 months, attenuated at ≥12 m. BMI also reduced. No significant subgroup moderators. Short-term effect modest; mild publication bias. | Intervention duration |
| [19] | Type of control group (wait-list/standard care vs. sham) Target population (general adults vs. overweight/obese) Risk of bias Duration of intervention Delivery mode Human support Outcome measurement (device-based vs. self-report for physical activity) | Holistic mHealth interventions (PA, diet, mental health) yielded significant weight loss (MD = −1.70 kg) and reduced stress vs. controls. No significant effect on physical activity or diet quality. Greater effect vs. wait-list/standard care. Most studies short-term; some/high risk of bias. | Control group type (for weight loss) Target population (for diet quality) |
| [20] | Type of intervention (device type: mobile phone, PDA) Duration of intervention (≤6 months, >6 months) | Mobile device interventions (phones, PDAs, apps) produced modest weight loss vs. controls (WMD = −1.09 kg). Greater loss with mobile phone interventions. No difference by duration. No publication bias. | Device type (mobile phone) |
| [21] | Type of intervention (IC-W, MC-W, GSH-W, SH-W, SH, wait-list) Feedback features (frequency/personalization, provider: human/machine) Duration of intervention Participant characteristics (age, baseline weight, sample size) Study quality (risk of bias) | Intensive Contact Web-based (IC-W) programs with frequent professional feedback were most effective (MD = −1.86 kg vs. wait-list). All web-based interventions outperformed wait-list. Only intervention length was a significant moderator. Long-term data limited; dropout rates similar. | Feedback frequency/personalization Intervention length |
| [9] | Type of intervention (web-based with behavioral features/feedback, education-only, control/minimal, face-to-face) Adherence/usage (log-ins, self-monitoring, chat/board attendance) Outcome focus (weight loss vs. maintenance) | Web-based interventions led to modest weight loss vs. minimal/control (SMD = 0.73; 95% CI: −0.06 to 1.51). Enhanced behavioral features increased efficacy (WMD = 2.24 kg). Similar maintenance to face-to-face. High attrition and short follow-up. | Behavioral features Feedback Adherence/usage |
| [22] | Type of intervention (web, mobile, computer, with/without non-eHealth components) Type of control group (control, minimal, standard care, face-to-face) Technological features (additional features/technologies, monitoring devices) Duration of intervention Study quality (ITT analysis, retention) Participant characteristics (publication year, continent, gender, baseline BMI) | eHealth interventions resulted in modest weight loss vs. controls (MD = −2.70 kg), stronger with additional non-eHealth features. Extra features (self-monitoring, feedback) increased effect. No significant effect on weight maintenance. No publication bias. | Intervention type Additional features Monitoring devices Intervention duration Study quality Intent-to-treat (ITT) analysis Retention Publication year Continent |
| [23] | Duration of follow-up (medium, long term) Population (adolescents, adults) Type of control group (no/minimal intervention) Outcomes measured (physical activity, anthropometric measures, quality of life, self-efficacy, well-being, dietary behavior, adverse events) | mHealth smartphone interventions in adults produced significant weight loss at medium- and long-term follow-up (WMD = −1.79 kg and −1.35 kg). Secondary improvements in physical activity, diet, and quality of life were mixed. Moderate-high heterogeneity; safe profile. | No significant moderators identified Subgroup and meta-regression analyses did not reveal significant effect modification by follow-up duration, population, type of control group, or outcome measured. |
| [24] | Participant characteristics (age, country: USA vs. other) Duration of intervention (12, 16, 24 weeks) Study quality/methodology (intention-to-treat analysis, Jadad score, drop-out rate) Intervention features (interactive web-based, feedback included, goal-setting included) | Web-based interventions showed no significant effect on weight loss vs. controls (WMD = 0.56 kg). Feedback and goal-setting features improved outcomes. Certainty limited by high heterogeneity and poor methodological quality. | Age group Intent-to-treat (ITT) analysis Feedback Goal-setting Jadad score Country (USA vs. others) |
| [25] | Continent (North America, Asia, Europe) Duration of intervention Contact type (face-to-face, stand-alone, remote, combined) Frequency of digital contact Participant characteristics (baseline BMI) Technological features (digital scale, self-monitoring of weight, provider type, tracking technologies) Type of control group (usual care, enhanced care, active) | Digital health lifestyle interventions (DHLI) for prediabetes yielded significant weight loss vs. non-digital (MD = −1.74 kg). Greater effect with face-to-face elements, higher BMI, and self-monitoring. Secondary benefits for BMI, waist, HbA1c. Most studies at risk of bias. | Continent Study duration Contact type Frequency of digital contact Baseline BMI Digital scale Self-monitoring of weight |
| [26] | Type of control group (active intervention vs. wait-list) Duration of follow-up (<6 vs. ≥6 months) | Web-based interventions had no significant difference vs. offline in weight loss (MD = −0.77 kg). Greater short-term (<6 m) effect. No long-term benefit or difference in other outcomes. Moderate certainty; high attrition. | Control group type Follow-up duration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Couto, F.d.F.S.; de Almeida, C.P.B. Mobile and Web Apps for Weight Management in Overweight and Obese Adults: An Updated Umbrella Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2025, 22, 1152. https://doi.org/10.3390/ijerph22071152
Couto FdFS, de Almeida CPB. Mobile and Web Apps for Weight Management in Overweight and Obese Adults: An Updated Umbrella Review and Meta-Analysis. International Journal of Environmental Research and Public Health. 2025; 22(7):1152. https://doi.org/10.3390/ijerph22071152
Chicago/Turabian StyleCouto, Felipe da Fonseca Silva, and Carlos Podalirio Borges de Almeida. 2025. "Mobile and Web Apps for Weight Management in Overweight and Obese Adults: An Updated Umbrella Review and Meta-Analysis" International Journal of Environmental Research and Public Health 22, no. 7: 1152. https://doi.org/10.3390/ijerph22071152
APA StyleCouto, F. d. F. S., & de Almeida, C. P. B. (2025). Mobile and Web Apps for Weight Management in Overweight and Obese Adults: An Updated Umbrella Review and Meta-Analysis. International Journal of Environmental Research and Public Health, 22(7), 1152. https://doi.org/10.3390/ijerph22071152







