Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission
Abstract
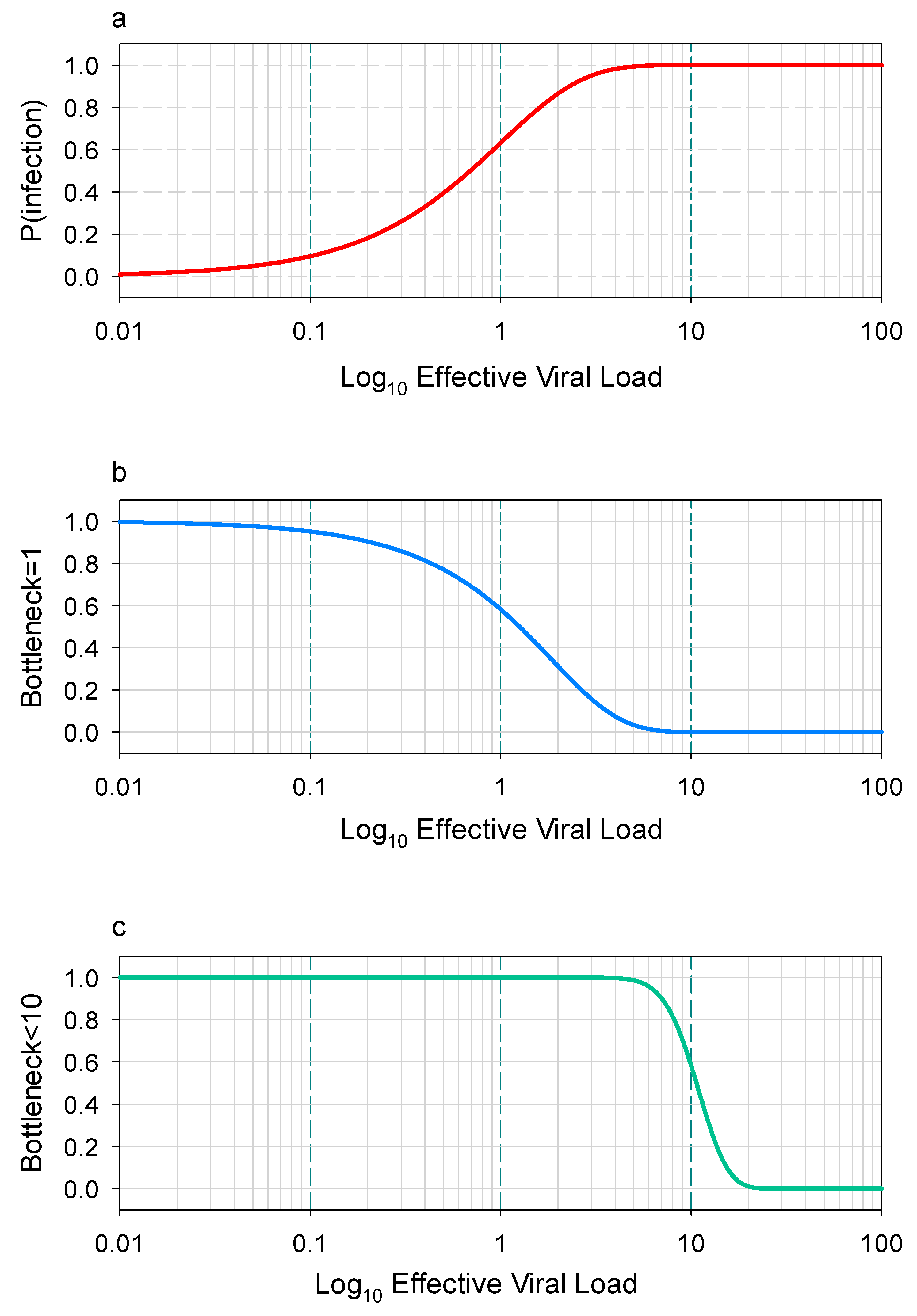
1. Introduction
2. Sampling Techniques
2.1. Active Sampling Techniques
2.2. Passive Sampling Techniques
2.3. Real-Time Monitoring Techniques
3. Virus Detection
4. Challenges
4.1. Technical Challenges
4.2. Data Interpretation and Model Construction
4.3. Ethical and Social Challenges
5. Future Research Directions
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jin, X.; Ren, J.; Li, R.; Gao, Y.; Zhang, H.; Li, J.; Zhang, J.; Wang, X.; Wang, G. Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine 2021, 37, 100986. [Google Scholar] [CrossRef] [PubMed]
- Paules, C.I.; Sullivan, S.G.; Subbarao, K.; Fauci, A.S. Chasing Seasonal Influenza—The Need for a Universal Influenza Vaccine. N. Engl. J. Med. 2018, 378, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health. 2019, 9, 020421. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Allen, J.; Bahnfleth, W.; Bluyssen, P.M.; Boerstra, A.; Buonanno, G.; Cao, J.; Dancer, S.J.; Floto, A.; Franchimon, F.; et al. A paradigm shift to combat indoor respiratory infection. Science 2021, 372, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Prather, K.A.; Sznitman, J.; Jimenez, J.L.; Lakdawala, S.S.; Tufekci, Z.; Marr, L.C. Airborne transmission of respiratory viruses. Science 2021, 373, eabd9149. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Morawska, L.; Milton, D.K. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ma, N.; Witt, C.; Rapp, S.; Wild, P.S.; Andreae, M.O.; Pöschl, U.; Su, H. Face masks effectively limit the probability of SARS-CoV-2 transmission. Science 2021, 372, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Teo, Z.W.; Wan, M.P.; Ng, B.F. Aerosols from speaking can linger in the air for up to nine hours. Build. Environ. 2021, 205, 108239. [Google Scholar] [CrossRef] [PubMed]
- Firatoglu, Z.A. The effect of natural ventilation on airborne transmission of the COVID-19 virus spread by sneezing in the classroom. Sci. Total Environ. 2023, 896, 165113. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.A.; Lauzardo, M.; Fan, Z.H.; Jutla, A.; Tilly, T.B.; Gangwar, M.; Usmani, M.; Shankar, S.N.; Mohamed, K.; Eiguren-Fernandez, A.; et al. Viable SARS-CoV-2 in the air of a hospital room with COVID-19 patients. Int. J. Infect. Dis. 2020, 100, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Blachere, F.M.; Lindsley, W.G.; Pearce, T.A.; Anderson, S.E.; Fisher, M.; Khakoo, R.; Meade, B.J.; Lander, O.; Davis, S.; Thewlis, R.E.; et al. Measurement of airborne influenza virus in a hospital emergency department. Clin. Infect. Dis. 2009, 48, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Leung, N.H.L.; Zhou, J.; Chu, D.K.W.; Yu, H.; Lindsley, W.G.; Beezhold, D.H.; Yen, H.-L.; Li, Y.; Seto, W.-H.; Peiris, J.S.M.; et al. Quantification of Influenza Virus RNA in Aerosols in Patient Rooms. PLoS ONE 2016, 11, e0148669. [Google Scholar] [CrossRef] [PubMed]
- Bastani, H.; Drakopoulos, K.; Gupta, V.; Vlachogiannis, I.; Hadjichristodoulou, C.; Lagiou, P.; Magiorkinis, G.; Paraskevis, D.; Tsiodras, S. Efficient and targeted COVID-19 border testing via reinforcement learning. Nature 2021, 599, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Frydas, I.S.; Kermenidou, M.; Karypidou, M.; Karakitsios, S.; Sarigiannis, D.A. SARS-CoV-2 airborne detection within different departments of a COVID-19 hospital building and evaluation of air cleaners in air viral load reduction. J. Aerosol Sci. 2025, 187, 106587. [Google Scholar] [CrossRef]
- Asif, M.; Algethami, J.S.; Alhamami, M.A.M.; Lie, P.; Shaomin, S.; Aziz, A. Redefining nanobiosensors in rapid detection of viral infections: Where are we now? Trends Environ. Anal. Chem. 2025, 46, e00263. [Google Scholar] [CrossRef]
- Li, B.; Lin, B.; Wang, Y.; Shi, Y.; Zeng, W.; Zhao, Y.; Gu, Y.; Liu, C.; Gao, H.; Cheng, H.; et al. Multi-scenario surveillance of respiratory viruses in aerosols with sub-single-copy spatial resolution. Nat. Commun. 2024, 15, 8770. [Google Scholar] [CrossRef] [PubMed]
- Kutter, J.S.; de Meulder, D.; Bestebroer, T.M.; Mulders, A.; Fouchier, R.A.M.; Herfst, S. Comparison of three air samplers for the collection of four nebulized respiratory viruses-Collection of respiratory viruses from air. Indoor Air. 2021, 31, 1874–1885. [Google Scholar] [CrossRef] [PubMed]
- Hogan CJJr Kettleson, E.M.; Lee, M.H.; Ramaswami, B.; Angenent, L.T.; Biswas, P. Sampling methodologies and dosage assessment techniques for submicrometre and ultrafine virus aerosol particles. J. Appl. Microbiol. 2005, 99, 1422–1434. [Google Scholar] [CrossRef] [PubMed]
- Verreault, D.; Moineau, S.; Duchaine, C. Methods for sampling of airborne viruses. Microbiol. Mol. Biol. Rev. 2008, 72, 413–444. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.; Hong, S.; Jang, J.; Han, C.-H.; Lee, J.; Jang, J. Recent advancements in the measurement of pathogenic airborne viruses. J. Hazard. Mater. 2021, 420, 126574. [Google Scholar] [CrossRef] [PubMed]
- Robotto, A.; Civra, A.; Quaglino, P.; Polato, D.; Brizio, E.; Lembo, D. SARS-CoV-2 airborne transmission: A validated sampling and analytical method. Environ. Res. 2021, 200, 111783. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Dong, L.; Zhang, J.; Wang, D.; Gao, Y. Reference material development for detection of human respiratory syncytial virus using digital PCR. Anal. Bioanal. Chem. 2023, 415, 3131–3135. [Google Scholar] [CrossRef] [PubMed]
- Pereira, L.; Lapinscki, B.; Debur, M.; Santos, J.; Petterle, R.; Nogueira, M.; Vidal, L.; De Almeida, S.; Raboni, S. Standardization of a high-performance RT-qPCR for viral load absolute quantification of influenza A. J. Virol. Methods. 2022, 301, 114439. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Yin, F.; Liang, M.; Zong, W.; Wang, Y.; Xiao, D.; Yin, J.; Mu, Y. Development of chamber-based digital polymerase chain reaction: From chip to device. Trends Anal. Chem. 2025, 191, 118299. [Google Scholar] [CrossRef]
- Kanki, P.J.; Hamel, D.J.; Riedel, S.; Dutta, S.; Cheng, A.; Chang, C.A.; Arnaout, R.; Kirby, J.E. SARS-CoV-2 live virus culture and sample freeze-thaw stability. Diagn. Microbiol. Infect. Dis. 2024, 109, 116282. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, A.; Pieri, M.; Sarubbi, S.; Pelagalli, M.; Calugi, G.; Tomassetti, F.; Bernardini, S.; Nuccetelli, M. Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring. Clin. Immunol. 2022, 234, 108918. [Google Scholar] [CrossRef] [PubMed]
- Ohno, M.; Sekiya, T.; Obeng-Kyeremeh, R.; Handabile, C.; Haruta, M.; Nomura, N.; Kawakita, T.; Shingai, M.; Kida, H. Optimization of the preparation method of inactivated intact virus particle vaccine for COVID-19. Vaccine. 2025, 56, 127173. [Google Scholar] [CrossRef] [PubMed]
- Lauring, A.S.; Tenforde, M.W.; Chappell, J.D.; Gaglani, M.; Ginde, A.A.; McNeal, T.; Ghamande, S.; Douin, D.J.; Talbot, H.K.; Casey, J.D.; et al. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: Prospective observational study. BMJ 2022, 376, e069761. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Jiang, W.; Huang, Z.; Yuan, Z.; Chen, Z.; Lin, J. Multiplex detection of respiratory RNA viruses without amplification based on CRISPR-Cas13a immunochromatographic test strips. Virol. J. 2025, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, S.; Ma, Y.; Jiang, Y.; Li, Y.; Shi, J.; Deng, G.; Tian, G.; Kong, H.; Wang, X. On-site and visual detection of the H5 subtype avian influenza virus based on RT-RPA and CRISPR/Cas12a. Viruses 2024, 16, 753. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, P.; Hu, X.; Huang, L.; Geng, Z.; Xu, H.; Hu, W.; Wang, L.; Wu, P.; Liu, G.L. An ultra-sensitive and specific nanoplasmonic-enhanced isothermal amplification platform for the ultrafast point-of-care testing of SARS-CoV-2. Chem. Eng. J. 2023, 451, 138822. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, R.; Li, K.; Zhu, Z.; Huang, X.; He, Q.; Sun, Z.; He, H.; Ge, Y.; Zhang, Q.; et al. On-mask detection of SARS-CoV-2 related substances by surface enhanced Raman scattering. Talanta 2024, 277, 126403. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.P.S.; Kong, S.L.Y.; Pang, J.K.S.; Soh, B.S. 3D Human Organoids: The Next "Viral" Model for the Molecular Basis of Infectious Diseases. Biomedicines 2022, 10, 1541. [Google Scholar] [CrossRef] [PubMed]
- Pratanwanich, P.N.; Yao, F.; Chen, Y.; Koh, C.W.Q.; Wan, Y.K.; Hendra, C.; Poon, P.; Goh, Y.T.; Yap, P.M.L.; Chooi, J.Y.; et al. Identification of differential RNA modifications from nanopore direct RNA sequencing with xPore. Nat. Biotechnol. 2021, 39, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, P.; Zhao, L.; Beggs, C.B.; Illingworth, C.J.R. The airborne transmission of viruses causes tight transmission bottlenecks. Nat. Commun. 2024, 15, 3540. [Google Scholar] [CrossRef] [PubMed]
| Sampler | Advantages | Disadvantages |
|---|---|---|
| Cyclone sampler | Enables size-fractionated aerosol collection and is scalable for parallel sampling, with a flexible duration and high efficiency. | Not intended for infectious virus isolation. |
| Cascade impactor | This method supports infectious virus isolation and particle size fractionation and offers customizable fraction collection with flexible material selection. | Constrained by anesthesia duration. Labor-intensive and less efficient at low viral concentrations. |
| Liquid impinger | Designed for infectious virus recovery using multistage liquid impinger technology with integrated aerosol particle size fractionation. | The high liquid volumes risk diluting low-concentration samples below detection limits, while prolonged collection risks solvent evaporation. Additionally, it demonstrates reduced efficiency for sub-0.3 μm particle capture. |
| Condensation sampler | Designed for low-volume infectious virus collection and recovery. | No size-based aerosol separation and a large physical size. |
| Filter samplers | With appropriate filter media, this method effectively recovers infectious viruses by maximizing aerosol–surface interactions. The design can be efficiently expanded for high-throughput applications. | This method cannot maintain viral infectivity (filters cause desiccation) and provides no particle size discrimination of aerosols. |
| Aerosol mass spectrometer | Provides simultaneous quantification of aerosol particle number concentration and chemical components. | This method cannot recover infectious viruses and collects aerosols non-specifically. |
| Technology | Detection Target | Sensitivity | Time Resolution | Live Virus Discrimination |
|---|---|---|---|---|
| qPCR | Viral RNA | 10 copies/m3 | 2–4 h | No |
| Viral Culture | Live Virus | 100 TCID50/m3 | 3–7 days | Yes |
| SERS Sensor | Viral Protein | 1 particle/m3 | 10 min | Partial |
| Microfluidic Chip | Intact Virus | 10 particles/m3 | Real-time | Yes |
| Challenge | Proposed Solution | Future Direction |
|---|---|---|
| Low sensitivity | Nanoplasmonic sensors, microfluidic chips | AI-enhanced signal amplification |
| Lack of standardization | Unified protocols (WHO and CDC collaboration) | Global data-sharing platforms |
| High costs | Portable FET/SERS devices | Low-cost CRISPR-based field tests |
| Viability detection | 3D organoid models, protein integrity assays | Rapid viability markers (e.g., viral fusion) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Q. Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission. Int. J. Environ. Res. Public Health 2025, 22, 1151. https://doi.org/10.3390/ijerph22071151
Liu Q. Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission. International Journal of Environmental Research and Public Health. 2025; 22(7):1151. https://doi.org/10.3390/ijerph22071151
Chicago/Turabian StyleLiu, Qingyang. 2025. "Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission" International Journal of Environmental Research and Public Health 22, no. 7: 1151. https://doi.org/10.3390/ijerph22071151
APA StyleLiu, Q. (2025). Early Warning Signs for Monitoring Airborne Respiratory Virus Transmission. International Journal of Environmental Research and Public Health, 22(7), 1151. https://doi.org/10.3390/ijerph22071151






