Personalised Nutrition Approaches in the Prevention and Management of Type 2 Diabetes: A Narrative Review of Evidence and Practice
Abstract
1. Introduction
2. Methodology
2.1. Literature Search Strategy
- “Personalized nutrition” OR “individualised dietary intervention”
- “Type 2 diabetes” OR “T2DM”
- “Glycaemic index” OR “GI”
- “Glycaemic load” OR “GL”
- “Food insulin index” OR “FII”.
2.2. Inclusion and Exclusion Criteria
- Publications from published in English from 2008 to 2025.
- Peer-reviewed articles including clinical trials (RCTs), reviews, as well as quantitative and qualitative studies, including book chapters.
- Grey literature and policy documents relevant to dietary strategies in diabetes prevention or management.
- Studies focusing on glycaemic control, behavioural adherence, and personalised nutrition interventions.
- Studies not focused on T2DM.
- Articles lacking dietary or nutritional intervention focus.
- Letters to the editor, editorials, and conference abstracts with insufficient data.
- Non-English language publications.
2.3. Study Selection Process
2.4. Integration of Grey Literature
2.5. Questions
- What role does personalised nutrition play in preventing and managing T2DM?
- How do GI, GL, and FII-based strategies affect glycaemic control?
- Which behavioural, cultural, or systemic factors influence adherence?
- What are the challenges and facilitators of implementing these strategies in low-resource settings?
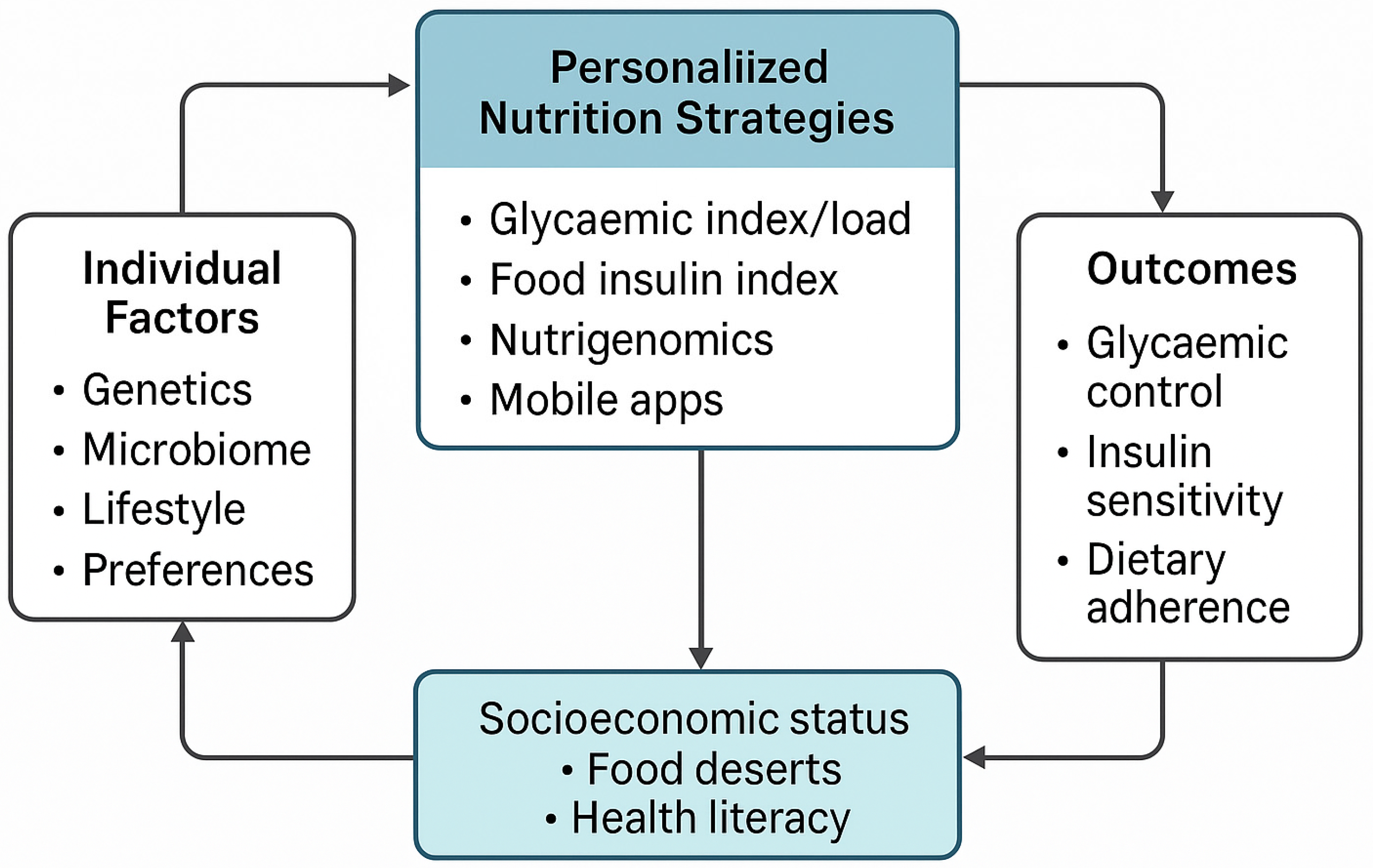
3. Nutrition and Its Role in Managing T2DM
4. Personalised Nutrition in T2DM: Strategies and Evidence
- Glycaemic index, glycaemic load, and food insulin index (FII): Low-GI and low-GL diets reduce postprandial glucose excursions and improve HbA1c and insulin sensitivity [8,20]. These measures have long been considered useful in guiding dietary choices for glycaemic control. While GL offers better predictive power than GI alone, FII remains underutilised due to limited data and clinical guidance. The FII captures the insulin response to all macronutrients, offering a more holistic assessment of dietary impact [9]. However, the FII clinical application is limited due to a lack of global standardisation, a narrow range of tested foods, exclusion from major nutrient databases, and unclear guidelines for practical use.
- Cultural and socioeconomic adaptation: Success in LMICs depends on affordability, local dietary norms, and food availability. Programmes must use culturally familiar foods and be adapted to local language and literacy levels [28].
5. Real-World Effectiveness of Personalised Nutrition
6. Mobile Apps for Diabetes Self-Management
7. Behavioural and Cultural Considerations in Personalised Nutrition for T2DM
8. Implementation Challenges of Personalised Nutrition in T2DM
8.1. Implementation Barriers
8.1.1. Healthcare Infrastructure and Human Resources
8.1.2. Digital Divide
8.1.3. Health Literacy
8.1.4. Policy and Sustainability Gaps
9. Integrating Personalised Nutrition into Primary Care for T2DM Management in LMICs
9.1. Task-Shifting and Capacity Building
9.2. Context-Specific, Standardised Tools
- Visual food charts with staples such as maize, samp, legumes, and sweet potatoes;
- Educational leaflets written in local languages with illustrations;
- Real-life examples and modified traditional recipes discussed during counselling sessions.
9.3. Policy Integration for Sustainability
9.4. Leveraging Technology in PHC
- Text message campaigns providing localised dietary advice in preferred languages;
- Mobile apps used by PHC workers to support point-of-care counselling;
- Continuous glucose monitors and wearables linked to clinic data for ongoing feedback.
10. Future Directions and Research Needs
10.1. Evaluating Long-Term (Above 12 Months) Outcomes in Real-World Settings
10.2. Cost-Effectiveness and Scalability
10.3. Gender-Sensitive and Culturally Tailored Interventions
10.4. Digital Integration for Wider Access
11. Conclusions
12. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- International Diabetes Federation (IDF). Diabetes Atlas, 11th Edition. 2025. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/?mc_cid=b5d31665df&mc_eid=672aa4d27c (accessed on 20 April 2025).
- Abdul Basith Khan, M.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of type 2 diabetes—Global burden of disease and forecasted trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef]
- Pastakia, S.D.; Pekny, C.R.; Manyara, S.M.; Fischer, L. Diabetes in sub-Saharan Africa–from policy to practice to progress: Targeting the existing gaps for future care for diabetes. Diabetes Metab. Syndr. Obes. Targets Ther. 2017, 10, 247–263. [Google Scholar] [CrossRef]
- Karachaliou, F.; Simatos, G.; Simatou, A. The challenges in the development of diabetes prevention and care models in low-income settings. Front. Endocrinol. 2020, 11, 518. [Google Scholar] [CrossRef]
- Minari, T.P.; Tácito, L.H.B.; Yugar, L.B.T.; Ferreira-Melo, S.E.; Manzano, C.F.; Pires, A.C.; Moreno, H.; Vilela-Martin, J.F.; Cosenso-Martin, L.N.; Yugar-Toledo, J.C. Nutritional strategies for the management of type 2 diabetes mellitus: A narrative review. Nutrients 2023, 15, 5096. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Ordovás, J.M.; Parks, E.J.; Anderson, C.A.M.; Barabási, A.L.; Clinton, S.K.; de la Haye, K.; Duffy, V.B.; Franks, P.W.; Ginexi, E.M.; et al. Research gaps and opportunities in precision nutrition: An NIH workshop report. Am. J. Clin. Nutr. 2022, 116, 1877–1900. [Google Scholar] [CrossRef] [PubMed]
- Shyam, S.; Lee, K.X.; Tan, A.S.W.; Khoo, T.A.; Harikrishnan, S.; Lalani, S.A.; Ramadas, A. Effect of personalized nutrition on dietary, physical activity, and health outcomes: A systematic review of randomized trials. Nutrients 2022, 14, 4104. [Google Scholar] [CrossRef] [PubMed]
- Vlachos, D.; Malisova, S.; Lindberg, F.A.; Karaniki, G. Glycemic index (GI) or glycemic load (GL) and dietary interventions for optimizing postprandial hyperglycemia in patients with T2 diabetes: A review. Nutrients 2020, 12, 1561. [Google Scholar] [CrossRef]
- Strydom, H.; Delport, E.; Muchiri, J.; White, Z. The Application of the Food Insulin Index in the Prevention and Management of Insulin Resistance and Diabetes: A Scoping Review. Nutrients 2024, 16, 584. [Google Scholar] [CrossRef]
- Salinari, A.; Machì, M.; Armas Diaz, Y.; Cianciosi, D.; Qi, Z.; Yang, B.; Ferreiro Cotorruelo, M.S.; Villar, S.G.; Dzul Lopez, L.A.; Battino, M.; et al. The application of digital technologies and artificial intelligence in healthcare: An overview on nutrition assessment. Diseases 2023, 11, 97. [Google Scholar] [CrossRef]
- Fenta, E.T.; Kidie, A.A.; Tiruneh, M.G.; Anagaw, T.F.; Bogale, E.K.; Dessie, A.A.; Worku, N.K.; Amera, M.G.; Tesfa, H.; Limenh, L.W.; et al. Exploring barriers of health literacy on non-communicable disease prevention and care among patients in north wollo zone public hospitals; Northeast, Ethiopia, 2023: Application of socio-ecological model. BMC Public Health 2024, 24, 971. [Google Scholar] [CrossRef]
- Sosa-Holwerda, A.; Park, O.H.; Albracht-Schulte, K.; Niraula, S.; Thompson, L.; Oldewage-Theron, W. The role of artificial intelligence in nutrition research: A scoping review. Nutrients 2024, 16, 2066. [Google Scholar] [CrossRef] [PubMed]
- Khazrai, Y.M.; Defeudis, G.; Pozzilli, P. Effect of diet on type 2 diabetes mellitus: A review. Diabetes/Metab. Res. Rev. 2014, 30, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.L.; Gong, Y.; Qi, Y.J.; Shao, Z.M.; Jiang, Y.Z. Effects of dietary intervention on human diseases: Molecular mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2024, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Diet, Nutrition and the Prevention of Chronic Diseases; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Ryan, D.H.; Yockey, S.R. Weight loss and improvement in comorbidity: Differences at 5%, 10%, 15%, and over. Curr. Obes. Rep. 2017, 6, 187–194. [Google Scholar] [CrossRef]
- Matusheski, N.V.; Caffrey, A.; Christensen, L.; Mezgec, S.; Surendran, S.; Hjorth, M.F.; McNulty, H.; Pentieva, K.; Roager, H.M.; Seljak, B.K.; et al. Diets, nutrients, genes and the microbiome: Recent advances in personalised nutrition. Br. J. Nutr. 2021, 126, 1489–1497. [Google Scholar] [CrossRef]
- Riley, H.L.; Kable, M.E.; Marco, M.L.; Keim, N.L. The role of the gut microbiome in predicting response to diet and the development of precision nutrition models. Part II: Results. Adv. Nutr. Int. Rev. J. 2019, 10, 979–998. [Google Scholar]
- Unwin, D.; Livesey, G.; Haslam, D. It is the glycaemic response to, not the carbohydrate content of food that matters in diabetes and obesity: The glycaemic index revisited. J. Insul. Resist. 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Gerontiti, E.; Shalit, A.; Stefanaki, K.; Kazakou, P.; Karagiannakis, D.S.; Peppa, M.; Psaltopoulou, T.; Paschou, S.A. The role of low glycemic index and load diets in medical nutrition therapy for type 2 diabetes: An update. Hormones 2024, 23, 655–665. [Google Scholar] [CrossRef]
- Berná, G.; Oliveras-López, M.J.; Jurado-Ruíz, E.; Tejedo, J.; Bedoya, F.; Soria, B.; Martín, F. Nutrigenetics and nutrigenomics insights into diabetes etiopathogenesis. Nutrients 2014, 6, 5338–5369. [Google Scholar] [CrossRef]
- Ramos-Lopez, O. Genotype-based precision nutrition strategies for the prediction and clinical management of type 2 diabetes mellitus. World J. Diabetes 2024, 15, 142. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized nutrition by prediction of glycemic responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Wolever, T.M.S. Personalized nutrition by prediction of glycaemic responses: Fact or fantasy? Eur. J. Clin. Nutr. 2016, 70, 411–413. [Google Scholar] [CrossRef]
- Vandeputte, D. Personalized nutrition through the gut microbiota: Current insights and future perspectives. Nutr. Rev. 2020, 78 (Suppl. 3), 66–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Choi, Y.; Park, J. The role of continuous glucose monitoring in physical activity and nutrition management: Perspectives on present and possible uses. Phys. Act. Nutr. 2023, 27, 44. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, N.; Al Zaghal, E. Continuous glucose monitoring as a behavior modification tool. Clin. Diabetes: A Publ. Am. Diabetes Assoc. 2020, 38, 126. [Google Scholar] [CrossRef] [PubMed]
- Masuku, M.M.; Bhengu, A.S. The value of indigenous foods in improving food security in Emaphephetheni rural setting. Indilinga Afr. J. Indig. Knowl. Syst. 2021, 20, 13–23. [Google Scholar]
- Zafar, M.I.; Mills, K.E.; Zheng, J.; Regmi, A.; Hu, S.Q.; Gou, L.; Chen, L.L. Low-glycemic index diets as an intervention for diabetes: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2019, 110, 891–902. [Google Scholar] [CrossRef]
- Solomon, T.P.; Haus, J.M.; Kelly, K.R.; Cook, M.D.; Filion, J.; Rocco, M.; Kashyap, S.R.; Watanabe, R.M.; Barkoukis, H.; Kirwan, J.P. A low–glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am. J. Clin. Nutr. 2010, 92, 1359–1368. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Bordoni, L.; Muscogiuri, G.; Colao, A.; Savastano, S. Obesity Programs of Nutrition Education Research Assessment (OPERA) Group Nutrigenetics—Personalized nutrition in obesity cardiovascular diseases. Int. J. Obes. Suppl. 2020, 10, 1–13. [Google Scholar] [CrossRef]
- Jonusas, J.; Aleknavicius, K.; Valinskas, S. Klinio mobile app for diabetes self-care: A pilot study of HbA1c improvement in type 2 diabetes patients. Smart Health 2023, 29, 100404. [Google Scholar] [CrossRef]
- Levinson, C.A.; Fewell, L.; Brosof, L.C. My Fitness Pal calorie tracker usage in the eating disorders. Eat. Behav. 2017, 27, 14–16. [Google Scholar] [CrossRef]
- Ehiem, C.I. Implementation of Mobile App Glucose Buddy to Self-Monitor and Manage Blood Glucose and Exercise to Reduce A1c Levels in Adults With Type 2 Diabetes Over Three Months. Doctoral Dissertation, University of Massachusetts Global, Aliso Viejo, CA, USA, 2023. [Google Scholar]
- Puoane, T.; Tsolekile, L.; Steyn, N. Perceptions about body image and sizes among black African girls living in Cape Town. Ethn. Dis. 2010, 20, 29–34. [Google Scholar] [PubMed]
- Pradeilles, R.; Holdsworth, M.; Olaitan, O.; Irache, A.; Osei-Kwasi, H.A.; Ngandu, C.B.; Cohen, E. Body size preferences for women and adolescent girls living in Africa: A mixed-methods systematic review. Public Health Nutr. 2022, 25, 738–759. [Google Scholar] [CrossRef]
- Sileo, K.M.; Kershaw, T.S. Dimensions of masculine norms, depression, and mental health service utilization: Results from a prospective cohort study among emerging adult men in the United States. Am. J. Men’s Health 2020, 14, 1557988320906980. [Google Scholar] [CrossRef] [PubMed]
- Rebe, S.; Cronje, N.; Redelinghuys, N.; Pretorius, W.; du Toit, A. Exploring the consumption of maize products, side dishes and snacks preferred by consumers at a public tertiary institution in South Africa. J. Consum. Sci. 2024, 1, 23–42. [Google Scholar] [CrossRef]
- Mayen, A.L.; Marques-Vidal, P.; Paccaud, F.; Bovet, P.; Stringhini, S. Socioeconomic determinants of dietary patterns in low-and middle-income countries: A systematic review. Am. J. Clin. Nutr. 2014, 100, 1520–1531. [Google Scholar] [CrossRef]
- Major-Smith, D.; Morgan, J.; Emmett, P.; Golding, J.; Northstone, K. Associations between religious/spiritual beliefs and behaviours and dietary patterns: Analysis of the parental generation in a prospective cohort study (ALSPAC) in Southwest England. Public Health Nutr. 2023, 26, 2895–2911. [Google Scholar] [CrossRef]
- Ordovas, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 361, k2173. [Google Scholar] [CrossRef]
- Carpio-Arias, T.V.; Crovetto-Mattassi, M.; Durán-Agüero, S.; Parra-Soto, S.; Landaeta-Díaz, L.; Cerezo de Ríos, S.; Bejarano-Roncancio, J.J.; Cordón-Arrivillaga, K.; Vitullo, M.; Perichart-Perera, O.; et al. Barriers and opportunities for clinical nutritionists in 13 latin american countries: A qualitative study. J. Prim. Care Community Health 2023, 14, 21501319231204580. [Google Scholar] [CrossRef]
- Atun, R.; Davies, J.I.; Gale, E.A.M.; Bärnighausen, T.; Beran, D.; Kengne, A.P.; Levitt, N.S.; Mangugu, F.W.; Nyirenda, M.J.; Ogle, G.D.; et al. Diabetes in sub-Saharan Africa: From clinical care to health policy. Lancet Diabetes Endocrinol. 2017, 5, 622–667. [Google Scholar] [CrossRef]
- Phillip, M.; Bergenstal, R.M.; Close, K.L.; Danne, T.; Garg, S.K.; Heinemann, L.; Hirsch, I.B.; Kovatchev, B.P.; Laffel, L.M.; Mohan, V.; et al. The digital/virtual diabetes clinic: The future is now—Recommendations from an international panel on diabetes digital technologies introduction. Diabetes Technol. Ther. 2021, 23, 146–154. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Livingstone, K.M.; Marsaux, C.F.; Macready, A.L.; Fallaize, R.; O’Donovan, C.B.; Woolhead, C.; Forster, H.; Walsh, M.C.; Navas-Carretero, S.; et al. Effect of personalised nutrition on health-related behaviour change: Evidence from the Food4Me trial. Int. J. Epidemiol. 2017, 46, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Sawesi, S.; Rashrash, M.; Phalakornkule, K.; Carpenter, J.S.; Jones, J.F. The impact of information technology on patient engagement and health behavior change: A systematic review of the literature. JMIR Med. Inform. 2016, 4, e4514. [Google Scholar] [CrossRef]
- Nnyepi, M.S.; Gwisai, N.; Lekgoa, M.; Seru, T. Evidence of nutrition transition in Southern Africa. Proc. Nutr. Soc. 2015, 74, 478–486. [Google Scholar] [CrossRef]
- Zerfu, T.A.; Umeta, M.; Baye, K. Dietary diversity during pregnancy is associated with reduced risk of maternal anemia, preterm delivery, and low birth weight. The Am. J. Clin. Nutr. 2016, 103, 1482–1488. [Google Scholar] [CrossRef]
- Dugani, S.B.; Mielke, M.M.; Vella, A. Burden and management of type 2 diabetes in rural United States. Diabetes/Metab. Res. Rev. 2021, 37, e3410. [Google Scholar] [CrossRef] [PubMed]
- Beaglehole, R.; Epping-Jordan, J.; Patel, V.; Chopra, M.; Ebrahim, S.; Kidd, M.; Haines, A. Improving the prevention and management of chronic disease in low-income and middle-income countries: A priority for primary health care. Lancet 2008, 372, 940–949. [Google Scholar] [CrossRef]
- Joshi, R.; Alim, M.; Kengne, A.P.; Jan, S.; Maulik, P.K.; Peiris, D.; Patel, A.A. Task shifting for non-communicable disease management in low and middle income countries—A systematic review. PLoS ONE 2014, 9, e103754. [Google Scholar] [CrossRef]
- Ebere, R.; Imungi, J.; Kimani, V. Glycemic index values of traditional Kenyan foods: The missing link in the effectiveness of dietary approach in the prevention and management of diabetes mellitus in Kenya. Afr. Health Sci. 2021, 21, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Mphasha, M.H.; Skaal, L.; Mothiba, T.M. Development of a Family-Centred Nutrition and Exercise Diabetes Care Programme for Better Diabetes Outcomes in Rural Areas of Limpopo: Practice-Oriented Theory. Open Public Health J. 2022, 15, e187494452205191. [Google Scholar] [CrossRef]
- Kempers, J.; Rotaru, C.; Topa, A.; Zarbailov, N.; Curteanu, A.; Prytherch, H. Does training on the WHO package of essential noncommunicable (PEN) disease interventions enhance consultation quality? A real-world assessment of adherence to PEN protocol in primary health centres in the Republic of Moldova. Glob. Health Action 2023, 16, 2285619. [Google Scholar] [CrossRef]
- Monteiro, C.A.; Moura, E.C.; Conde, W.L.; Popkin, B.M. Socioeconomic status and obesity in adult populations of developing countries: A review. Bull. World Health Organ. 2004, 82, 940–946. [Google Scholar] [PubMed]
- Mphasha, M.H.; Skaal, L.; Mothiba, T.M.; Ngoatle, C.; Hlahla, L.S. Primary health care–family partnership for better diabetes outcomes of patients: A systematic review. J. Endocrinol. Metab. Diabetes 2023, 28, 1–6. [Google Scholar] [CrossRef]
- Abaza, H.; Marschollek, M. mHealth application areas and technology combinations. Methods Inf. Med. 2017, 56, e105–e122. [Google Scholar] [CrossRef] [PubMed]
- Mphasha, M.H.P. Cost-Effective Interventions to Curb Cardiovascular Diseases in Africa. In Novel Pathogenesis and Treatments for Cardiovascular Disease; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Dolman, R.C.; Stonehouse, W.; van’t Riet, H.; Badham, J.; Jerling, J.C. Beliefs of South Africans regarding food and cardiovascular health. Public Health Nutr. 2008, 11, 946–954. [Google Scholar] [CrossRef]
| Strategy | Description | Strengths | Limitations |
|---|---|---|---|
| Glycaemic index | Ranks foods by glucose impact | Simple, practical | Ignores portion sizes |
| Glycaemic load | Considers GI and quantity | Better prediction of glucose levels | Slightly more complex for patients |
| Food insulin index | Assesses insulin response from all macros | Captures full insulin dynamics | Limited data, less familiar |
| Nutrigenomics | Tailors diet using genetic markers | Highly individualised | Expensive and less accessible |
| Barrier | Description | Suggested Solutions |
|---|---|---|
| Lack of trained staff | Too few dietitians in clinics and PHC | Train and support CHWs |
| Digital divide | CGMs and apps are expensive and limited | Government subsidies, partnerships |
| Health iliteracy | Complex terms confuse many patients | Simplify materials; adapt to local contexts |
| Healthcare infrastructure | Limited access to diagnostic tools | Use mobile-friendly or basic diagnostic tools |
| Policy and sustainability gaps | PN is not integrated into national health policies; nutrition is often sidelined in PHC | Embed PN into NCD strategies and PHC protocols; ensure dedicated funding |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mphasha, M.; Mothiba, T. Personalised Nutrition Approaches in the Prevention and Management of Type 2 Diabetes: A Narrative Review of Evidence and Practice. Int. J. Environ. Res. Public Health 2025, 22, 1047. https://doi.org/10.3390/ijerph22071047
Mphasha M, Mothiba T. Personalised Nutrition Approaches in the Prevention and Management of Type 2 Diabetes: A Narrative Review of Evidence and Practice. International Journal of Environmental Research and Public Health. 2025; 22(7):1047. https://doi.org/10.3390/ijerph22071047
Chicago/Turabian StyleMphasha, Mabitsela, and Tebogo Mothiba. 2025. "Personalised Nutrition Approaches in the Prevention and Management of Type 2 Diabetes: A Narrative Review of Evidence and Practice" International Journal of Environmental Research and Public Health 22, no. 7: 1047. https://doi.org/10.3390/ijerph22071047
APA StyleMphasha, M., & Mothiba, T. (2025). Personalised Nutrition Approaches in the Prevention and Management of Type 2 Diabetes: A Narrative Review of Evidence and Practice. International Journal of Environmental Research and Public Health, 22(7), 1047. https://doi.org/10.3390/ijerph22071047






