Bone Mineral Density in Children and Adolescents of the Abay Region, Kazakhstan: Prevalence and Associated Risk Factors
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Groups
2.2. Survey
2.3. DXA Measurement
2.4. Biochemical Blood Analysis
2.5. Statistical Data Processing
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bachrach, L.K.; Gordon, C.M. Bone Densitometry in Children and Adolescents. Pediatrics 2016, 138, e20162398. [Google Scholar] [CrossRef] [PubMed]
- Abed, M.N.; Alassaf, F.A.; Qazzaz, M.E. Exploring the Interplay Between Vitamin D, Insulin Resistance, Obesity and Skeletal Health. J. Bone Metab. 2024, 31, 75–89. [Google Scholar] [CrossRef]
- Formosa, M.M.; Christou, M.A.; Mäkitie, O. Bone fragility and osteoporosis in children and young adults. J. Endocrinol. Investig. 2024, 47, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Zavala, R.; Bou-Torrent, R.; Magallares-López, B.; Mir-Perelló, C.; Palmou-Fontana, N.; Sevilla-Pérez, B.; Medrano-San Ildefonso, M.; González-Fernández, M.I.; Román-Pascual, A.; Alcañiz-Rodríguez, P.; et al. Expert panel consensus recommendations for diagnosis and treatment of secondary osteoporosis in children. Pediatr. Rheumatol. Online J. 2020, 24, 20. [Google Scholar] [CrossRef] [PubMed]
- Ciancia, S.; van Rijn, R.R.; Högler, W.; Appelman-Dijkstra, N.M.; Boot, A.M.; Sas, T.C.J.; Renes, J.S. Osteoporosis in children and adolescents: When to suspect and how to diagnose it. Eur. J. Pediatr. 2022, 181, 2549–2561. [Google Scholar] [CrossRef]
- Al-Bari, A.A.; Al Mamun, A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020, 19, 668–679. [Google Scholar] [CrossRef]
- Diemar, S.S.; Lylloff, L.; Rønne, M.S.; Møllehave, L.T.; Heidemann, M.; Thuesen, B.H.; Johannesen, J.; Schou, A.J.; Husby, S.; Wedderkopp, N.; et al. Reference intervals in Danish children and adolescents for bone turnover markers carboxy-terminal cross-linked telopeptide of type I collagen (β-CTX), pro-collagen type I N-terminal propeptide (PINP), osteocalcin (OC) and bone-specific alkaline phosphatase (bone ALP). Bone 2021, 146, 115879. [Google Scholar] [CrossRef]
- López-Peralta, S.; Romero-Velarde, E.; Vásquez-Garibay, E.M.; González-Hita, M.; Robles-Robles, L.C.; Ruiz-González, F.J.; Pérez-Romero, M.A. Bone mineral density and body composition in normal weight, overweight and obese children. BMC Pediatr. 2022, 22, 249. [Google Scholar] [CrossRef]
- Gómez-Alonso, C. Paediatric Metabolic Bone Disease: A Lifetime Ahead. Adv. Ther. 2020, 37 (Suppl. 2), 38–46. [Google Scholar] [CrossRef]
- Hart, N.H.; Newton, R.U.; Tan, J.; Rantalainen, T.; Chivers, P.; Siafarikas, A.; Nimphius, S. Biological basis of bone strength: Anatomy, physiology and measurement. J. Musculoskelet. Neuronal Interact. 2020, 20, 347–371. [Google Scholar] [PubMed Central]
- Rosendahl, K.; Lundestad, A.; Bjørlykke, J.A.; Lein, R.K.; Angenete, O.; Augdal, T.A.; Müller, L.O.; Jaramillo, D. Revisiting the radiographic assessment of osteoporosis-Osteopenia in children 0–2 years of age. A systematic review. PLoS ONE 2020, 15, e0241635. [Google Scholar] [CrossRef]
- Zhumalina, A.; Tusupkaliev, B.; Mania, A.; Kim, I.; Zharlykasinova, M. The Importance of Determining the Level of Bone Metabolism Markers and Vitamin D in the First Year of Life in the Kazakh Population. J. Pediatr. Pharmacol. Ther. 2024, 29, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, N.J.; Arabi, A.; Bachrach, L.K.; Fewtrell, M.; Fuleihan, G.E.-H.; Kecskemethy, H.H.; Jaworski, M.; Gordon, C.M.; International Society for Clinical Densitometry. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: The revised 2013 ISCD Pediatric Official Positions. J. Clin. Densitom. 2014, 17, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, P.J.; Cooper, C.; Dawson-Hughes, B.; Gordon, C.M.; Rizzoli, R. Life-course approach to nutrition. Osteoporos. Int. 2015, 26, 2723–2742. [Google Scholar] [CrossRef]
- Geserick, M.; Vogel, M.; Eckelt, F.; Schlingmann, M.; Hiemisch, A.; Baber, R.; Thiery, J.; Körner, A.; Kiess, W.; Kratzsch, J. Children and adolescents with obesity have reduced serum bone turnover markers and 25-hydroxyvitamin D but increased parathyroid hormone concentrations-Results derived from new pediatric reference ranges. Bone 2020, 132, 115124. [Google Scholar] [CrossRef] [PubMed]
- Al Nozha, O.M.; El Tarhouny, S.; Taha, I.; Sultan, I.; Abdu Allah, A.M.; Hammoda, M.A.; Elmehallawy, G.; Elmehallawy, Y.; Eysawi, E.A.; Desouky, M.K. Association Between Vitamin D Level and Z-Score Changes of Bone Density in College-Age Saudi Girls: A Cross-Sectional Study. Int. J. Gen. Med. 2023, 16, 865–874. [Google Scholar] [CrossRef]
- Chhantyal, K.; He, L.; Mo, J.; Yin, M.; He, T.; Chen, Y.; Yang, Y.; Zhang, L.; Rong, L. Free vitamin D correlate better with bone mineral density and thoracolumbar junction osteoporotic vertebral fractures than serum vitamin D. BMC Musculoskelet. Disord. 2020, 21, 164. [Google Scholar] [CrossRef]
- Jang, M.J.; Shin, C.; Kim, S.; Lee, J.W.; Chung, N.G.; Cho, B.; Jung, M.H.; Suh, B.K.; Ahn, M.B. Factors affecting bone mineral density in children and adolescents with secondary osteoporosis. Ann. Pediatr. Endocrinol. Metab. 2023, 28, 34–41. [Google Scholar] [CrossRef]
- Khalatbari, H.; Binkovitz, L.A.; Parisi, M.T. Dual-energy X-ray absorptiometry bone densitometry in pediatrics: A practical review and update. Pediatr. Radiol. 2021, 51, 25–39. [Google Scholar] [CrossRef]
- Tan, L.O.; Lim, S.Y.; Vasanwala, R.F. Primary osteoporosis in children. BMJ Case Rep. 2017, 2017, bcr2017220700. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Guss, C.E.; McAllister, A.; Gordon, C.M. DXA in Children and Adolescents. J. Clin. Densitom. 2021, 24, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, J.; Koes, B.W.; Paulis, W.D.; van Middelkoop, M. Differences in bone mineral density between normal-weight children and children with overweight and obesity: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 526–546. [Google Scholar] [CrossRef]
- Pimentel, D.V.; Suttkus, A.; Vogel, M.; Lacher, M.; Jurkutat, A.; Poulain, T.; Ceglarek, U.; Kratzsch, J.; Kiess, W.; Körner, A.; et al. Effect of physical activity and BMI SDS on bone metabolism in children and adolescents. Bone 2021, 153, 116131. [Google Scholar] [CrossRef] [PubMed]
- Mansur, J.L.; Oliveri, B.; Giacoia, E.; Fusaro, D.; Costanzo, P.R. Vitamin D: Before, during and after Pregnancy: Effect on Neonates and Children. Nutrients 2022, 14, 1900. [Google Scholar] [CrossRef]
- Dai, Z.; Butler, L.M.; van Dam, R.M.; Ang, L.W.; Yuan, J.M.; Koh, W.P. Adherence to a vegetable-fruit-soy dietary pattern or the Alternative Healthy Eating Index is associated with lower hip fracture risk among Singapore Chinese. J. Nutr. 2014, 144, 511–518. [Google Scholar] [CrossRef]
- Liu, X.; Wu, Y.; Bennett, S.; Zou, J.; Xu, J.; Zhang, L. The Effects of Different Dietary Patterns on Bone Health. Nutrients 2024, 16, 2289. [Google Scholar] [CrossRef]
- Villareal, D.T.; Fontana, L.; Das, S.K.; Redman, L.; Smith, S.R.; Saltzman, E.; Bales, C.; Rochon, J.; Pieper, C.; Huang, M.; et al. Effect of Two-Year Caloric Restriction on Bone Metabolism and Bone Mineral Density in Non-Obese Younger Adults: A Randomized Clinical Trial. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2016, 31, 40–51. [Google Scholar] [CrossRef]
- Sayar, Y.; Arikan, F.I.; Taşar, M.A.; Dallar, Y. Effect of Sportive Activity on Bone Mineral Density during Adolescence. Türk. Fiz. Tip. Rehabil. Derg. 2015, 61, 120–124. [Google Scholar] [CrossRef]
- Yang, X.; Zhai, Y.; Zhang, J.; Chen, J.Y.; Liu, D.; Zhao, W.H. Combined effects of physical activity and calcium on bone health in children and adolescents: A systematic review of randomized controlled trials. World J. Pediatr. 2020, 16, 356–365. [Google Scholar] [CrossRef]
- Muscogiuri, G. Vitamin D: Past, present and future perspectives in the prevention of chronic diseases. Eur. J. Clin. Nutr. 2018, 72, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Amanzholkyzy, A.; Nurgaliyeva, R.E.; Kaldybayeva, A.T.; Batyrova, T.Z.; Balmaganbetova, F.K.; Aibassova, Z.A. Biochemical variability of vitamin D receptor (VDR) gene and its relationship with bone mineral density in children of the Western Region of the Republic of Kazakhstan. Res. J. Pharm. Technol. 2019, 12, 735–740. [Google Scholar] [CrossRef]
- Marwaha, R.K.; Garg, M.K.; Mithal, A.; Gupta, S.; Shukla, M.; Chadha, A. Effect of Vitamin D Supplementation on Bone Turnover Markers in Children and Adolescents from North India. Indian J. Endocrinol. Metab. 2019, 23, 27–34. [Google Scholar] [CrossRef] [PubMed]
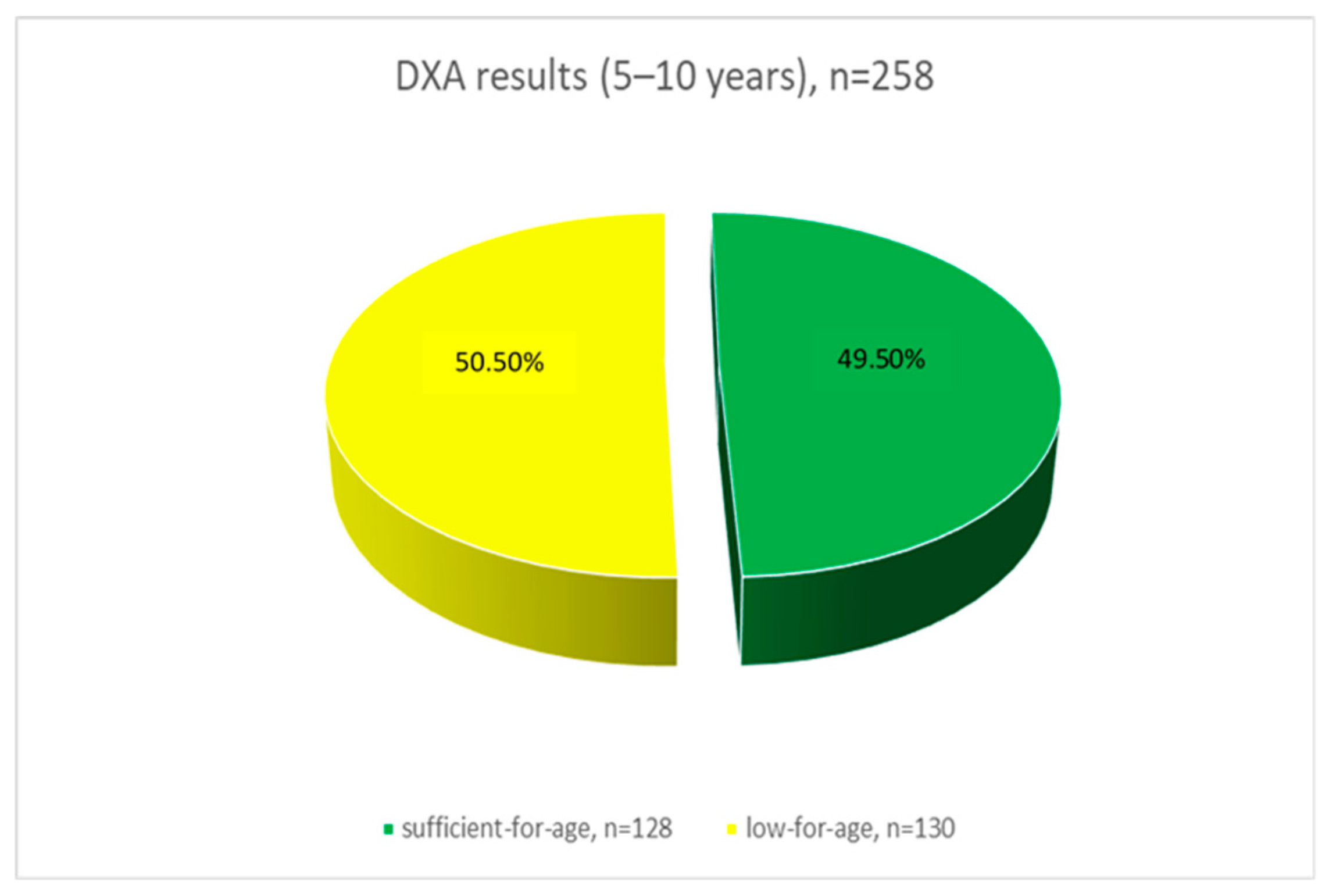

| Parameter | Total (n = 509) | 5–10 Years (n = 258) | 11–17 Years (n = 251) | Statistical Criterion | Effect Size | |
|---|---|---|---|---|---|---|
| t-Test | p-Value | Cohen’s d | ||||
| Age (Years) | 10.7 ± 3.4 | 7.8 ± 1.6 | 13.6 ± 1.9 | 37.3 | 0.000 | 3.31 |
| BMI (kg/m2) | 17.8 ± 3.9 | 16.2 ± 2.9 | 19.4 ± 4.3 | 9.8 | 0.000 | 0.87 |
| Weight (kg) | 36.9 ± 15.5 | 26.14 ± 7.47 | 47.91 ± 13.79 | 22.1 | 0.000 | 1.96 |
| Height (cm) | 141.1 ± 19.8 | 126.35 ± 12.27 | 156.35 ± 13.61 | 26.1 | 0.000 | 2.31 |
| n (%) | n (%) | n (%) | χ2 | OR (95% CI) | ||
| Male | 268 (52.7%) | 132 (51.2%) | 136 (54.2%) | 0.466 | 0.495 | 0.89 (0.63; 1.26) |
| Female | 241 (47.3%) | 126 (48.8%) | 115 (45.8%) | |||
| Not breastfed | 35 (6.9%) | 17 (6.6%) | 18 (7.2%) | 0.067 | 0.795 | 0.91 (0.46; 1.82) |
| * Fractures | 81 (15.9) | 34 (13.2%) | 47 (18.7%) | 2.925 | 0.087 | 0.66 (0.41; 1.07) |
| ** Heredity | 25 (4.9%) | 14 (5.4%) | 11 (4.4%) | 0.297 | 0.586 | 1.25 (0.56; 2.81) |
| Parents’ fractures | 96 (18.9%) | 41 (15.9%) | 55 (21.9%) | 3.014 | 0.083 | 0.67 (0.43; 1.05) |
| Behavioral factors | ||||||
| 1 Vitamin D | 370 (72.7%) | 185 (71.7%) | 185 (73.7%) | 0.256 | 0.613 | 0.91 (0.62; 1.34) |
| 1 Calcium suppl. | 410 (80.6%) | 206 (79.8%) | 204 (81.3%) | 0.166 | 0.684 | 0.91 (0.58;1.41) |
| 2 Sport sections | 290 (57.0) | 149 (57.8%) | 141 (51,66.2) | 0.129 | 0.719 | 1.07 (0.75; 151) |
| 3 Daily walks | 38 (7.5%) | 17 (6.6%) | 21 (8.4%) | 0.563 | 0.453 | 0.78 (0.40; 1.51) |
| Nutritional intake | ||||||
| 4 Milk and dairy products | 40 (7.9%) | 14 (5.4%) | 26 (10.4%) | 4.27 | 0.04 | 0.49 (0.25; 0.98) |
| 4 Green vegetables | 223 (43.8%) | 119 (46.1%) | 104 (41.4) | 1.14 | 0.29 | 1.21 (0.85; 1.72) |
| 4 Meat | 27 (5.3%) | 15 (5.8%) | 12 (4.8%) | 0.28 | 0.60 | 1.23 (0.56; 2.69) |
| 4 Fish and seafood | 98 (19.3%) | 49 (19.1%) | 49 (19.5%) | 0.01 | 0.91 | 0.98 (0.63; 1.52) |
| 4 Dried fruits and nuts | 88 (17.3%) | 47 (18.3%) | 41 (16.3%) | 0.34 | 0.56 | 1.15 (0.72; 1.82) |
| 4 Eggs | 59 (11.6%) | 31 (12%) | 28 (11.2%) | 0.09 | 0.76 | 1.08 (0.63; 1.87) |
| 5 Soda | 117 (23.0%) | 61 (23.7%) | 56 (22.3%) | 0.15 | 0.70 | 1.08 (0.72; 1.64) |
| 5 Fast food | 56 (11.0%) | 26 (10.1%) | 30 (12.0%) | 0.44 | 0.51 | 0.83 (0.48; 1.45) |
| Blood analysis | (n = 186) | (n = 82) | (n = 104) | U | p | Cohen’s d |
| Vitamin D, ng/mL | 16.19 ± 9.37 | 17.01 ± 10.53 | 14.87 ± 7.35 | 3271.5 | 0.006 | 0.41 |
| Alkaline phosphatase, U/L | 226.56 ± 135.38 | 239.93 ± 87.83 | 182.17 ± 172.67 | 3310.5 | 0.009 | 0.39 |
| Ionized calcium, mmol/L | 1.27 ± 0.07 | 1.28 ± 0.05 | 1.26 ± 0.08 | 3244.5 | 0.005 | 0.42 |
| Parameter | Total | Sufficient for Age Z ≥ −1.0 | Low for Age Z < −1.0 | Statistical Criterion | Effect Size | |
|---|---|---|---|---|---|---|
| (n = 509) | (n = 230) | (n = 279) | t-Test | p-Value | Cohen’s d | |
| Age (Years) | 10.7 ± 3.4 | 10.21 ± 3.29 | 11.04 ± 3.48 | 2.74 | 0.006 | 0.25 |
| Weight (kg) | 36.9 ± 15.5 | 35.58 ± 15.16 | 37.95 ± 15.74 | 1.72 | 0.09 | 0.15 |
| Height (cm) | 141.1 ± 19.8 | 139.18 ± 19.33 | 142.76 ± 20.1 | 2.04 | 0.04 | 0.18 |
| BMI (kg/m2) | 17.8 ± 3.9 | 17.62 ± 4.03 | 17.91 ± 3.97 | 0.84 | 0.41 | 0.07 |
| n (%) | n (%) | n (%) | χ2 | OR (95% CI) | ||
| Male | 268 (52.7%) | 127 (55.2%) | 141 (50.5%) | 1.108 | 0.293 | 1.21 (0.85; 1.71) |
| Female | 241 (47.3%) | 103 (44.8%) | 138 (49.5%) | |||
| Not breastfed | 35 (6.9%) | 21 (9.1%) | 14 (5.0%) | 3.330 | 0.068 | 1.90 (0.94; 3.83) |
| * Fractures | 81 (15.9%) | 32 (13.9%) | 49 (17.6%) | 1.255 | 0.263 | 0.75 (0.47; 1.23) |
| ** Heredity | 25 (4.9%) | 12 (5.2%) | 13 (4.7%) | 0.084 | 0.772 | 1.12 (0.50; 2.52) |
| Parents’ fractures | 96 (18.9%) | 42 (18.3%) | 54 (19.4%) | 0.099 | 0.75 | 0.93 (0.59; 1.46) |
| Behavioral factors | ||||||
| 1 Vitamin D | 370 (72.7%) | 170 (73.9%) | 200 (71.7%) | 0.315 | 0.574 | 0.89 (0.60; 1.32) |
| 1 Calcium suppl. | 410 (80.6%) | 189 (82.2%) | 221 (79.2%) | 0.706 | 0.401 | 0.82 (0.53; 1.29) |
| 2 Sport sections | 290 (57.0%) | 126 (54.8%) | 164 (58.8%) | 0.822 | 0.364 | 0.85 (0.60; 1.21) |
| 3 Daily walks | 38 (7.5%) | 17 (7.4%) | 21 (7.6%) | 0.005 | 0.945 | 0.98 (0.50; 1.90) |
| Nutritional intake | ||||||
| 4 Milk and dairy products | 40 (7.9%) | 16 (7.0%) | 24 (8.6%) | 0.471 | 0.492 | 0.79 (0.41; 1.53) |
| 4 Green vegetables | 223 (43.8%) | 116 (50.4%) | 107 (38.4%) | 7.48 | 0.006 | 1.64 (1.14; 2.33) |
| 4 Meat | 27 (5.3%) | 12 (5.2%) | 15 (5.4%) | 0.008 | 0.929 | 0.97 (0.44; 2.11) |
| 4 Fish and seafood | 98 (19.3%) | 49 (21.3%) | 49 (17.7%) | 1.053 | 0.305 | 1.25 (0.81; 1.96) |
| 4 Dried fruits and nuts | 88 (17.3%) | 49 (21.3%) | 39 (14.0%) | 4.652 | 0.031 | 1.93 (1.22; 3.05) |
| 4 Eggs | 59 (11.6%) | 27 (11.7%) | 32 (11.5%) | 0.009 | 0.925 | 1.02 (0.60; 1.77) |
| 5 Soda | 117 (23%) | 51 (22.2%) | 66 (23.7%) | 0.17 | 0.676 | 0.91 (0.60; 1.39) |
| 5 Fast food | 56 (11.0%) | 26 (11.3%) | 30 (10.8%) | 0.034 | 0.854 | 1.05 (0.60; 1.84) |
| Blood analysis | (n = 186) | (n = 70) | (n = 116) | U | p | Cohen’s d |
| Vitamin D, ng/mL | 16.19 ± 9.37 | 16.87 ± 8.47 | 15.32 ± 10.28 | 3458.0 | 0.091 | 0.25 |
| Alkaline phosphatase, U/L | 226.56 ± 135.38 | 235.93 ± 123.64 | 225.82 ± 154.93 | 3873.0 | 0.599 | 0.08 |
| Ionized calcium, mmol/L | 1.27 ± 0.07 | 1.26 ± 0.08 | 1.27 ± 0.08 | 3251.5 | 0.023 | 0.34 |
| Parameter | OR | 95% CI | p | AOR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | 1.07 | 1.02; 1.13 | 0.007 | 1.21 | 1.05; 1.41 | 0.013 |
| Weight (kg) | 1.01 | 0.99; 1.022 | 0.087 | 0.97 | 0.94; 1.00 | 0.047 |
| Height (cm) | 1.009 | 1.00; 1.02 | 0.043 | - | - | - |
| BMI (kg/m2) | 1.02 | 0.98; 1.07 | 0.404 | - | - | - |
| Sex, male | 0.83 | 0.58; 1.18 | 0.29 | - | - | - |
| 1 Vitamin D | 0.89 | 0.61; 1.32 | 0.574 | - | - | - |
| 1 Calcium suppl. | 0.83 | 0.53; 1.29 | 0.401 | - | - | - |
| 2 Sport sections | 1.18 | 0.83; 1.68 | 0.37 | - | - | - |
| 3 Daily walks | 1.02 | 0.53; 1.99 | 0.945 | - | - | - |
| 4 Milk and dairy products | 1.26 | 0.65; 2.43 | 0.493 | - | - | - |
| 4 Green vegetables | 0.61 | 0.43; 0.87 | 0.006 | 0.46 | 0.24; 0.87 | 0.017 |
| 4 Meat | 1.04 | 0.48; 2.26 | 0.929 | - | - | - |
| 4 Fish and seafood | 0.79 | 0.51; 1.23 | 0.305 | - | - | - |
| 4 Dried fruits/nuts | 0.60 | 0.38; 0.96 | 0.032 | - | - | - |
| 4 Eggs | 0.97 | 0.57; 1.68 | 0.925 | - | - | - |
| 5 Soda | 1.09 | 0.72; 1.66 | 0.676 | - | - | - |
| 5 Fast food | 0.95 | 0.54; 1.66 | 0.854 | - | - | - |
| Blood analysis (n = 186) | ||||||
| Alkaline phosphatase, U/L | 1.001 | 0.998; 1.004 | 0.374 | - | - | - |
| Vitamin D, ng/mL | 0.98 | 0.95; 1.003 | 0.084 | - | - | - |
| Ionized calcium, mmol/L | 632.5 | 4.09; 97,767.8 | 0.012 | 2099.9 | 7.77; 567,476 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madiyeva, M.; Kanapiyanova, G.; Bersimbekova, G.; Prilutskaya, M.; Kaskabayeva, A.; Rymbayeva, T.; Dyussupov, A. Bone Mineral Density in Children and Adolescents of the Abay Region, Kazakhstan: Prevalence and Associated Risk Factors. Int. J. Environ. Res. Public Health 2025, 22, 949. https://doi.org/10.3390/ijerph22060949
Madiyeva M, Kanapiyanova G, Bersimbekova G, Prilutskaya M, Kaskabayeva A, Rymbayeva T, Dyussupov A. Bone Mineral Density in Children and Adolescents of the Abay Region, Kazakhstan: Prevalence and Associated Risk Factors. International Journal of Environmental Research and Public Health. 2025; 22(6):949. https://doi.org/10.3390/ijerph22060949
Chicago/Turabian StyleMadiyeva, Madina, Gulnur Kanapiyanova, Gulzhan Bersimbekova, Mariya Prilutskaya, Alida Kaskabayeva, Tamara Rymbayeva, and Altay Dyussupov. 2025. "Bone Mineral Density in Children and Adolescents of the Abay Region, Kazakhstan: Prevalence and Associated Risk Factors" International Journal of Environmental Research and Public Health 22, no. 6: 949. https://doi.org/10.3390/ijerph22060949
APA StyleMadiyeva, M., Kanapiyanova, G., Bersimbekova, G., Prilutskaya, M., Kaskabayeva, A., Rymbayeva, T., & Dyussupov, A. (2025). Bone Mineral Density in Children and Adolescents of the Abay Region, Kazakhstan: Prevalence and Associated Risk Factors. International Journal of Environmental Research and Public Health, 22(6), 949. https://doi.org/10.3390/ijerph22060949






