Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health
Abstract
1. Introduction
2. Mechanisms of Epigenetic Modification of PFAS-Induced Disruptions
2.1. Properties, Structure, and Epigenetic Fundamentals of PFAS
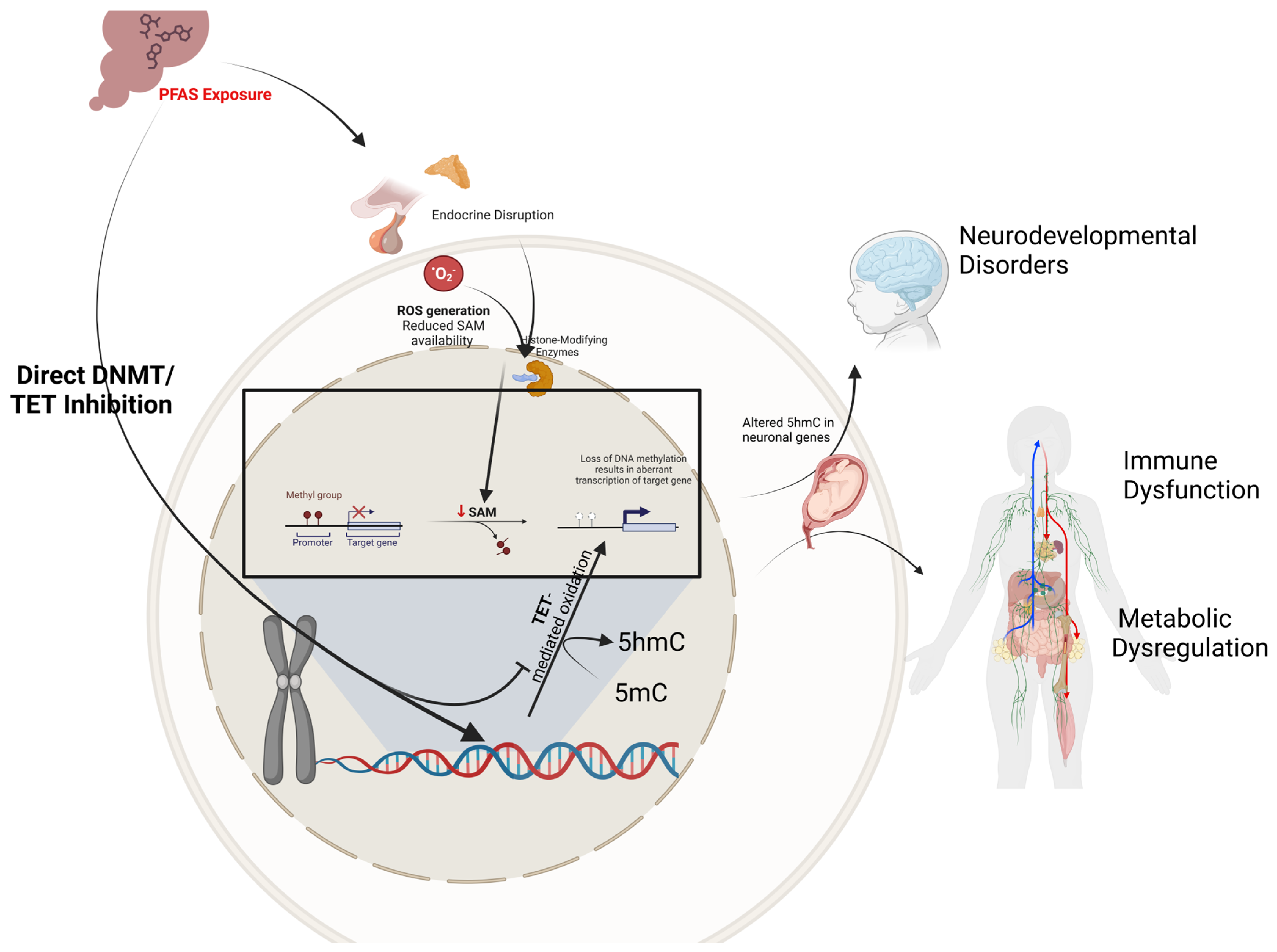
2.2. PFAS Exposure and DNA Methylation: A Nexus of Environmental and Epigenetic Impact
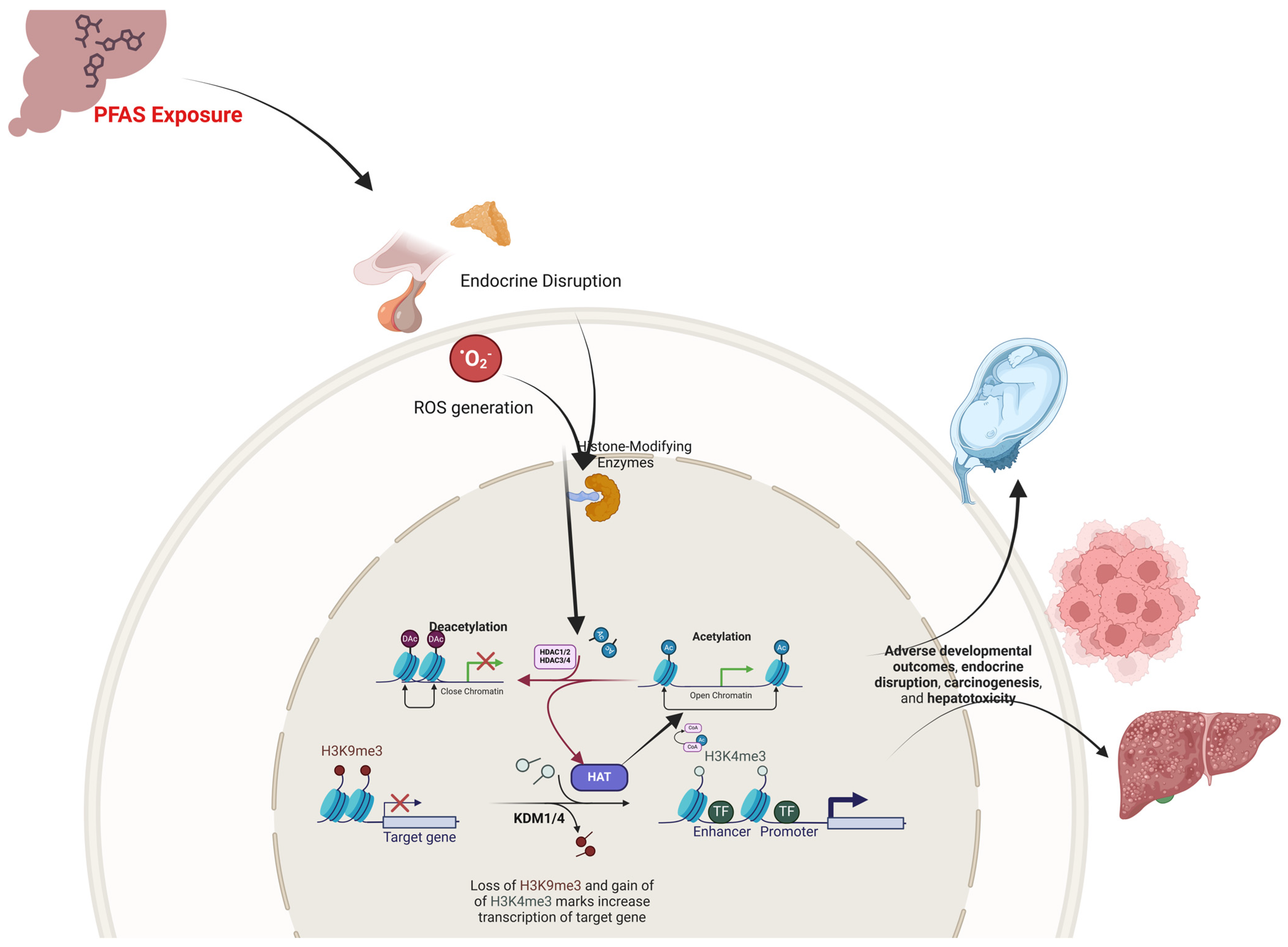
2.3. PFAS Exposure and Histone Modifications: Epigenetic Regulators of Developmental Toxicity
2.4. Non-Coding RNA Networks in PFAS-Mediated Epigenetic Perturbations
3. Prenatal PFAS Exposure and Epigenetic Programming
3.1. Epigenetics and Development
Epigenetic Changes in the Placenta and Embryos
4. Health Risks Associated with PFAS-Induced Epigenetic Changes In Utero
4.1. An Integrated Epidemiological and Mechanistic Perspective of Epigenetic Dysregulation of Maternal PFAS Exposure and Low Birth Weight
4.2. Molecular Mechanisms and Neurodevelopmental Impact of Maternal PFAS Epigenetic Disruption
4.3. PFAS-Induced Epigenetic Reprogramming and Metabolic Dysregulation In Utero
4.4. Cardiovascular and Cardiometabolic Diseases
4.4.1. PFAS Exposure Epigenetics and the Pathogenesis of Preeclampsia
4.4.2. Bridging Maternal Exposure to Chronic Disease Risk
4.5. PFAS-Mediated Epigenetic Modifications and Immune Dysfunction
5. Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PFAS | Per- and polyfluoroalkyl substances |
| PFBS | Perfluorobutane sulfonate |
| PFHpA | Perfluoroheptanoic acid |
| PFHpS | Perfluoroheptane sulfonic acid |
| PFHxA | Perfluorohexanoic acid |
| PFHxS | Perfluorohexane sulfonic acid |
| PFNA | Perfluorononanoic acid |
| PFOA | Perfluorooctanoic acid |
| PFOSA | Perfluorooctane sulfonamide |
| PFOS | Perfluorooctane sulfonic acid |
| PFPeA | Perfluoropentanoic acid |
| PFDS | Perfluorodecane sulfonic acid |
| PFDoDA | Perfluorododecanoic acid |
| PFTrDA | Perfluorotridecanoic acid |
| PFUnDA | Perfluoroundecanoic acid |
| MeFOSAA | Methyl-perfluorooctane sulfonamido acetic acid |
| EtPFOSAA | Ethyl-perfluorooctane sulfonamido acetic acid |
| HFPO-DA | Hexafluoropropylene oxide dimer acid |
| HFPO-TA | Hexafluoropropylene oxide trimer acid |
| HATs | Histone acetyltransferases |
| HDACs | Histone deacetylases |
| IGF2 | Insulin-like growth factor 2 |
| MEST | Mesoderm-specific transcript |
| lncRNAs | Long non-coding RNAs |
| DNMTs | DNA methyltransferases |
| DMRs | Differentially methylated regions |
| PPAR-α | Peroxisome proliferator-activated receptor—alpha |
| MEG3 | Maternally expressed gene 3 |
| PTX3 | Pentraxin 3 |
| ROS | Reactive oxygen species |
| BDNF | Brain-derived neurotrophic factor |
| CREB | cAMP-responsive element binding protein |
| ER | Estrogen receptor |
| DOHaD | Developmental Origins of Health and Disease |
| HFPO-TA | Hexafluoropropylene oxide trimer acid |
| WGBS | Whole-genome bisulfite sequencing |
| UPLC–MS/MS | Ultra-performance liquid chromatography–tandem mass spectrometry |
| EWAS | High-resolution epigenome-wide association studies |
| CHD | Congenital heart disease |
| RRBS | Reduced representation bisulfite sequencing |
| ChIP-seq | Chromatin immunoprecipitation sequencing |
| SAM | S-adenosylmethionine |
| SUMO | Small ubiquitin-related modifier |
References
- US-EPA. Our Current Understanding of the Human Health and Environmental Risks of PFAS|US EPA. Available online: https://www.epa.gov/pfas/our-current-understanding-human-health-and-environmental-risks-pfas?utm_source=chatgpt.com (accessed on 22 January 2025).
- Verma, S.; Lee, T.; Sahle-Demessie, E.; Ateia, M.; Nadagouda, M.N. Recent advances on PFAS degradation via thermal and nonthermal methods. Chem. Eng. J. Adv. 2023, 13, 100421. [Google Scholar] [CrossRef] [PubMed]
- Szilagyi, J.T.; Avula, V.; Fry, R.C. Perfluoroalkyl Substances (PFAS) and Their Effects on the Placenta, Pregnancy, and Child Development: A Potential Mechanistic Role for Placental Peroxisome Proliferator-Activated Receptors (PPARs). Curr. Environ. Health Rep. 2020, 7, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Cousins, I.T.; Dewitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Miller, M.; Ng, C.A.; Scheringer, M.; Vierke, L.; et al. Strategies for grouping per- and polyfluoroalkyl substances (PFAS) to protect human and environmental health. Environ. Sci. Process. Impacts 2020, 22, 1444–1460. [Google Scholar] [CrossRef]
- Cousins, I.T.; DeWitt, J.C.; Glüge, J.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Scheringer, M.; Wang, Z. The high persistence of PFAS is sufficient for their management as a chemical class. Environ. Sci. Process. Impacts 2020, 22, 2307–2312. [Google Scholar] [CrossRef]
- Haimbaugh, A.; Meyer, D.N.; Connell, M.L.; Blount-Pacheco, J.; Tolofari, D.; Gonzalez, G.; Banerjee, D.; Norton, J.; Miller, C.J.; Baker, T.R. Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies. Int. J. Environ. Res. Public Health 2024, 21, 1615. [Google Scholar] [CrossRef]
- Eick, S.M.; Hom Thepaksorn, E.K.; Izano, M.A.; Cushing, L.J.; Wang, Y.; Smith, S.C.; Gao, S.; Park, J.-S.; Padula, A.M.; DeMicco, E.; et al. Associations between prenatal maternal exposure to per- and polyfluoroalkyl substances (PFAS) and polybrominated diphenyl ethers (PBDEs) and birth outcomes among pregnant women in San Francisco. Environ. Health 2020, 19, 100. [Google Scholar] [CrossRef]
- Liao, Q.; Huang, H.; Tang, P.; Liang, J.; Chen, J.; Lei, L.; Song, Y.; Pan, D.; Lin, M.; Lv, F.; et al. Association Between Prenatal Per- and Polyfluoroalkyl Substance Exposure and Maternal Serum Total Bile Acid Levels During Pregnancy: Effect Modification by Infant Sex and Maternal Prepregnancy BMI. Expo. Health 2024, 16, 727–744. [Google Scholar] [CrossRef]
- Sebe, G.O.; Anyaogu, E.V.; Ntomchukwu, A.D.A.R.C.; Oghenerhoro, S.O.; Jonathan, O.E. Health impacts and mechanisms of per-and polyfluoroalkyl substances (PFAS) from epidemiological to toxicological. J. Biosci. Med. 2023, 11, 218–240. [Google Scholar] [CrossRef]
- Olsen, G.W.; Lange, C.C.; Ellefson, M.E.; Mair, D.C.; Church, T.R.; Goldberg, C.L.; Herron, R.M.; Medhdizadehkashi, Z.; Nobiletti, J.B.; Rios, J.A.; et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ. Sci. Technol. 2012, 46, 6330–6338. [Google Scholar] [CrossRef]
- Barlas, R. The Role of Epigenetic Modifications in Cellular Differentiation and the Development of Complex Diseases. Next Front. Life Sci. AI 2024, 8, 199. [Google Scholar] [CrossRef]
- Klibaner-Schiff, E.; Simonin, E.M.; Akdis, C.A.; Cheong, A.; Johnson, M.M.; Karagas, M.R.; Kirsh, S.; Kline, O.; Mazumdar, M.; Oken, E.; et al. Environmental exposures influence multigenerational epigenetic transmission. Clin. Epigenetics 2024, 16, 145. [Google Scholar] [CrossRef] [PubMed]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Hamilton, J.P. Epigenetics: Principles and practice. Dig. Dis. 2011, 29, 130–135. [Google Scholar] [CrossRef]
- Perng, W.; Nakiwala, D.; Goodrich, J.M. What Happens in Utero Does Not Stay in Utero: A Review of Evidence for Prenatal Epigenetic Programming by Per- and Polyfluoroalkyl Substances (PFAS) in Infants, Children, and Adolescents. Curr. Environ. Health Rep. 2023, 10, 35–44. [Google Scholar] [CrossRef]
- Everson, T.M.; Sehgal, N.; Campbell, K.; Barr, D.B.; Panuwet, P.; Yakimavets, V.; Chen, K.; Perez, C.; Shankar, K.; Eick, S.M.; et al. Placental PFAS concentrations are associated with perturbations of placental DNA methylation. Environ. Pollut. 2025, 368, 125737. [Google Scholar] [CrossRef]
- Tsai, W.-J.; Hsieh, W.-S.; Chen, P.-C.; Liu, C.-Y. Prenatal Perfluoroalkyl Substance Exposure in Association with Global Histone Post-Translational Methylation in 2-Year-Old Children. Toxics 2024, 12, 876. [Google Scholar] [CrossRef]
- Aldhous, M.C.; Hor, K.; Reynolds, R.M. Epigenetics and Diet in Pregnancy. In Handbook of Nutrition and Pregnancy; Lammi-Keefe, C.J., Couch, S.C., Kirwan, J.P., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 163–181. [Google Scholar]
- Petroff, R.L.; Cavalcante, R.G.; Langen, E.S.; Dolinoy, D.C.; Padmanabhan, V.; Goodrich, J.M. Mediation effects of DNA methylation and hydroxymethylation on birth outcomes after prenatal per- and polyfluoroalkyl substances (PFAS) exposure in the Michigan mother–infant Pairs cohort. Clin. Epigenetics 2023, 15, 49. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Zhang, Y.; Guan, S.; Gong, X.; Wang, X. Maternal exposure to perfluorooctanoic acid (PFOA) causes liver toxicity through PPAR-α pathway and lowered histone acetylation in female offspring mice. Environ. Sci. Pollut. Res. 2019, 26, 18866–18875. [Google Scholar] [CrossRef]
- Wang, L.; Mao, B.; Fan, K.; Sun, R.; Zhang, J.; Liang, H.; Liu, Y. ROS attenuates TET2-dependent ZO-1 epigenetic expression in cerebral vascular endothelial cells. Fluids Barriers CNS 2022, 19, 73. [Google Scholar] [CrossRef]
- Xie, X.; Weng, X.; Liu, S.; Chen, J.; Guo, X.; Gao, X.; Fei, Q.; Hao, G.; Jing, C.; Feng, L. Perfluoroalkyl and polyfluoroalkyl substance exposure and association with sex hormone concentrations: Results from the NHANES 2015–2016. Environ. Sci. Eur. 2021, 33, 69. [Google Scholar] [CrossRef] [PubMed]
- Giesy, J.P.; Kannan, K. Global Distribution of Perfluorooctane Sulfonate in Wildlife. Environ. Sci. Technol. 2001, 35, 1339–1342. [Google Scholar] [CrossRef] [PubMed]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; de Voogt, P.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef] [PubMed]
- Roesch, P.; Vogel, C.; Simon, F.G. Reductive Defluorination and Mechanochemical Decomposition of Per- and Polyfluoroalkyl Substances (PFASs): From Present Knowledge to Future Remediation Concepts. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An overview of the uses of per- and polyfluoroalkyl substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Kim, S.; Thapar, I.; Brooks, B.W. Epigenetic changes by per- and polyfluoroalkyl substances (PFAS). Environ. Pollut. 2021, 279, 116929. [Google Scholar] [CrossRef]
- ITRC. PFAS Technical and Regulatory Guidance Document and Fact Sheets PFAS-1. Available online: https://pfas-1.itrcweb.org/2-2-chemistry-terminology-and-acronyms/ (accessed on 25 December 2024).
- Wang, Z.; DeWitt, J.C.; Higgins, C.P.; Cousins, I.T. A Never-Ending Story of Per- and Polyfluoroalkyl Substances (PFASs)? Environ. Sci. Technol. 2017, 51, 2508–2518. [Google Scholar] [CrossRef]
- Allis, C.D.; Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016, 17, 487–500. [Google Scholar] [CrossRef]
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef]
- Kishi, R.; Araki, A.; Minatoya, M.; Hanaoka, T.; Miyashita, C.; Itoh, S.; Kobayashi, S.; Ait Bamai, Y.; Yamazaki, K.; Miura, R.; et al. The Hokkaido Birth Cohort Study on Environment and Children’s Health: Cohort profile—Updated 2017. Environ. Health Prev. Med. 2017, 22, 46. [Google Scholar] [CrossRef]
- Braun, J.M.; Chen, A.; Romano, M.E.; Calafat, A.M.; Webster, G.M.; Yolton, K.; Lanphear, B.P. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity 2016, 24, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: Patterns and paradigms. Nat. Rev. Genet. 2009, 10, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, T.; Workman, J.L. Signals and combinatorial functions of histone modifications. Annu. Rev. Biochem. 2011, 80, 473–499. [Google Scholar] [CrossRef]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef]
- Bird, A. DNA methylation patterns and epigenetic memory. Genes. Dev. 2002, 16, 6–21. [Google Scholar] [CrossRef]
- Li, E.; Zhang, Y. DNA methylation in mammals. Cold Spring Harb. Perspect. Biol. 2014, 6, a019133. [Google Scholar] [CrossRef]
- He, Y.F.; Li, B.Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L.; et al. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar] [CrossRef]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Svoboda, L.K.; Ishikawa, T.; Dolinoy, D.C. Developmental toxicant exposures and sex-specific effects on epigenetic programming and cardiovascular health across generations. Environ. Epigenetics 2022, 8, dvac017. [Google Scholar] [CrossRef]
- Rappazzo, K.M.; Coffman, E.; Hines, E.P. Exposure to Perfluorinated Alkyl Substances and Health Outcomes in Children: A Systematic Review of the Epidemiologic Literature. Int. J. Environ. Res. Public Health 2017, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, R47–R58. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Ueda, Y.; Dodge, J.E.; Wang, Z.; Li, E. Establishment and maintenance of genomic methylation patterns in mouse embryonic stem cells by Dnmt3a and Dnmt3b. Mol. Cell. Biol. 2003, 23, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.D.; Chan, M.M.; Mikkelsen, T.S.; Gu, H.; Gnirke, A.; Regev, A.; Meissner, A. A unique regulatory phase of DNA methylation in the early mammalian embryo. Nature 2012, 484, 339–344. [Google Scholar] [CrossRef]
- Bartolomei, M.S. Genomic imprinting: Employing and avoiding epigenetic processes. Genes. Dev. 2009, 23, 2124–2133. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- David, S. Per-and Polyfluorinated Substances (PFAS); a Literature Review. Undergrad. J. Public Health 2024, 8. [Google Scholar] [CrossRef]
- Sae-Lee, C.; Barrow, T.M.; Colicino, E.; Choi, S.H.; Rabanal-Ruiz, Y.; Green, D.; Korolchuk, V.I.; Mathers, J.C.; Byun, H.-M. Genomic targets and selective inhibition of DNA methyltransferase isoforms. Clin. Epigenetics 2022, 14, 103. [Google Scholar] [CrossRef]
- Xu, Y.; Lindh, C.H.; Fletcher, T.; Jakobsson, K.; Engström, K. Perfluoroalkyl substances influence DNA methylation in school-age children highly exposed through drinking water contaminated from firefighting foam: A cohort study in Ronneby, Sweden. Environ. Epigenetics 2022, 8, dvac004. [Google Scholar] [CrossRef]
- Boyd, R.I.; Ahmad, S.; Singh, R.; Fazal, Z.; Prins, G.S.; Madak Erdogan, Z.; Irudayaraj, J.; Spinella, M.J. Toward a Mechanistic Understanding of Poly- and Perfluoroalkylated Substances and Cancer. Cancers 2022, 14, 2919. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, R.A.; Fuso, A.; d’Erme, M.; Miraglia, N.; Martire, S.; Scarpa, S.; Mosca, L. Role of S-adenosylmethionine in the Modulation of Oxidative Stress- Related Neurodegeneration. Int. J. Clin. Nutr. Diet. 2016, 2, 109. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lewis, N.; Abdulkadir, A.; Kandel, S.; Rosby, R.; Hossain, E. Per- and Polyfluoroalkyl Substances (PFAS) as Emerging Obesogens: Mechanisms, Epidemiological Evidence, and Regulatory Challenges. Physiologia 2024, 4, 517–567. [Google Scholar] [CrossRef]
- Tricotteaux-Zarqaoui, S.; Lahimer, M.; Abou Diwan, M.; Corona, A.; Candela, P.; Cabry, R.; Bach, V.; Khorsi-Cauet, H.; Benkhalifa, M. Endocrine disruptor chemicals exposure and female fertility declining: From pathophysiology to epigenetic risks. Front. Public Health 2024, 12, 1466967. [Google Scholar] [CrossRef]
- Singh, D.D. Epigenetic Mechanisms of Endocrine-Disrupting Chemicals in Breast Cancer and Their Impact on Dietary Intake. J. Xenobiotics 2025, 15, 1. [Google Scholar] [CrossRef]
- García-Carpizo, V.; Ruiz-Llorente, L.; Fraga, M.; Aranda, A. The growing role of gene methylation on endocrine function. J. Mol. Endocrinol. 2011, 47, R75–R89. [Google Scholar] [CrossRef]
- Zhuchen, H.-Y.; Wang, J.-Y.; Liu, X.-S.; Shi, Y.-W. Research Progress on Neurodevelopmental Toxicity in Offspring after Indirect Exposure to PFASs in Early Life. Toxics 2023, 11, 571. [Google Scholar] [CrossRef]
- Liu, Y.; Eliot, M.N.; Papandonatos, G.D.; Kelsey, K.T.; Fore, R.; Langevin, S.; Buckley, J.; Chen, A.; Lanphear, B.P.; Cecil, K.M.; et al. Gestational Perfluoroalkyl Substance Exposure and DNA Methylation at Birth and 12 Years of Age: A Longitudinal Epigenome-Wide Association Study. Environ. Health Perspect. 2022, 130, 37005. [Google Scholar] [CrossRef]
- Tahara, T.; Hirata, I.; Nakano, N.; Nagasaka, M.; Nakagawa, Y.; Shibata, T.; Ohmiya, N. Comprehensive DNA Methylation Profiling of Inflammatory Mucosa in Ulcerative Colitis. Inflamm. Bowel Dis. 2016, 23, 165–173. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, F.; Paterson, A.D.; Lachin, J.M.; Zhang, L.; Schones, D.E.; Wu, X.; Wang, J.; Tompkins, J.D.; Genuth, S.; et al. Epigenomic profiling reveals an association between persistence of DNA methylation and metabolic memory in the DCCT/EDIC type 1 diabetes cohort. Proc. Natl. Acad. Sci. USA 2016, 113, E3002–E3011. [Google Scholar] [CrossRef]
- Lutz, A.-K.; Pérez Arévalo, A.; Ioannidis, V.; Stirmlinger, N.; Demestre, M.; Delorme, R.; Bourgeron, T.; Boeckers, T.M. SHANK2 Mutations Result in Dysregulation of the ERK1/2 Pathway in Human Induced Pluripotent Stem Cells-Derived Neurons and Shank2(−/−) Mice. Front. Mol. Neurosci. 2021, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, W.; Sun, X.; Xie, S.; Xu, X.; Liu, M.; Yang, C.; Li, M.; Zhang, W.; Liu, W.; et al. NudCL2 regulates cell migration by stabilizing both myosin-9 and LIS1 with Hsp90. Cell Death Dis. 2020, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Arand, J.; Chiang, H.R.; Martin, D.; Snyder, M.P.; Sage, J.; Reijo Pera, R.A.; Wossidlo, M. Tet enzymes are essential for early embryogenesis and completion of embryonic genome activation. EMBO Rep. 2022, 23, e53968. [Google Scholar] [CrossRef]
- Villanger, G.D.; Weyde, K.V.F.; Olsen, A.K.; Duale, N.; Kamstra, J.H.; Skogheim, T.S.; Winterton, A.; Caspersen, I.H.; Engel, S.M.; Thomsen, C.; et al. Gestational blood levels of PFAS mixtures and associations with global DNA methylation in pregnant women and their infants. ISEE Conf. Abstr. 2023, 2023. [Google Scholar] [CrossRef]
- Robinson, S.L.; Zeng, X.; Guan, W.; Sundaram, R.; Mendola, P.; Putnick, D.L.; Waterland, R.A.; Gunasekara, C.J.; Kannan, K.; Gao, C.; et al. Perfluorooctanoic acid (PFOA) or perfluorooctane sulfonate (PFOS) and DNA methylation in newborn dried blood spots in the Upstate KIDS cohort. Environ. Res. 2021, 194, 110668. [Google Scholar] [CrossRef]
- Miura, R.; Araki, A.; Miyashita, C.; Kobayashi, S.; Kobayashi, S.; Wang, S.L.; Chen, C.H.; Miyake, K.; Ishizuka, M.; Iwasaki, Y.; et al. An epigenome-wide study of cord blood DNA methylations in relation to prenatal perfluoroalkyl substance exposure: The Hokkaido study. Environ. Int. 2018, 115, 21–28. [Google Scholar] [CrossRef]
- Liu, Y.; Eliot Melissa, N.; Papandonatos George, D.; Kelsey Karl, T.; Langevin, S.; Buckley, J.; Chen, A.; Lanphear Bruce, P.; Cecil Kim, M.; Yolton, K.; et al. Longitudinal analysis of DNA methylation in relation to gestational perfluoroalkyl substance exposure: An epigenome-wide association study. ISEE Conf. Abstr. 2021, 2021. [Google Scholar] [CrossRef]
- Xie, Z.; Sun, S.; Ji, H.; Miao, M.; He, W.; Song, X.; Cao, W.; Wu, Q.; Liang, H.; Yuan, W. Prenatal exposure to per- and polyfluoroalkyl substances and DNA methylation in the placenta: A prospective cohort study. J. Hazard. Mater. 2024, 463, 132845. [Google Scholar] [CrossRef]
- Starling, A.P.; Liu, C.; Shen, G.; Yang, I.V.; Kechris, K.; Borengasser, S.J.; Boyle, K.E.; Zhang, W.; Smith, H.A.; Calafat, A.M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances, Umbilical Cord Blood DNA Methylation, and Cardio-Metabolic Indicators in Newborns: The Healthy Start Study. Environ. Health Perspect. 2020, 128, 127014. [Google Scholar] [CrossRef]
- Kobayashi, S.; Azumi, K.; Goudarzi, H.; Araki, A.; Miyashita, C.; Kobayashi, S.; Itoh, S.; Sasaki, S.; Ishizuka, M.; Nakazawa, H.; et al. Effects of prenatal perfluoroalkyl acid exposure on cord blood IGF2/H19 methylation and ponderal index: The Hokkaido Study. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 251–259. [Google Scholar] [CrossRef]
- Henikoff, S.; Smith, M.M. Histone variants and epigenetics. Cold Spring Harb. Perspect. Biol. 2015, 7, a019364. [Google Scholar] [CrossRef]
- Abraham, K.; Mielke, H.; Fromme, H.; Völkel, W.; Menzel, J.; Peiser, M.; Zepp, F.; Willich, S.N.; Weikert, C. Internal exposure to perfluoroalkyl substances (PFASs) and biological markers in 101 healthy 1-year-old children: Associations between levels of perfluorooctanoic acid (PFOA) and vaccine response. Arch. Toxicol. 2020, 94, 2131–2147. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, B.; Zhao, B.; Li, Y.; Zhao, X.; Yuan, Z. Blueberry anthocyanin alleviate perfluorooctanoic acid-induced toxicity in planarian (Dugesia japonica) by regulating oxidative stress biomarkers, ATP contents, DNA methylation and mRNA expression. Environ. Pollut. 2019, 245, 957–964. [Google Scholar] [CrossRef]
- Lau, C. Perfluoroalkyl acids: Recent research highlights. Reprod. Toxicol. 2012, 33, 405–409. [Google Scholar] [CrossRef]
- Schier, A.F. The maternal-zygotic transition: Death and birth of RNAs. Science 2007, 316, 406–407. [Google Scholar] [CrossRef]
- Feldman, N.; Gerson, A.; Fang, J.; Li, E.; Zhang, Y.; Shinkai, Y.; Cedar, H.; Bergman, Y. G9a-mediated irreversible epigenetic inactivation of Oct-3/4 during early embryogenesis. Nat. Cell Biol. 2006, 8, 188–194. [Google Scholar] [CrossRef]
- Alam, M.N.; Han, X.; Nan, B.; Liu, L.; Tian, M.; Shen, H.; Huang, Q. Chronic low-level perfluorooctane sulfonate (PFOS) exposure promotes testicular steroidogenesis through enhanced histone acetylation. Environ. Pollut. 2021, 284, 117518. [Google Scholar] [CrossRef]
- Pierozan, P.; Cattani, D.; Karlsson, O. Perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) induce epigenetic alterations and promote human breast cell carcinogenesis in vitro. Arch. Toxicol. 2020, 94, 3893–3906. [Google Scholar] [CrossRef]
- Li, C.H.; Ren, X.M.; Cao, L.Y.; Qin, W.P.; Guo, L.H. Investigation of binding and activity of perfluoroalkyl substances to the human peroxisome proliferator-activated receptor β/δ. Environ. Sci. Process. Impacts 2019, 21, 1908–1914. [Google Scholar] [CrossRef]
- Li, F.; Yang, R.; Lu, L.; Hua, W.; Sun, Y.; Tian, M.; Lu, Y.; Huang, Q. Comparative steroidogenic effects of hexafluoropropylene oxide trimer acid (HFPO-TA) and perfluorooctanoic acid (PFOA): Regulation of histone modifications. Environ. Pollut. 2024, 350, 124030. [Google Scholar] [CrossRef]
- Wen, Y.; Rashid, F.; Fazal, Z.; Singh, R.; Spinella, M.J.; Irudayaraj, J. Nephrotoxicity of perfluorooctane sulfonate (PFOS)—Effect on transcription and epigenetic factors. Environ. Epigenetics 2022, 8, dvac010. [Google Scholar] [CrossRef] [PubMed]
- Kaikkonen, M.U.; Lam, M.T.; Glass, C.K. Non-coding RNAs as regulators of gene expression and epigenetics. Cardiovasc. Res. 2011, 90, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Miguel, V.; Lamas, S.; Espinosa-Diez, C. Role of non-coding-RNAs in response to environmental stressors and consequences on human health. Redox Biol. 2020, 37, 101580. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Quan, X.; Lei, S.; Chen, G.; Hong, J.; Huang, Z.; Wang, Q.; Song, W.; Yang, X. LncRNA MEG3 alleviates PFOS induced placental cell growth inhibition through its derived miR-770 targeting PTX3. Environ. Pollut. 2022, 293, 118542. [Google Scholar] [CrossRef]
- Ng, C.A.; Hungerbühler, K. Bioconcentration of perfluorinated alkyl acids: How important is specific binding? Environ. Sci. Technol. 2013, 47, 7214–7223. [Google Scholar] [CrossRef]
- Pierozan, P.; Höglund, A.; Theodoropoulou, E.; Karlsson, O. Perfluorooctanesulfonic acid (PFOS) induced cancer related DNA methylation alterations in human breast cells: A whole genome methylome study. Sci. Total Environ. 2024, 949, 174864. [Google Scholar] [CrossRef]
- Revill, K.; Wang, T.; Lachenmayer, A.; Kojima, K.; Harrington, A.; Li, J.; Hoshida, Y.; Llovet, J.M.; Powers, S. Genome-wide methylation analysis and epigenetic unmasking identify tumor suppressor genes in hepatocellular carcinoma. Gastroenterology 2013, 145, 1424–1435. [Google Scholar] [CrossRef]
- Ren, W.; Yuan, Y.; Chen, X.; Zhai, H.; An, Y.; Tang, L.; Wang, J.; Zhang, D.; Zhang, L.; Cheng, W.; et al. Identification and Validation of Long Non-Coding RNA LCIIAR as a Biomarker in LUAD. Front. Oncol. 2022, 12, 933071. [Google Scholar] [CrossRef]
- Yu, X.; Zheng, H.; Tse, G.; Zhang, L.; Wu, W.K.K. CASC2: An emerging tumour-suppressing long noncoding RNA in human cancers and melanoma. Cell Prolif. 2018, 51, e12506. [Google Scholar] [CrossRef]
- Xu, Y.; Jurkovic-Mlakar, S.; Li, Y.; Wahlberg, K.; Scott, K.; Pineda, D.; Lindh, C.H.; Jakobsson, K.; Engström, K. Association between serum concentrations of perfluoroalkyl substances (PFAS) and expression of serum microRNAs in a cohort highly exposed to PFAS from drinking water. Environ. Int. 2020, 136, 105446. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Gunasekaran, K.; Sasidharan, S.; Jeyamanickavel Mathan, V.; Perumal, E. MicroRNAs and Xenobiotic Toxicity: An Overview. Toxicol. Rep. 2020, 7, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, Q.Z.; Wu, C.Q.; Pan, X.Y.; Wang, J.; Tan, Y.; Shan, X.Y.; Zeng, H.C. PFOS Disturbs BDNF-ERK-CREB Signalling in Association with Increased MicroRNA-22 in SH-SY5Y Cells. BioMed Res. Int. 2015, 2015, 302653. [Google Scholar] [CrossRef] [PubMed]
- Rauti, R.; Cellot, G.; D’Andrea, P.; Colliva, A.; Scaini, D.; Tongiorgi, E.; Ballerini, L. BDNF impact on synaptic dynamics: Extra or intracellular long-term release differently regulates cultured hippocampal synapses. Mol. Brain 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Castrén, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Lu, Y. BDNF and synaptic plasticity, cognitive function, and dysfunction. Handb. Exp. Pharmacol. 2014, 220, 223–250. [Google Scholar] [CrossRef]
- Niu, Z.S.; Wang, W.H.; Dong, X.N.; Tian, L.M. Role of long noncoding RNA-mediated competing endogenous RNA regulatory network in hepatocellular carcinoma. World J. Gastroenterol. 2020, 26, 4240–4260. [Google Scholar] [CrossRef]
- Ala, U. Competing Endogenous RNAs, Non-Coding RNAs and Diseases: An Intertwined Story. Cells 2020, 9, 1574. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Beedle, A.S. Non-genomic transgenerational inheritance of disease risk. Bioessays 2007, 29, 145–154. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Jirtle, R.L.; Skinner, M.K. Environmental epigenomics and disease susceptibility. Nat. Rev. Genet. 2007, 8, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Waterland, R.A.; Michels, K.B. Epigenetic epidemiology of the developmental origins hypothesis. Annu. Rev. Nutr. 2007, 27, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Reik, W.; Walter, J. Evolution of imprinting mechanisms: The battle of the sexes begins in the zygote. Nat. Genet. 2001, 27, 255–256. [Google Scholar] [CrossRef]
- Seisenberger, S.; Peat, J.R.; Hore, T.A.; Santos, F.; Dean, W.; Reik, W. Reprogramming DNA methylation in the mammalian life cycle: Building and breaking epigenetic barriers. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20110330. [Google Scholar] [CrossRef]
- Ku, M.-S.; Pan, W.-C.; Huang, Y.-T.; Hsieh, W.-S.; Hsu, Y.-H.; Chen, P.-C.; Liu, C.-Y. Associations between prenatal exposure to perfluoroalkyl substances, hypomethylation of MEST imprinted gene and birth outcomes. Environ. Pollut. 2022, 304, 119183. [Google Scholar] [CrossRef]
- Ferguson-Smith, A.C. Genomic imprinting: The emergence of an epigenetic paradigm. Nat. Rev. Genet. 2011, 12, 565–575. [Google Scholar] [CrossRef]
- Gabory, A.; Roseboom, T.J.; Moore, T.; Moore, L.G.; Junien, C. Placental contribution to the origins of sexual dimorphism in health and diseases: Sex chromosomes and epigenetics. Biol. Sex. Differ. 2013, 4, 5. [Google Scholar] [CrossRef]
- Court, F.; Tayama, C.; Romanelli, V.; Martin-Trujillo, A.; Iglesias-Platas, I.; Okamura, K.; Sugahara, N.; Simón, C.; Moore, H.; Harness, J.V.; et al. Genome-wide parent-of-origin DNA methylation analysis reveals the intricacies of human imprinting and suggests a germline methylation-independent mechanism of establishment. Genome Res. 2014, 24, 554–569. [Google Scholar] [CrossRef]
- El Hajj, N.; Pliushch, G.; Schneider, E.; Dittrich, M.; Müller, T.; Korenkov, M.; Aretz, M.; Zechner, U.; Lehnen, H.; Haaf, T. Metabolic programming of MEST DNA methylation by intrauterine exposure to gestational diabetes mellitus. Diabetes 2013, 62, 1320–1328. [Google Scholar] [CrossRef]
- Leung, D.; Du, T.; Wagner, U.; Xie, W.; Lee, A.Y.; Goyal, P.; Li, Y.; Szulwach, K.E.; Jin, P.; Lorincz, M.C.; et al. Regulation of DNA methylation turnover at LTR retrotransposons and imprinted loci by the histone methyltransferase Setdb1. Proc. Natl. Acad. Sci. USA 2014, 111, 6690–6695. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xu, X.; Li, J.; Jiang, Y.; Hong, X.; Rexrode, K.M.; Wang, G.; Hu, F.B.; Zhang, H.; Karmaus, W.J.; et al. Sex differences in the intergenerational link between maternal and neonatal whole blood DNA methylation: A genome-wide analysis in 2 birth cohorts. Clin. Epigenetics 2023, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.M.; Fry, R.C. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu. Rev. Public Health 2018, 39, 309–333. [Google Scholar] [CrossRef]
- Leung, Y.-K.; Ouyang, B.; Niu, L.; Xie, C.; Ying, J.; Medvedovic, M.; Chen, A.; Weihe, P.; Valvi, D.; Grandjean, P.; et al. Identification of sex-specific DNA methylation changes driven by specific chemicals in cord blood in a Faroese birth cohort. Epigenetics 2018, 13, 290–300. [Google Scholar] [CrossRef]
- Kobayashi, S.; Sata, F.; Ikeda-Araki, A.; Miyashita, C.; Goudarzi, H.; Iwasaki, Y.; Nakajima, T.; Kishi, R. Relationships between maternal perfluoroalkyl substance levels, polymorphisms of receptor genes, and adverse birth outcomes in the Hokkaido birth cohort study, Japan. Reprod. Toxicol. 2022, 107, 112–122. [Google Scholar] [CrossRef]
- Salinas, I.; Sinha, N.; Sen, A. Androgen-induced epigenetic modulations in the ovary. J. Endocrinol. 2021, 249, R53–R64. [Google Scholar] [CrossRef]
- Yu, X.; Xu, J.; Song, B.; Zhu, R.; Liu, J.; Liu, Y.F.; Ma, Y.J. The role of epigenetics in women’s reproductive health: The impact of environmental factors. Front. Endocrinol. 2024, 15, 1399757. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Yang, J.; Wang, Y.; Du, H.; Han, M.; Xu, L.; Liu, S.; Yi, J.; Chen, Y.; et al. Gestational exposure to perfluoroalkyl substances is associated with placental DNA methylation and birth size. Sci. Total Environ. 2023, 858, 159747. [Google Scholar] [CrossRef]
- von der Lippe, C.; Tveten, K.; Prescott, T.E.; Holla, Ø.L.; Busk, Ø.L.; Burke, K.B.; Sansbury, F.H.; Baptista, J.; Fry, A.E.; Lim, D.; et al. Heterozygous variants in cause a neurodevelopmental disorder associated with symptomatic overgrowth of pharyngeal lymphoid tissue, macrocephaly, and elevated fetal hemoglobin. Am. J. Med. Genet. Part A 2022, 188, 272–282. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Z.; Wang, X.; Hou, Y.; Bai, Y.; Tian, Y. Hypoxia-Regulated lncRNA USP2-AS1 Drives Head and Neck Squamous Cell Carcinoma Progression. Cells 2022, 11, 3407. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Tong, H.; Li, S.; Yan, Y. TCP11L2 promotes bovine skeletal muscle-derived satellite cell migration and differentiation via FMNL2. J. Cell. Physiol. 2020, 235, 7183–7193. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, T.E.; Serafini, T.; de la Torre, J.R.; Tessier-Lavigne, M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell 1994, 78, 425–435. [Google Scholar] [CrossRef]
- Serafini, T.; Colamarino, S.A.; Leonardo, E.D.; Wang, H.; Beddington, R.; Skarnes, W.C.; Tessier-Lavigne, M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell 1996, 87, 1001–1014. [Google Scholar] [CrossRef]
- Larrivée, B.; Freitas, C.; Trombe, M.; Lv, X.; Delafarge, B.; Yuan, L.; Bouvrée, K.; Bréant, C.; Del Toro, R.; Bréchot, N.; et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007, 21, 2433–2447. [Google Scholar] [CrossRef]
- Honeycutt, S.E.; N’Guetta, P.Y.; Hardesty, D.M.; Xiong, Y.; Cooper, S.L.; Stevenson, M.J.; O’Brien, L.L. Netrin 1 directs vascular patterning and maturity in the developing kidney. Development 2023, 150, dev201886. [Google Scholar] [CrossRef]
- Hallberg, I.; Persson, S.; Olovsson, M.; Moberg, M.; Ranefall, P.; Laskowski, D.; Damdimopoulou, P.; Sirard, M.-A.; Rüegg, J.; Sjunnesson, Y.C.B. Bovine oocyte exposure to perfluorohexane sulfonate (PFHxS) induces phenotypic, transcriptomic, and DNA methylation changes in resulting embryos in vitro. Reprod. Toxicol. 2022, 109, 19–30. [Google Scholar] [CrossRef]
- Lendvai, Á.; Deutsch, M.J.; Plösch, T.; Ensenauer, R. The peroxisome proliferator-activated receptors under epigenetic control in placental metabolism and fetal development. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E797–E810. [Google Scholar] [CrossRef]
- Skinner, M.K.; Manikkam, M.; Guerrero-Bosagna, C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol. Metab. 2010, 21, 214–222. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, B.N.; Lee, Y.A.; Shin, C.H.; Hong, Y.C.; Døssing, L.D.; Hildebrandt, G.; Lim, Y.H. Association between early-childhood exposure to perfluoroalkyl substances and ADHD symptoms: A prospective cohort study. Sci. Total Environ. 2023, 879, 163081. [Google Scholar] [CrossRef]
- Oh, J.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Roa, D.L.; Schmidt, R.J.; Hertz-Picciotto, I.; Shin, H.M. Prenatal exposure to per- and polyfluoroalkyl substances in association with autism spectrum disorder in the MARBLES study. Environ. Int. 2021, 147, 106328. [Google Scholar] [CrossRef]
- Vuong, A.M.; Yolton, K.; Xie, C.; Dietrich, K.N.; Braun, J.M.; Webster, G.M.; Calafat, A.M.; Lanphear, B.P.; Chen, A. Childhood exposure to per- and polyfluoroalkyl substances (PFAS) and neurobehavioral domains in children at age 8 years. Neurotoxicol. Teratol. 2021, 88, 107022. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.L.; Sharma, V.; Lyall, K. Effects of Early-life PFAS Exposure on Child Neurodevelopment: A Review of the Evidence and Research gaps. Curr. Environ. Health Rep. 2025, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Blake, B.E.; Fenton, S.E. Early life exposure to per- and polyfluoroalkyl substances (PFAS) and latent health outcomes: A review including the placenta as a target tissue and possible driver of peri- and postnatal effects. Toxicology 2020, 443, 152565. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.C.; Dassuncao, C.; Tokranov, A.K.; Wagner, C.C.; Allen, J.G. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 131–147. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Ferguson, K.K.; Meeker, J.D.; McElrath, T.F.; Cantonwine, D.E. Maternal levels of perfluoroalkyl substances (PFAS) during early pregnancy in relation to preeclampsia subtypes and biomarkers of preeclampsia risk. Environ. Health Perspect. 2021, 129, 107004. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, J.; Wu, S.; Sánchez, O.F.; Zhang, X.; Freeman, J.L.; Yuan, C. Pre-differentiation exposure of PFOA induced persistent changes in DNA methylation and mitochondrial morphology in human dopaminergic-like neurons. Environ. Pollut. 2022, 308, 119684. [Google Scholar] [CrossRef]
- Fan, X.; Tang, S.; Wang, Y.; Fan, W.; Ben, Y.; Naidu, R.; Dong, Z. Global exposure to per-and polyfluoroalkyl substances and associated burden of low birthweight. Environ. Sci. Technol. 2022, 56, 4282–4294. [Google Scholar] [CrossRef]
- Wikström, S.; Lin, P.-I.; Lindh, C.H.; Shu, H.; Bornehag, C.-G. Maternal serum levels of perfluoroalkyl substances in early pregnancy and offspring birth weight. Pediatr. Res. 2020, 87, 1093–1099. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Q.; Zhang, L.; Li, W.; Yin, S.; Li, F.; Xu, C. In utero exposure to per-/polyfluoroalkyl substances (PFASs): Preeclampsia in pregnancy and low birth weight for neonates. Chemosphere 2023, 313, 137490. [Google Scholar] [CrossRef]
- Shoaff, J.; Papandonatos, G.D.; Calafat, A.M.; Chen, A.; Lanphear, B.P.; Ehrlich, S.; Kelsey, K.T.; Braun, J.M. Prenatal exposure to perfluoroalkyl substances: Infant birth weight and early life growth. Environ. Epidemiol. 2018, 2, e010. [Google Scholar] [CrossRef]
- Engström, K.; Axmon, A.; Nielsen, C.; Rignell-Hydbom, A. High in utero exposure to perfluoroalkyl substances from drinking water and birth weight: A cohort study among infants in Ronneby, Sweden. Int. J. Environ. Res. Public Health 2022, 19, 2385. [Google Scholar] [CrossRef]
- Steenland, K.; Barry, V.; Savitz, D. Serum Perfluorooctanoic Acid and Birthweight: An Updated Meta-analysis With Bias Analysis. Epidemiology 2018, 29, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.M.; Lee, A.L.; Rappazzo, K.M.; Ru, H.; Radke, E.G.; Bateson, T.F. Systematic review and meta-analysis of birth weight and PFNA exposures. Environ. Res. 2023, 222, 115357. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Gao, Y.; Zhang, Y.; Qin, K.; Liew, Z.; Tian, Y. Associations of paternal and maternal per- and polyfluoroalkyl substances exposure with cord serum reproductive hormones, placental steroidogenic enzyme and birth weight. Chemosphere 2021, 285, 131521. [Google Scholar] [CrossRef] [PubMed]
- Morales-Grahl, E.; Hilz, E.N.; Gore, A.C. Regrettable Substitutes and the Brain: What Animal Models and Human Studies Tell Us about the Neurodevelopmental Effects of Bisphenol, Per- and Polyfluoroalkyl Substances, and Phthalate Replacements. Int. J. Mol. Sci. 2024, 25, 6887. [Google Scholar] [CrossRef]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef]
- Lee, H.; Sung, E.J.; Seo, S.; Min, E.K.; Lee, J.-Y.; Shim, I.; Kim, P.; Kim, T.-Y.; Lee, S.; Kim, K.-T. Integrated multi-omics analysis reveals the underlying molecular mechanism for developmental neurotoxicity of perfluorooctanesulfonic acid in zebrafish. Environ. Int. 2021, 157, 106802. [Google Scholar] [CrossRef]
- Currie, S.D.; Wang, J.-S.; Tang, L. Impacts of PFAS Exposure on Neurodevelopment: A Comprehensive Literature Review. Environments 2024, 11, 188. [Google Scholar] [CrossRef]
- Shin, H.M.; Bennett, D.H.; Calafat, A.M.; Tancredi, D.; Hertz-Picciotto, I. Modeled prenatal exposure to per- and polyfluoroalkyl substances in association with child autism spectrum disorder: A case-control study. Environ. Res. 2020, 186, 109514. [Google Scholar] [CrossRef]
- Gui, S.Y.; Chen, Y.N.; Wu, K.J.; Liu, W.; Wang, W.J.; Liang, H.R.; Jiang, Z.X.; Li, Z.L.; Hu, C.Y. Association Between Exposure to Per- and Polyfluoroalkyl Substances and Birth Outcomes: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 855348. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Chen, H.; Weng, J. Multifaceted paternal exposures before conception and their epigenetic impact on offspring. J. Assist. Reprod. Genet. 2024, 41, 2931–2951. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Xie, J.; Zhao, H.; Zhao, X.; Sánchez, O.F.; Rochet, J.-C.; Freeman, J.L.; Yuan, C. Developmental neurotoxicity of PFOA exposure on hiPSC-derived cortical neurons. Environ. Int. 2024, 190, 108914. [Google Scholar] [CrossRef] [PubMed]
- Spratlen, M.J.; Perera, F.P.; Lederman, S.A.; Rauh, V.A.; Robinson, M.; Kannan, K.; Trasande, L.; Herbstman, J. The association between prenatal exposure to perfluoroalkyl substances and childhood neurodevelopment. Environ. Pollut. 2020, 263, 114444. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.-x.; Zuo, Q.-l.; Fu, X.-h.; Song, L.-l.; Cen, M.-q.; Wu, J. Association between prenatal exposure to per- and polyfluoroalkyl substances and neurodevelopment in children: Evidence based on birth cohort. Environ. Res. 2023, 236, 116812. [Google Scholar] [CrossRef]
- Varsi, K.; Torsvik, I.K.; Huber, S.; Averina, M.; Brox, J.; Bjørke-Monsen, A.-L. Impaired gross motor development in infants with higher PFAS concentrations. Environ. Res. 2022, 204, 112392. [Google Scholar] [CrossRef]
- Viberg, H.; Lee, I.; Eriksson, P. Adult dose-dependent behavioral and cognitive disturbances after a single neonatal PFHxS dose. Toxicology 2013, 304, 185–191. [Google Scholar] [CrossRef]
- Johansson, N.; Fredriksson, A.; Eriksson, P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology 2008, 29, 160–169. [Google Scholar] [CrossRef]
- Niu, J.; Liang, H.; Tian, Y.; Yuan, W.; Xiao, H.; Hu, H.; Sun, X.; Song, X.; Wen, S.; Yang, L. Prenatal plasma concentrations of Perfluoroalkyl and polyfluoroalkyl substances and neuropsychological development in children at four years of age. Environ. Health 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Kelly, T.K.; De Carvalho, D.D.; Jones, P.A. Epigenetic modifications as therapeutic targets. Nat. Biotechnol. 2010, 28, 1069–1078. [Google Scholar] [CrossRef]
- Durham, J.; Tessmann, J.W.; Deng, P.; Hennig, B.; Zaytseva, Y.Y. The role of perfluorooctane sulfonic acid (PFOS) exposure in inflammation of intestinal tissues and intestinal carcinogenesis. Front. Toxicol. 2023, 5, 1244457. [Google Scholar] [CrossRef]
- Ho, T.C.; Wan, H.T.; Lee, W.K.; Lam, T.K.Y.; Lin, X.; Chan, T.F.; Lai, K.P.; Wong, C.K.C. Effects of In Utero PFOS Exposure on Epigenetics and Metabolism in Mouse Fetal Livers. Environ. Sci. Technol. 2023, 57, 14892–14903. [Google Scholar] [CrossRef]
- Imir, O.B. The Effects of Per- and Polyfluoroalkyl Substances (PFAS) and High Fat Diet on Metabolism, Reproductive Health & Prostate Cancer Progression. Master’s Thesis, University of Illinois at Urbana-Champaign, Champaign, IL, USA, 2021. [Google Scholar]
- Papadopoulou, E.; Stratakis, N.; Basagaña, X.; Brantsæter, A.L.; Casas, M.; Fossati, S.; Gražulevičienė, R.; Småstuen Haug, L.; Heude, B.; Maitre, L.; et al. Prenatal and postnatal exposure to PFAS and cardiometabolic factors and inflammation status in children from six European cohorts. Environ. Int. 2021, 157, 106853. [Google Scholar] [CrossRef]
- Maxwell, D.L.; Oluwayiose, O.A.; Houle, E.; Roth, K.; Nowak, K.; Sawant, S.; Paskavitz, A.L.; Liu, W.; Gurdziel, K.; Petriello, M.C.; et al. Mixtures of per- and polyfluoroalkyl substances (PFAS) alter sperm methylation and long-term reprogramming of offspring liver and fat transcriptome. Environ. Int. 2024, 186, 108577. [Google Scholar] [CrossRef]
- Fangninou, F.F.; Yu, Z.; Li, W.; Xue, L.; Yin, D. Metastatic effects of perfluorooctanoic acid (PFOA) on Drosophila melanogaster with metabolic reprogramming and dysrhythmia in a multigenerational exposure scenario. Sci. Total Environ. 2024, 912, 169305. [Google Scholar] [CrossRef]
- US. EPA. FINAL: Human Health Toxicity Assessment for Perfluorooctanoic Acid (PFOA) and Related Salts; EPA Document Number: 815R24006; 2024. Available online: https://www.epa.gov/system/files/documents/2024-05/final-human-health-toxicity-assessment-pfoa.pdf (accessed on 1 November 2024).
- Bushong, A.; Sepúlveda, M.; Scherer, M.; Valachovic, A.C.; Neill, C.M.; Horn, S.; Choi, Y.; Lee, L.S.; Baloni, P.; Hoskins, T. Effects of Perfluorinated Alkyl Substances (PFAS) on Amphibian Body and Liver Conditions: Is. Lipid Metabolism Being Perturbed throughout Metamorphosis? Toxics 2024, 12, 732. [Google Scholar] [CrossRef]
- Meng, X.; Yu, G.; Luo, T.; Zhang, R.; Zhang, J.; Liu, Y. Transcriptomics integrated with metabolomics reveals perfluorobutane sulfonate (PFBS) exposure effect during pregnancy and lactation on lipid metabolism in rat offspring. Chemosphere 2023, 341, 140120. [Google Scholar] [CrossRef]
- González, M.C. Prenatal exposure to persistent organic pollutants as a risk factor of offspring metabolic syndrome development during childhood. Rev. Environ. Health 2022, 37, 61–70. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Zhang, M.; Shama; Cheng, K.K.Y.; Jia, W. The interplay between the muscle and liver in the regulation of glucolipid metabolism. J. Mol. Cell Biol. 2023, 15, mjad073. [Google Scholar] [CrossRef]
- Haverinen, E.; Fernandez, M.F.; Mustieles, V.; Tolonen, H. Metabolic Syndrome and Endocrine Disrupting Chemicals: An Overview of Exposure and Health Effects. Int. J. Environ. Res. Public Health 2021, 18, 13047. [Google Scholar] [CrossRef]
- Yu, G.; Wang, J.; Liu, Y.; Luo, T.; Meng, X.; Zhang, R.; Huang, B.; Sun, Y.; Zhang, J. Metabolic perturbations in pregnant rats exposed to low-dose perfluorooctanesulfonic acid: An integrated multi-omics analysis. Environ. Int. 2023, 173, 107851. [Google Scholar] [CrossRef]
- Chao, W.; D’Amore, P.A. IGF2: Epigenetic regulation and role in development and disease. Cytokine Growth Factor Rev. 2008, 19, 111–120. [Google Scholar] [CrossRef]
- Holly, J.M.P.; Biernacka, K.; Perks, C.M. The Neglected Insulin: IGF-II, a Metabolic Regulator with Implications for Diabetes, Obesity, and Cancer. Cells 2019, 8, 1207. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Keating, S.T.; El-Osta, A. Epigenetics and metabolism. Circ. Res. 2015, 116, 715–736. [Google Scholar] [CrossRef]
- He, X.; Liu, Y.; Xu, B.; Gu, L.; Tang, W. PFOA is associated with diabetes and metabolic alteration in US men: National Health and Nutrition Examination Survey 2003–2012. Sci. Total Environ. 2018, 625, 566–574. [Google Scholar] [CrossRef]
- Chang, S.; Fulmer, D.; Hur, S.K.; Thorvaldsen, J.L.; Li, L.; Lan, Y.; Rhon-Calderon, E.A.; Leu, N.A.; Chen, X.; Epstein, J.A.; et al. Dysregulated H19/Igf2 expression disrupts cardiac-placental axis during development of Silver-Russell syndrome-like mouse models. Elife 2022, 11, e78754. [Google Scholar] [CrossRef]
- Schillemans, T.; Donat-Vargas, C.; Åkesson, A. Per-and polyfluoroalkyl substances and cardiometabolic diseases: A review. Basic Clin. Pharmacol. Toxicol. 2024, 134, 141–152. [Google Scholar] [CrossRef]
- Schlezinger, J.J.; Gokce, N. Perfluoroalkyl/Polyfluoroalkyl Substances: Links to Cardiovascular Disease Risk. Circ. Res. 2024, 134, 1136–1159. [Google Scholar] [CrossRef]
- Takacs, M.L.; Abbott, B.D. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 2007, 95, 108–117. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Rifas-Shiman, S.L.; Mora, A.M.; Calafat, A.M.; Ye, X.; Luttmann-Gibson, H.; Gillman, M.W.; Oken, E.; Sagiv, S.K. Early-Life Exposure to Perfluoroalkyl Substances and Childhood Metabolic Function. Environ. Health Perspect. 2017, 125, 481–487. [Google Scholar] [CrossRef]
- Braun, J.M.; Kalloo, G.; Chen, A.; Dietrich, K.N.; Liddy-Hicks, S.; Morgan, S.; Xu, Y.; Yolton, K.; Lanphear, B.P. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int. J. Epidemiol. 2017, 46, 24. [Google Scholar] [CrossRef]
- Braun, J.M.; Buckley, J.P.; Cecil, K.M.; Chen, A.; Kalkwarf, H.J.; Lanphear, B.P.; Xu, Y.; Woeste, A.; Yolton, K. Adolescent follow-up in the Health Outcomes and Measures of the Environment (HOME) Study: Cohort profile. BMJ Open 2020, 10, e034838. [Google Scholar] [CrossRef]
- Conley, J.M.; Lambright, C.S.; Evans, N.; Farraj, A.K.; Smoot, J.; Grindstaff, R.D.; Hill, D.; McCord, J.; Medlock-Kakaley, E.; Dixon, A.; et al. Dose additive maternal and offspring effects of oral maternal exposure to a mixture of three PFAS (HFPO-DA, NBP2, PFOS) during pregnancy in the Sprague-Dawley rat. Sci. Total Environ. 2023, 892, 164609. [Google Scholar] [CrossRef]
- Lin, T.A.; Huang, C.W.; Wei, C.C. Early-life perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) exposure cause obesity by disrupting fatty acids metabolism and enhancing triglyceride synthesis in Caenorhabditis elegans. Aquat. Toxicol. 2022, 251, 106274. [Google Scholar] [CrossRef]
- Caroccia, B.; Caputo, I.; Rossi, F.B.; Piazza, M.; Pallafacchina, G.; Paolo Rossi, G. Endocrine disruptors and arterial hypertension: A developing story. Steroids 2023, 199, 109292. [Google Scholar] [CrossRef]
- Gump, B.B.; Hill, D.T.; Robinson, M.; Kannan, K.; Heffernan, K.; Atallah-Yunes, N.H.; Brann, L.; Parsons, P.J.; Palmer, C.D.; MacKenzie, J.A.; et al. Perfluoroalkyl substances (PFAS) and lead (Pb) as “cardiovascular disruptors” in 9–11-year-old children living in Syracuse, New York, United States. Environ. Res. 2023, 236, 116758. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y. Role of Mammalian DNA Methyltransferases in Development. Annu. Rev. Biochem. 2020, 89, 135–158. [Google Scholar] [CrossRef]
- Blaustein, J.R.; Quisel, M.J.; Hamburg, N.M.; Wittkopp, S. Environmental Impacts on Cardiovascular Health and Biology: An Overview. Circ. Res. 2024, 134, 1048–1060. [Google Scholar] [CrossRef]
- Qiu, M.; Chen, J.; Liu, M.; Shi, Y.; Nie, Z.; Dong, G.; Li, X.; Chen, J.; Ou, Y.; Zhuang, J. Reprogramming of DNA methylation patterns mediates perfluorooctane sulfonate-induced fetal cardiac dysplasia. Sci. Total Environ. 2024, 919, 170905. [Google Scholar] [CrossRef]
- Ou, Y.; Zeng, X.; Lin, S.; Bloom, M.S.; Han, F.; Xiao, X.; Wang, H.; Matala, R.; Li, X.; Qu, Y.; et al. Gestational exposure to perfluoroalkyl substances and congenital heart defects: A nested case-control pilot study. Environ. Int. 2021, 154, 106567. [Google Scholar] [CrossRef]
- Canova, C.; Barbieri, G.; Jeddi, M.Z.; Gion, M.; Fabricio, A.; Daprà, F.; Russo, F.; Fletcher, T.; Pitter, G. Associations between perfluoroalkyl substances and lipid profile in a highly exposed young adult population in the Veneto Region. Environ. Int. 2020, 145, 106117. [Google Scholar] [CrossRef]
- Tain, Y.-L.; Hsu, C.-N. The Impact of the Aryl Hydrocarbon Receptor on Antenatal Chemical Exposure-Induced Cardiovascular–Kidney–Metabolic Programming. Int. J. Mol. Sci. 2024, 25, 4599. [Google Scholar] [CrossRef]
- Hall, S.M.; Zhang, S.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of per- and polyfluoroalkyl substances (PFAS) in human placental tissues and associations with birth outcomes. Chemosphere 2022, 295, 133873. [Google Scholar] [CrossRef]
- Porfirio, K.; Yadav, P.; Dangudubiyyam, S.V.; Hofmann, A.; Mishra, J.S.; Kumar, S. Perfluorooctane Sulfonate Exposure Induces Cardiovascular Dysfunction in Female Rats: Role of Ovaries. Cardiol. Cardiovasc. Med. 2024, 8, 275–284. [Google Scholar] [CrossRef]
- Wen, Z.J.; Wei, Y.J.; Zhang, Y.F.; Zhang, Y.F. A review of cardiovascular effects and underlying mechanisms of legacy and emerging per- and polyfluoroalkyl substances (PFAS). Arch. Toxicol. 2023, 97, 1195–1245. [Google Scholar] [CrossRef]
- Feng, X.; Long, G.; Zeng, G.; Zhang, Q.; Song, B.; Wu, K.-H. Association of increased risk of cardiovascular diseases with higher levels of perfluoroalkylated substances in the serum of adults. Environ. Sci. Pollut. Res. 2022, 29, 89081–89092. [Google Scholar] [CrossRef]
- Watkins, D.J.; Wellenius, G.A.; Butler, R.A.; Bartell, S.M.; Fletcher, T.; Kelsey, K.T. Associations between serum perfluoroalkyl acids and LINE-1 DNA methylation. Environ. Int. 2014, 63, 71–76. [Google Scholar] [CrossRef]
- Schmidt, S. Marks and Mechanisms: Unraveling Potential Health Impacts of PFAS via DNA Methylation. Environ. Health Perspect. 2022, 130, 054001. [Google Scholar] [CrossRef]
- Jiao, X.; Zhao, L.; Xu, Y.; Gao, J.; Tang, W.; Wu, Y.; Yang, L.; Huang, J.; Sun, K.; Guo, Y. Environmental Exposure to Per-and Polyfluoroalkyl Substances and Childhood Congenital Heart Disease: A Mixed Analysis. Available SSRN 4928728 2024. [Google Scholar] [CrossRef]
- Zachariah, J.P.; Jone, P.N.; Agbaje, A.O.; Ryan, H.H.; Trasande, L.; Perng, W.; Farzan, S.F. Environmental Exposures and Pediatric Cardiology: A Scientific Statement From the American Heart Association. Circulation 2024, 149, e1165–e1175. [Google Scholar] [CrossRef]
- Ganakumar, V.; Sruthi, K.; Ghatnatti, V.B.; Goroshi, M. Endocrine Disruptors and the Heart: Unraveling the Cardiovascular Impact. Indian J. Cardiovasc. Dis. Women 2024, 9, 230–240. [Google Scholar] [CrossRef]
- Savitz, D.A.; Stein, C.R.; Bartell, S.M.; Elston, B.; Gong, J.; Shin, H.-M.; Wellenius, G.A. Perfluorooctanoic acid exposure and pregnancy outcome in a highly exposed community. Epidemiology 2012, 23, 386–392. [Google Scholar] [CrossRef]
- Hirke, A.; Varghese, B.; Varade, S.; Adela, R. Exposure to endocrine-disrupting chemicals and risk of gestational hypertension and preeclampsia: A systematic review and meta-analysis. Environ. Pollut. 2023, 317, 120828. [Google Scholar] [CrossRef]
- Ebel, M.; Rylander, L.; Fletcher, T.; Jakobsson, K.; Nielsen, C. Gestational hypertension, preeclampsia, and gestational diabetes mellitus after high exposure to perfluoroalkyl substances from drinking water in Ronneby, Sweden. Environ. Res. 2023, 239, 117316. [Google Scholar] [CrossRef]
- Yue, X.; Sun, Y.; Zhong, M.; Ma, Y.; Wei, Y.; Sun, F.; Xiao, L.; Liu, M.; Chen, J.; Lai, Y.; et al. Decreased expression of fibroblast growth factor 13 in early-onset preeclampsia is associated with the increased trophoblast permeability. Placenta 2018, 62, 43–49. [Google Scholar] [CrossRef]
- Legault, L.-M.; Breton-Larrivée, M.; Langford-Avelar, A.; Lemieux, A.; McGraw, S. Sex-based disparities in DNA methylation and gene expression in late-gestation mouse placentas. Biol. Sex Differ. 2024, 15, 2. [Google Scholar] [CrossRef]
- Wikström, S.; Lindh, C.H.; Shu, H.; Bornehag, C.-G. Early pregnancy serum levels of perfluoroalkyl substances and risk of preeclampsia in Swedish women. Sci. Rep. 2019, 9, 9179. [Google Scholar] [CrossRef]
- Rylander, L.; Lindh, C.H.; Hansson, S.R.; Broberg, K.; Källén, K. Per- and Polyfluoroalkyl Substances in Early Pregnancy and Risk for Preeclampsia: A Case-Control Study in Southern Sweden. Toxics 2020, 8, 43. [Google Scholar] [CrossRef]
- Qian, Y.; Ducatman, A.; Ward, R.; Leonard, S.; Bukowski, V.; Lan Guo, N.; Shi, X.; Vallyathan, V.; Castranova, V. Perfluorooctane sulfonate (PFOS) induces reactive oxygen species (ROS) production in human microvascular endothelial cells: Role in endothelial permeability. J. Toxicol. Environ. Health A 2010, 73, 819–836. [Google Scholar] [CrossRef]
- Kim, S.; Stroski, K.M.; Killeen, G.; Smitherman, C.; Simcik, M.F.; Brooks, B.W. 8:8 Perfluoroalkyl phosphinic acid affects neurobehavioral development, thyroid disruption, and DNA methylation in developing zebrafish. Sci. Total Environ. 2020, 736, 139600. [Google Scholar] [CrossRef]
- Imir, O.B.; Kaminsky, A.Z.; Zuo, Q.-Y.; Liu, Y.-J.; Singh, R.; Spinella, M.J.; Irudayaraj, J.; Hu, W.-Y.; Prins, G.S.; Madak Erdogan, Z. Per- and Polyfluoroalkyl Substance Exposure Combined with High-Fat Diet Supports Prostate Cancer Progression. Nutrients 2021, 13, 3902. [Google Scholar] [CrossRef]
- Zhang, X. Assessing the Correlation Between Per- and Polyfluoroalkyl Substances (pfas) Exposure and Cancer Incidence in the US. Master’s Thesis, Yale University, New Haven, CT, USA, 2024. [Google Scholar]
- Ayodele, A.; Obeng-Gyasi, E. Exploring the Potential Link between PFAS Exposure and Endometrial Cancer: A Review of Environmental and Sociodemographic Factors. Cancers 2024, 16, 983. [Google Scholar] [CrossRef]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, Adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Ahmad, S.; Wen, Y.; Irudayaraj, J.M.K. PFOA induces alteration in DNA methylation regulators and SARS-CoV-2 targets Ace2 and Tmprss2 in mouse lung tissues. Toxicol. Rep. 2021, 8, 1892–1898. [Google Scholar] [CrossRef]
- Bulka, C.M.; Enggasser, A.E.; Fry, R.C. Epigenetics at the Intersection of COVID-19 Risk and Environmental Chemical Exposures. Curr. Environ. Health Rep. 2022, 9, 477–489. [Google Scholar] [CrossRef]
- Liang, L.; Pan, Y.; Bin, L.; Liu, Y.; Huang, W.; Li, R.; Lai, K.P. Immunotoxicity mechanisms of perfluorinated compounds PFOA and PFOS. Chemosphere 2022, 291, 132892. [Google Scholar] [CrossRef]
- Rudzanová, B.; Thon, V.; Vespalcová, H.; Martyniuk, C.J.; Piler, P.; Zvonař, M.; Klánová, J.; Bláha, L.; Adamovsky, O. Altered Transcriptome Response in PBMCs of Czech Adults Linked to Multiple PFAS Exposure: B Cell Development as a Target of PFAS Immunotoxicity. Environ. Sci. Technol. 2024, 58, 90–98. [Google Scholar] [CrossRef]
- Niemiec, S.S.; Kechris, K.; Pattee, J.; Yang, I.V.; Adgate, J.L.; Calafat, A.M.; Dabelea, D.; Starling, A.P. Prenatal exposures to per- and polyfluoroalkyl substances and epigenetic aging in umbilical cord blood: The Healthy Start study. Environ. Res. 2023, 231, 116215. [Google Scholar] [CrossRef]
- Sevelsted, A.; Pedersen, C.-E.T.; Gürdeniz, G.; Rasmussen, M.A.; Schullehner, J.; Sdougkou, K.; Martin, J.W.; Lasky-Su, J.; Morin, A.; Ober, C.; et al. Exposures to perfluoroalkyl substances and asthma phenotypes in childhood: An investigation of the COPSAC2010 cohort. eBioMedicine 2023, 94, 104699. [Google Scholar] [CrossRef]
- van Larebeke, N.; Koppen, G.; De Craemer, S.; Colles, A.; Bruckers, L.; Den Hond, E.; Govarts, E.; Morrens, B.; Schettgen, T.; Remy, S.; et al. Per- and polyfluoroalkyl substances (PFAS) and immune system-related diseases: Results from the Flemish Environment and Health Study (FLEHS) 2008–2014. Environ. Sci. Eur. 2023, 35, 28. [Google Scholar] [CrossRef]
- Chen, L.; Tong, C.; Huo, X.; Zhang, J.; Tian, Y. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: A longitudinal cohort with repeated measurements. Chemosphere 2021, 267, 128899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Rifas-Shiman, S.L.; Aris, I.; Fleisch, A.; Oken, E.; Hivert, M.-F. Abstract 64: Prenatal Per- and Polyfluoroalkyl Substance (PFAS) Exposures, Individually and as a Mixture, Are Associated With Obesity Risk at 16-20 Years in the Project Viva Prospective Cohort: Implications for PFAS as Hazardous Substances for Developmental Health. Circulation 2023, 147, A64. [Google Scholar] [CrossRef]
| Author(s), Year. [Reference] | DNA Methylation Measurement | Primary Findings | Study Design | Population Characteristics | Exposure |
| Villanger et al., 2023 [67] | - Biological sample used: Blood from pregnant women and cord blood from newborn children - Specific methylation types measured: 5-methylcytosine (5-mC) and 5-hydroxymethylcytosine (5-hmC) - Adjusted elastic net regression and quantile g-computation approach were used for analysis | - Specific PFAS compounds showing significant associations: PFHxS (important for 5-mC in both mothers and infants), PFOS (important for 5-hmC in mothers) - No joint effect of PFAS mixtures on DNA methylation markers - Subgroup analyses: Relationship varied with seafood intake and PFDA concentrations for mothers; maternal education level, seafood intake, and smoking during pregnancy for infants - No specific numbers of statistically significant methylation sites, direction of changes, statistical significance levels, or effect sizes/regression coefficients provided | Prospective cohort study; Mother-infant cohort study (part of the Norwegian Mother, Father, and Child Cohort Study) | - Total number of participants: 634 pregnant women - Number of mother-infant pairs: 634 - Geographical location of the study: Norway Recruitment period: 2010–2014 - Maternal characteristics: Gestation week ~18 | - Specific PFAS compounds measured: PFHxS, PFOS (seven PFAS measured, but only these two specified) - Biological sample used for PFAS measurement: maternal blood, cord blood - Timing of exposure measurement: gestation week ~ 18 |
| Robinson et al., 2021 [68] | - Biological sample used: DNA extracted from dried blood spots (DBS) - Measurement technique: Infinium MethylationEPIC BeadChip - Specific genomic regions or genes analyzed: Individual CpG sites, including cg15557840 near SCRT2, SRXN1; cg19039925 in GVIN1 in boys; cg05754408 in ZNF26 in girls; cg03278866 within PTBP1 - Methylation quantification method: Robust linear regression examining associations with DNA methylation at individual CpG sites - Unique aspect: Analysis included 2242 CpG sites identified as Correlated Regions of Systemic Interindividual Variation (CoRSIVs) | - Number of statistically significant methylation sites: 4 (cg15557840, cg19039925, cg05754408, cg03278866) - Specific PFAS compounds showing significant associations: PFOA and PFOS - Statistical significance levels: FDR < 0.05 - Sex-specific analyses: PFOS associated with cg19039925 in boys and cg05754408 in girls - Notable non-significant trends: Limited evidence of association overall | Cohort study; Mother-infant cohort study | - Recruitment period: 2008 and 2010 - Total number of participants: 597 neonates - Infant characteristics: Sex and plurality mentioned as covariates | - Specific PFAS compounds measured: PFOA, PFOS - Biological sample used for PFAS measurement: Newborn dried blood spots (DBS) - Measurement method/technique: High-performance liquid chromatography/tandem mass spectrometry (LC-MS/MS) - Timing of exposure measurement: At birth (implied from newborn DBS) - Concentration ranges or summary statistics: >90th percentile concentrations were analyzed |
| Miura et al., 2018 [69] | - Biological sample used: Cord blood - Specific methylation types measured: 5-methylcytosine - Measurement technique: Illumina HumanMethylation 450 BeadChip - Specific genomic regions or genes analyzed: 485,577 CpGs across the genome - Methylation quantification method: Beta-values calculated from signal intensities - Unique aspects: Identification of differentially methylated regions (DMRs) using bumphunter function | - Number of statistically significant methylation sites: Four DMPs with FDR < 0.05 - Specific PFAS compounds showing significant associations: PFOS and PFOA - Direction of methylation changes: Up-methylation for PFOS, down-methylation for PFOA - Statistical significance levels: FDR < 0.05 for DMPs, FWER < 0.1 for DMRs | Prospective cohort study; Mother-child cohort study | Number of mother-infant pairs: 190 - Geographical location of the study: Sapporo, Japan - Recruitment period: 2002–2005 Inclusion/exclusion criteria: Inclusion—pregnant women at 23–35 weeks of gestation; Exclusion—miscarriage, stillbirth, relocation, voluntary withdrawal, multiple births - Maternal characteristics: Average age 29.7 ± 4.8 years - Infant characteristics: Sex distribution—44.2% male | - Specific PFAS compounds measured: PFOS, PFOA - Biological sample used for PFAS measurement: Maternal serum - Measurement method/technique: Column-switching liquid chromatography-tandem mass spectrometry (LC-MS/MS) - Timing of exposure measurement: Between 24 and 41 weeks of gestational age - Concentration ranges or summary statistics: Median PFOS: 5.2 ng/mL (3.8 to 7.1), Median PFOA: 1.4 ng/mL (0.9 to 2.1) |
| Liu et al., 2021 [70] | - Biological sample used: Cord blood at delivery and peripheral leukocyte DNA at age 12 years - Measurement technique: Illumina HumanMethylation EPIC BeadChip - Specific genomic regions or genes analyzed: Loci mapped to genes such as AGAP1, HPSE2, HABP2, RNF13, RADIL, and TMEM56 - Methylation quantification method: Associations analyzed using generalized estimating equations | - Number of statistically significant methylation sites: 35 - Specific PFAS compounds showing significant associations: PFOS (5 loci), PFOA (10 loci), PFHxS (7 loci), PFNA (13 loci) - Statistical significance levels: q-value 0.05 for overall loci, q-value 0.01 for specific loci | Prospective mother-child cohort study | - Total number of participants: 532 (266 mothers and 266 children) - Number of mother-infant pairs: 266 - Geographical location of the study: Cincinnati, OH - Recruitment period: 2003–2006) - Maternal characteristics: Gestational age at serum measurement ~ 16 weeks | - Specific PFAS compounds measured: PFOA, PFOS, PFNA, PFHxS - Biological sample used for PFAS measurement: Maternal serum - Timing of exposure measurement: ~16 weeks gestation |
| Everson et al., 2025 [17] | - Biological sample used: Human placental tissues - Specific methylation types measured: Implied 5-methylcytosine (via bisulfite conversion) - Measurement technique: Illumina MethylationEPIC Beadarray - Specific genomic regions or genes analyzed: Epigenome-wide (broad analysis across the genome) - Methylation quantification method: Functional normalization and beta-mixture quantile (BMIQ) normalization | - Number of statistically significant methylation sites: 23 loci - Specific PFAS compounds showing significant associations: PFHxS (11 loci), PFNA (5 loci), PFOS (4 loci), PFOA (2 loci), PFDA (1 locus) - Direction of methylation changes: Both increased and decreased (6 loci increased, 6 loci decreased) - Statistical significance levels: FDR q-values < 0.05 - Sex-specific analyses: More methylation perturbations in females than males, particularly for PFHxS and PFOS | Prospective longitudinal observational cohort study; Mother-infant cohort study | - Total number of participants: 151 - Number of mother-infant pairs: 151 - Geographical location of the study: Little Rock, Arkansas - Recruitment period: 2010 to 2014 - Inclusion/exclusion criteria: Inclusion—mothers at least 21 years old, second parity; Exclusion—pre-existing medical conditions, sexually transmitted infections, medical complications, smoking or alcohol use during pregnancy, medication use known to influence fetal growth, conceptions aided with fertility treatment - Maternal characteristics: Mean age 30.5 years (SD = 3.42), most had a college degree, most self-identified as White - Infant characteristics: 63 females and 88 males, average gestation of 39.3 weeks (range 36.4–41.4 weeks) | - Specific PFAS compounds measured: PFHxA, PFHxS, PFHpA, PFOA, PFOS, PFOSA, MePFOSAA, PFNA, PFDA, PFDS, PFUnDA, PFDoDA, PFPeA, EtPFOSAA, HFPO-DA (Gen X), PFHpS, PFBS - Biological sample used for PFAS measurement: Human placental tissue - Measurement method/technique: High-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) with electrospray ionization - Timing of exposure measurement: Recruitment of pregnant women prior to gestational week 10 (2010–2014) - Concentration ranges or summary statistics: Five PFAS (PFHxS, PFOS, PFOA, PFNA, and PFDA) were detectable in over 70% of placental samples; PFOS had the highest average concentrations |
| Xie et al., 2024 [71] | - Biological sample used: Placental tissue - Specific methylation types measured: Likely 5-methylcytosine (inferred from bisulfite sequencing) - Measurement technique: Reduced representation bisulfite sequencing for genome-wide analysis; bisulfite amplicon sequencing for targeted gene analysis - Specific genomic regions or genes analyzed: CHST7, FGF13, IRS4, PHOX2A, PLXDC1 - Methylation quantification method: Sequencing | - Specific PFAS compounds showing significant associations: PFOA, PFNA, PFTrDA, PFDoA - Direction of methylation changes: - PFOA associated with hypomethylation of IRS4 and PLXDC1 - PFNA associated with hypomethylation of PLXDC1 - Positive associations (increased methylation) of CHST7 with PFTrDA and IRS4 with PFDoA and PFTrDA | Prospective cohort study; Mother-infant cohort study | - Total number of participants: implied 690 from 345 mother-infant pairs) - Recruitment period: April–December 2012 - Number of mother-infant pairs: 345 - Maternal characteristics: (PFAS measured during early pregnancy) - Infant characteristics: (development assessed at six months) | - Specific PFAS compounds measured: PFOA, PFNA, PFTrDA, PFDoA - Biological sample used for PFAS measurement: maternal plasma - Timing of exposure measurement: Early pregnancy |
| Liu et al., 2021 [61] | - Biological sample used: Cord blood and peripheral leukocytes at 12 years of age - Specific methylation types measured: likely 5-methylcytosine at CpG sites - Measurement technique: Illumina HumanMethylation EPIC BeadChip - Specific genomic regions or genes analyzed: CpG sites associated with cancers, cognitive health, cardiovascular disease, and kidney function - Methylation quantification method: Associations analyzed using linear regression with generalized estimating equations | - Number of statistically significant methylation sites: 435 CpG sites - Specific PFAS compounds showing significant associations: PFOS (2 CpGs), PFOA (12 CpGs), PFHxS (8 CpGs), PFNA (413 CpGs) - Statistical significance levels: q < 0.05 - Subgroup analyses: Little evidence of age-specific differences | Prospective longitudinal mother-child cohort study | - Geographical location of the study: Cincinnati, Ohio - Recruitment period: 2003–2006 | - Specific PFAS compounds measured: PFOA, PFOS, PFNA, PFHxS - Biological sample used for PFAS measurement: Maternal serum - Timing of exposure measurement: During pregnancy |
| Petroff et al., 2023 [20] | - Biological sample: Cord blood (nucleated cells such as leukocytes and nucleated red blood cells) - Specific methylation types measured: 5-methylcytosine (5-mC) and 5-hydroxymethylcytosine (5-hmC) - Measurement technique: Illumina MethylationEPIC BeadChip - Specific genomic regions or genes analyzed: Over 850,000 CpG sites - Methylation quantification method: MLML method for estimating 5-mC, 5-hmC, and unmethylated cytosines - Unique aspects: Use of oxidative bisulfite conversion to specifically measure 5-hmC | - Number of statistically significant methylation sites: - Total methylation: PFHxS 12 sites; PFOS 19 sites; PFOA 2 sites; PFNA 3 sites; PFDA 4 sites. - 5-mC and 5-hmC: Thousands of sites for PFHxS, PFOS, PFNA, PFDA, PFUnDA, and MeFOSAA. - Specific PFAS compounds showing significant associations: PFHxS, PFOS, PFOA, PFNA, PFDA, PFUnDA, MeFOSAA. - Direction of methylation changes: Decreased 5-hmC and increased 5-mC. - Statistical significance levels: q < 0.05. - Sex-specific analyses: Significant sex interactions for all PFAS; specific numbers of significant sites in males and females | Prospective birth cohort study; Mother-infant cohort study | - Total number of participants: 309 - Number of mother-infant pairs: 288 - Geographical location of the study: University of Michigan Von Voigtlander Women’s Hospital - Recruitment period: 2010 to 2019 - Inclusion/exclusion criteria: Inclusion—at least 18 years old, singleton pregnancy, between 8 and 14 weeks gestation, intended delivery at the University of Michigan Hospital - Maternal characteristics: Average age: 31.8 years, Mean baseline weight: 69–70 kg, Average baseline BMI: 25.5–25.8 - Infant characteristics: Sex distribution—Female: 72, Male: 69 | - Specific PFAS compounds measured: MeFOSAA, PFOSA, PFHxS, PFHpA, PFOA, PFOS, PFDA, PFNA, PFUnDA - Biological sample used for PFAS measurement: Maternal plasma - Measurement method/technique: On-line solid phase extraction coupled with high-performance liquid chromatography-isotope dilution tandem mass spectrometry (LC-MS/MS) - Timing of exposure measurement: First trimester - Concentration ranges or summary statistics: Geometric mean concentrations were 3.2 µg/L for PFHxS, 5.3 µg/L for PFOS, 1.1 µg/L for PFOA, 0.37 µg/L for PFNA, and 0.12 µg/L for PFDA |
| Starling et al., 2020 [72] | - Biological sample: Umbilical cord blood - Measurement technique: Illumina HumanMethylation450 array - Specific genomic regions or genes analyzed: DMPs and DMRs, including genes such as TJAP1, RPTOR, PON1, PON3, CIDEB, NR1H2, RASL11B, RNF39 - Methylation quantification method: Evaluation of DMPs at FDR < 0.05 and identification of DMRs using comb-p with Šidák-adjusted p < 0.05 | - Number of statistically significant methylation sites: 1 DMP (cg18587484) - Specific PFAS compounds showing significant associations: PFOA is mentioned - Statistical significance levels: FDR < 0.05 for DMP; Šidák-adjusted p < 0.05 for DMRs | Prospective cohort study; Mother-infant cohort study | - Total number of participants: 583 mother-infant pairs - Number of mother-infant pairs: 583 - Geographical location of the study: (suggested U.S. based on context) - Recruitment period: 2009–2014 - Maternal characteristics: PFAS measured at median 27 weeks of gestation | - Specific PFAS compounds measured: PFOA is mentioned - Biological sample used for PFAS measurement: Maternal serum - Timing of exposure measurement: Median 27 weeks of gestation - Concentration ranges or summary statistics: Below the median for females in the U.S. general population |
| Kobayashi et al., 2017 [73] | - Biological sample used: Cord blood DNA - Specific methylation types measured: likely 5-methylcytosine due to bisulfite sequencing - Measurement technique: Bisulfite pyrosequencing - Specific genomic regions or genes analyzed: Two differentially methylated regions (DMRs) within IGF2/H19 locus, as well as LINE1 - Methylation quantification method: Bisulfite pyrosequencing | - Number of statistically significant methylation sites: 1 (IGF2) - Specific PFAS compounds showing significant associations: PFOA - Direction of methylation changes: Decreased - Statistical significance levels: ß = −1.61, 95% CI: −3.00 to −0.22 (for PFOA and IGF2) - Effect sizes or regression coefficients: ß = −1.61 for IGF2 with PFOA - No sex-specific or subgroup analyses were mentioned | Prospective mother-child cohort study | - Total number of participants: 514 pregnant women - Number of mother-infant pairs: 235 - Geographical location of the study: Sapporo, Japan - Recruitment period: 2002–2005 | - Specific PFAS compounds measured: PFOA, PFOS - Biological sample used for PFAS measurement: maternal serum - Measurement method/technique: LC–MS/MS - Concentration ranges or summary statistics: Median concentrations of PFOS: 5.0 ng/mL, PFOA: 1.4 ng/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulkadir, A.; Kandel, S.; Lewis, N.; D’Auvergne, O.; Rosby, R.; Hossain, E. Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health. Int. J. Environ. Res. Public Health 2025, 22, 917. https://doi.org/10.3390/ijerph22060917
Abdulkadir A, Kandel S, Lewis N, D’Auvergne O, Rosby R, Hossain E. Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health. International Journal of Environmental Research and Public Health. 2025; 22(6):917. https://doi.org/10.3390/ijerph22060917
Chicago/Turabian StyleAbdulkadir, Abubakar, Shila Kandel, Niya Lewis, Oswald D’Auvergne, Raphyel Rosby, and Ekhtear Hossain. 2025. "Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health" International Journal of Environmental Research and Public Health 22, no. 6: 917. https://doi.org/10.3390/ijerph22060917
APA StyleAbdulkadir, A., Kandel, S., Lewis, N., D’Auvergne, O., Rosby, R., & Hossain, E. (2025). Epigenetic Consequences of In Utero PFAS Exposure: Implications for Development and Long-Term Health. International Journal of Environmental Research and Public Health, 22(6), 917. https://doi.org/10.3390/ijerph22060917






