4.2. Heavy Metal Concentrations in Water, Sediment and O. niloticus
HM levels in the water exceeded Thailand’s surface water quality standards. Cd concentrations in sediment samples surpassed Thailand’s soil quality standards. HM concentrations were higher in sediment than in water, likely due to human activities and ecological factors around the landfill, which contribute to HM accumulation in sediments. Additionally,
O. niloticus from the reference site exhibited higher HM concentrations than those from the landfill site. Notably, Pb levels (0.69 mg/kg) (
Table 6) in
O. niloticus exceeded Thailand’s food quality standards (0.5 mg/kg) [
32]. Consequently, these HMs are deposited in sediments and accumulate in
O. niloticus.
There are two main ways that HMs can enter aquatic organisms: either by direct ingestion of water and biota or by non-dietary absorption through the skin and gills [
11]. According to research, fish exposed to HM may experience changes in DNA structure and chromosomal damage [
12,
13]. Furthermore, by upsetting the equilibrium between the generation and removal of free radicals, HMs cause oxidative stress [
14].
Numerous studies have documented HM accumulation in water, sediment, and fish. Intamat et al. [
39] also reported that As levels in landfill-associated fish species, such as
Barbonymus gonionotus,
Raiamas tornieri,
Anabas testudineus, and
Oreochromis niloticus, exceeded Thailand’s food quality standards. Additionally, Noudeng et al. [
40] found that while leachate metal concentrations remained within international standards, Cd, Cr, and Pb levels in fish from a Laos landfill exceeded permissible limits. Sriuttha et al. [
41] reported that Cr concentrations in
A. testudineus and
R. tornieri, along with Cd levels in
B. gonionotus and
O. niloticus, exceeded international standards set by organizations such as the FAO and USA. The basic principles of EU legislation on contaminants in food are laid down in Council Regulation 315/93/EEC: Food. This regulation contains contaminants and their unacceptable amounts from the public health viewpoint and, in particular, at a toxicological level. Maximum levels must be set for certain contaminants in order to protect public health. Maximum levels for certain contaminants in food are set in Commission Regulation (EU) 2023/915. Maximum levels in certain foods are set for the following contaminants: metals (Pb, Cd, Hg, As). Conversely, Aytekin et al. [
42] observed the lowest accumulation of Cd, Cr, and Pb in fish muscle. Additionally, Sriuttha et al. [
41] found that Pb levels in
R. tornieri,
A. testudineus,
O. niloticus, and
B. gonionotus surpassed international guidelines. Aly et al. [
43] noted that Fe, Zn, Cu, Cd, and Pb levels in
O. niloticus remained within WHO standards; however, Pb concentrations in water samples exceeded the Egyptian chemical standards (1993) at Ismailia Canal, Egypt. Waichman et al. [
44] investigated HM concentrations (Cd, Mn, Fe, Ni, Cu, Zn, As, Cr, Pb, and Hg) in 11 fish samples from the Brazilian Amazon River and found that Cr and Hg levels in fish meat exceeded the Brazilian safety limit for human consumption.
4.3. BAFs of Heavy Metals in the Fish Samples
The BAF is more ecologically relevant as it accounts for environmental exposure. The relative order of BAF values for HMs in fish absorbed from water was Cr > Pb > As > Cd. When calculating the BAF for water, fish containing Cr exhibited a BAF value greater than 1. This suggests that Cr has the potential for bioaccumulation from the water. As can damage the integument, nervous system, and digestive organs [
45]. Thitiyan et al. [
46] reported that
Barbonymus gonionotus can accumulate low concentrations of As, Cr, Cd, and Pb from both soil and water. Similarly, Sriuttha et al. [
41] observed BAF values exceeding 1 for Cd, Cr, and Pb in
Anabas testudineus,
Rasbora tornieri, and
Oreochromis niloticus in a reservoir near a municipal landfill. Vaseem et al. [
47] conducted an experiment to examine the effects of Cr, Pb, and Cu contamination in water on their accumulation in the gills, skin, muscle, and liver of
Labeo rohita. These findings highlight the potential health risks posed by HM accumulation in fish, particularly for consumers who regularly ingest contaminated fish. Thus, a comprehensive health risk assessment is crucial to ensure food safety by estimating HM levels in consumed fish [
48,
49,
50,
51]. Kumar and Sharma [
52] reported that Cd can damage genetic material, impair development and fertility, and negatively affect the nervous system. Cr exposure can cause acute symptoms such as vomiting, abdominal pain, diarrhea, and stomach bleeding. Chronic exposure may lead to skin irritation, ulcers, osteoporosis, or cancer. The toxicity of Cr depends on its oxidation state, with Cr⁶
+ being more harmful than Cr
3+ [
53,
54]. Additionally, prolonged exposure to Cr can result in lung cancer and death. Pb can also cause significant harm, affecting the kidneys, intestines, hemoglobin production, brain, and nerve cells [
55].
4.4. Possible Harmful Effects of Heavy Metals on Health via Fish Consumption
Heavy metal (HM) pollution poses health risks through fish consumption, a key food source in Thailand. As, Cd, Cr, and Pb are particularly hazardous metals [
3]. The relative EDI values followed the order: Cr > Pb > Cd > As. All EDI values were below the provisional maximum tolerable daily intake levels for As (0.42 μg/kg/day), Cd (1 μg/kg/day), Cr (100 μg/kg/day), and Pb (3.57 μg/kg/day). This indicates no significant health risk from HM exposure through the sampled fish. For
O. niloticus consumption, all detected HM levels had HRI values below 1, based on the oral reference dose, suggesting it is generally safe for the local population. However, Pb concentrations in
O. niloticus exceeded the recommended food safety levels, warranting caution. HM contamination in Thailand’s Nam Phong River, which is located near a municipal landfill. Local residents frequently consume aquatic plants and animals from the river, leading to HM accumulation in their bloodstream [
56,
57,
58]. The United States Environmental Protection Agency (USEPA) classifies As and Pb as carcinogenic based on their CR values. According to USEPA (2015), CR values exceeding 1 × 10
−4 are generally considered unacceptable [
59]. In this study, the CR value of Pb was above the unacceptable threshold, while the CR value of As remained below it. Specifically, Pb contamination in
O. niloticus exceeded the acceptable limit, whereas As levels did not pose a significant risk. Even low-level Pb exposure can lead to subtle neurological changes and has been associated with various cancers, including those of the skin, lungs, liver, prostate, and bladder. Additionally, Pb toxicity has been linked to diabetes, neurological disorders, cardiovascular diseases, and reproductive issues [
60,
61]. Therefore, in this study area, local residents consuming
O. niloticus from the Nam Phong River are at risk of developing cancer due to Pb contamination. The CR values suggest that consuming
O. niloticus near the landfill site may pose significant health risks to the local population.
4.5. Genetic Differentiation
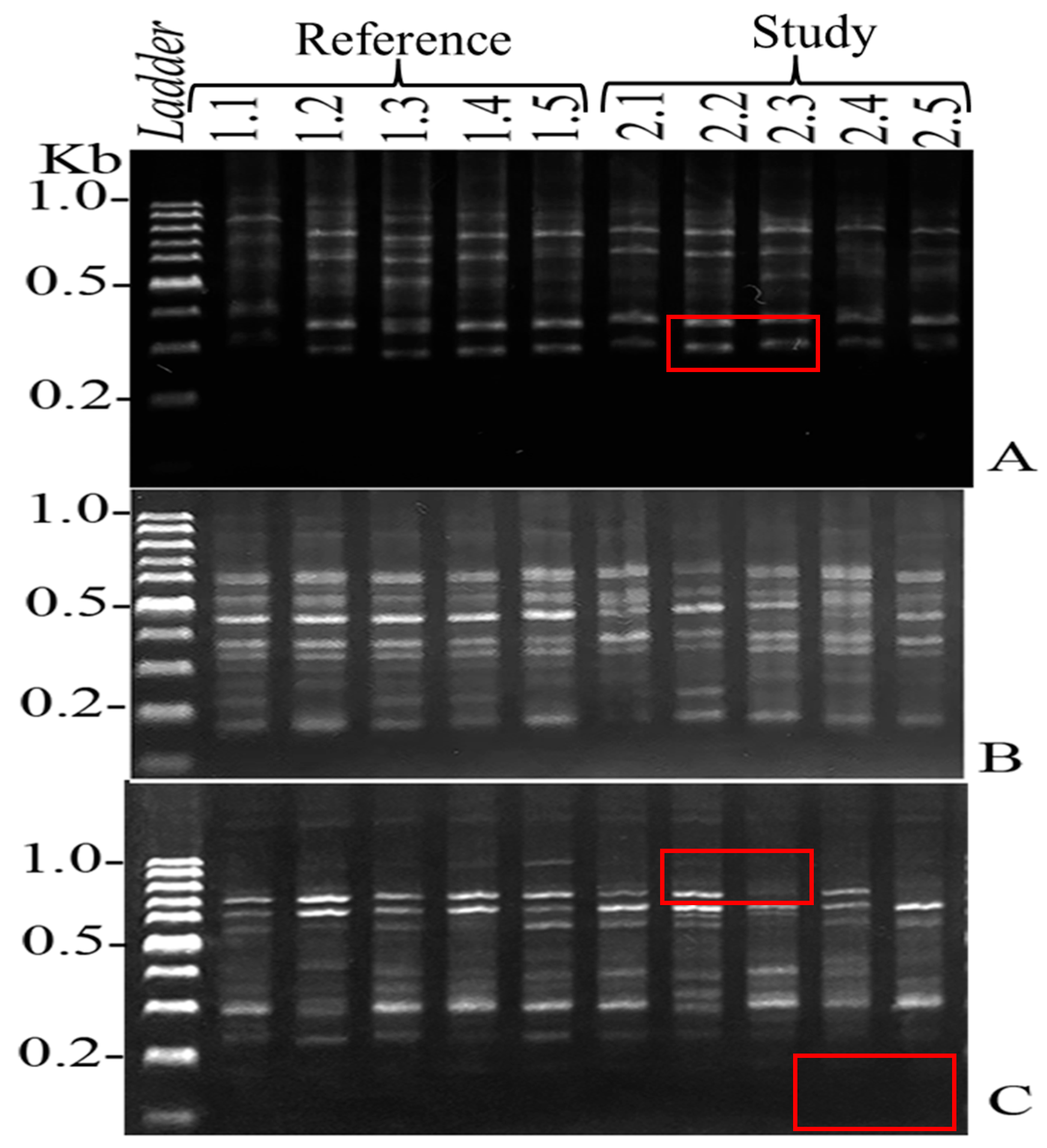
A total of 621 DNA bands were produced by the ISSR patterns of 13 successful primers, of which 56 bands were unique. The %GTS of each individual
O. niloticus in the reference area and municipal landfill is displayed in
Table 9.
O. niloticus from the municipal landfill had a %GTS range of 46.34 to 71.67%, whereas
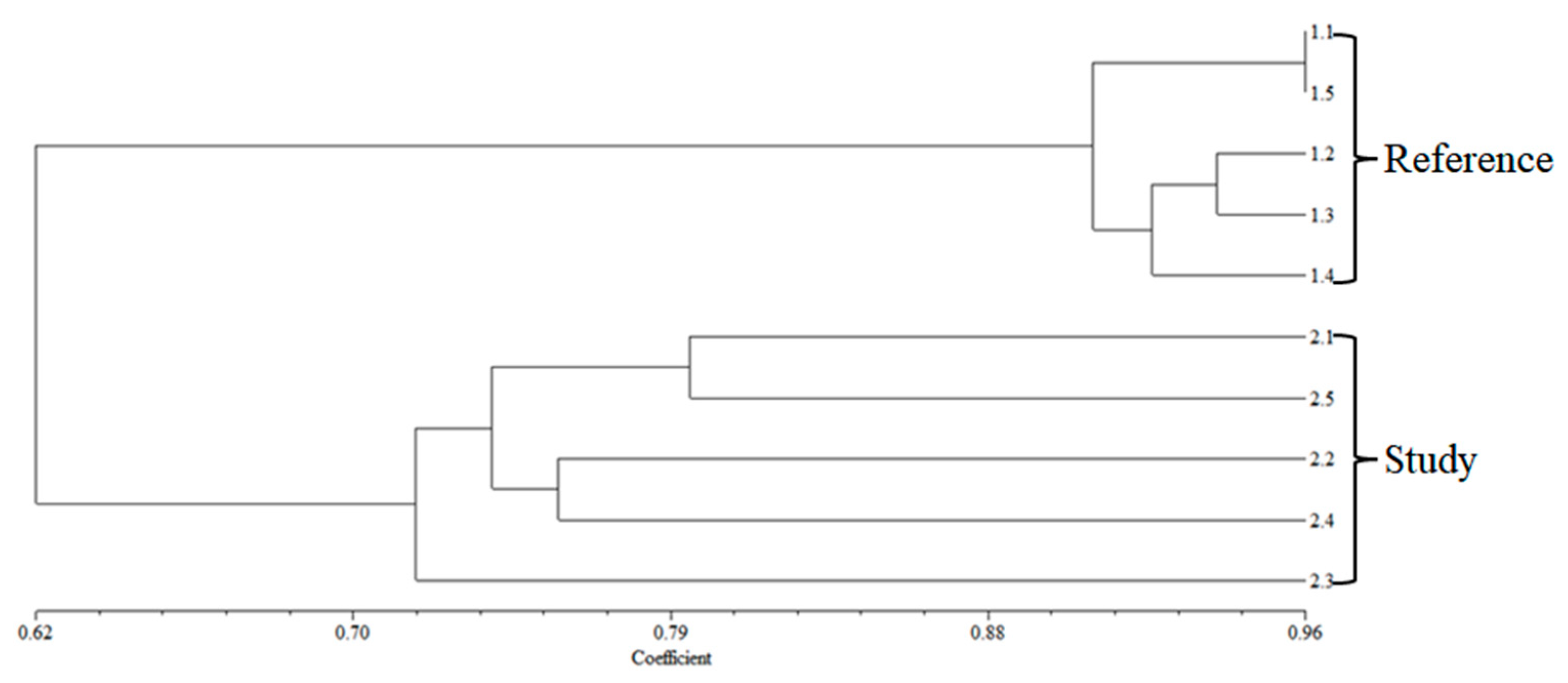
O. niloticus from the reference area had a range of 87.34 to 96.00%. In this study, the dendrogram results categorized the fish DNA samples into two distinct groups according to the sampling locations examined in
Figure 2 and
Figure 3. The findings indicate that metal accumulations in fish from landfills may impact their genotoxicity, as evidenced by alterations in the DNA bands. In environments contaminated with HMs, the metal exposure can result in genotoxic effects in fish, characterized by various forms of DNA damage. These effects may include single- and double-strand breaks, disruptions in DNA repair mechanisms, oxidation of nucleobases, and the formation of DNA-protein cross-links [
62,
63,
64]. Studies on the interaction of HMs with cell membranes demonstrated genotoxicity through multiple mechanisms. For instance, Jha et al. [
65] reported that As induced DNA alterations in
Channa punctatus. Cd has been linked to oxidative stress, ploidy changes, gene base oxidation, DNA damage, mutagenesis, deletions, and point mutations [
66,
67]. Cr has been shown to cause nitrogenous base alterations, DNA strand breaks, and the formation of protein-Cr-DNA adducts [
68,
69,
70]. Pb has been associated with carcinogenic events, DNA damage, oxidative stress, changes in gene transcription, and increased mitogenesis [
71]. The loss of DNA structural or functional integrity in exposed organisms could have adverse effects at both individual and population levels, particularly in terms of growth and reproduction [
71,
72,
73]. In this study, experimental
O. niloticus demonstrates potential as a valuable genotoxic indicator in aquatic ecosystems.
4.6. Oxidative Stress
Oxidative stress is the result of an imbalance between antioxidants and ROS products and affects DNA molecules and cellular enzymes [
26]. Accumulation of HMs and metalloid ions in organisms induces free radicals such as superoxide, hydrogen peroxide, and hydrogen ions, which can have potential effects on the health of organisms [
25,
74]. In this study, the liver of fish will be measured for MDA, H
2O
2, SOD, and CAT as oxidative stress biomarkers to monitor the extent of oxidative damage to the fish liver by HMs. The levels of MDA, H
2O
2, SOD, and CAT in fish collected from the landfill site were significantly different (
p < 0.05) from those in fish collected from the reference area (
Table 10).
MDA is a major byproduct of polyunsaturated fatty acid peroxidation in cellular membranes, and its levels rise with increased free radical activity. Measuring MDA concentration provides insight into the extent of oxidative damage caused by toxic substances [
75,
76]. This study found that fish from the landfill site had higher MDA concentrations compared to those from the reference site. Research has shown that toxic substances can accumulate in organisms, leading to elevated MDA levels [
25,
77,
78]. Thitiyan et al. [
46] observed a significant increase in liver MDA levels in
Barbonymus gonionotus after exposure to As, Cd, Cr, and Pb.
H
2O
2 is a crucial precursor to the hydroxyl radical (OH), the most harmful reactive oxygen species (ROS) within cells [
26]. This study revealed that fish from the landfill site had higher H
2O
2 concentrations than those from the reference site. The accumulation of HMs in fish contributed to increased H
2O
2 levels. Thitiyan et al. [
46] reported that
B. gonionotus from a municipal landfill site exhibited higher H
2O
2 concentrations than fish from a reference site, with an increasing trend linked to elevated As, Cd, Cr, and Pb levels. Similarly, Cd accumulation in
Channa striata led to higher H
2O
2 concentrations at the study site compared to the reference site [
26]. Additionally, Cd (VI) exposure resulted in increased H
2O
2 levels in
Channa punctatus [
30,
79].
CAT and SOD levels were lower in the liver of
O. niloticus from the landfill site compared to those from the reference site. These enzymes play a crucial role in the antioxidant defense system of organisms, helping to counteract the harmful effects of free radicals on vital biomolecules and tissues [
80]. Specifically, SOD catalyzes the dismutation of superoxide anions into H
2O
2, while CAT subsequently breaks down H
2O
2 into oxygen and water [
81].
Weber et al. [
82] reported that
Hoplias intermedius exposed to As, Pb, and Ni from tin mining waste exhibited reduced SOD and CAT levels compared to reference fish. As oxidative stress biomarkers influenced by As, Cd, Cr, and Pb, the levels of SOD, CAT, and MDA in
Barbonymus gonionotus from a reservoir near a municipal landfill could serve as indicators for monitoring metal- and metalloid-induced oxidative stress. Similarly, Fatima et al. [
83] found that
Channa striata and
Heteropneustes fossilis accumulated Pb, Cd, Cr, and Ni from the Kali River in Northern India, showing decreased SOD and CAT levels compared to reference fish.
Additionally, variations in oxidative stress biomarkers among fish species can be influenced by habitat differences, water quality parameters, feeding habits, species-specific responses, and the concentration and toxicity of contaminants.
A review of the literature data show that HMs, particularly Pb, can easily be absorbed and harms fish due to several mechanisms and factors, including high bioavailability in water. Pb exists in various soluble forms (e.g., Pb
2+ ions) that can easily be absorbed by fish through their gills and digestive systems. It has an affinity for biological molecules. Pb binds readily to proteins, enzymes, and cellular components, interfering with normal biological processes and making it harder for fish to excrete [
84]. Pb also has an affinity for biological molecules. Pb binds readily to proteins, enzymes, and cellular components, interfering with normal biological processes and making it harder for fish to excrete [
85]. HM concentrations are also impacted by environmental conditions, including pH levels, water hardness, and temperature. Lower pH increases metal solubility, enhancing absorption. Soft water has fewer competing ions (e.g., Ca
2+, Mg
2+), making heavy metals more bioavailable. Higher temperatures increase fish metabolism and uptake rates [
86]. Pb is also persistent in the environment. Unlike some metals that can be naturally cycled or diluted, Pb is highly persistent in ecosystems, leading to continuous exposure for fish populations [
87].