Exposure to Air Pollution and Changes in Resting Blood Pressure from Morning to Evening: The MobiliSense Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Resting BP Assessment
2.3. Exposure to PM2.5, NO, NO2, CO and O3
2.4. Accelerometry
2.5. Activity Profiling
2.6. Noise Exposure
Noise Prediction for Day 2
2.7. Other Covariates
2.8. Statistical Analysis
2.8.1. Linear Multilevel Models
2.8.2. Mixture Models
2.8.3. Sensitivity Analysis
3. Results
3.1. Population Characteristics
3.2. Linear Multilevel Models
3.3. Mixture Models
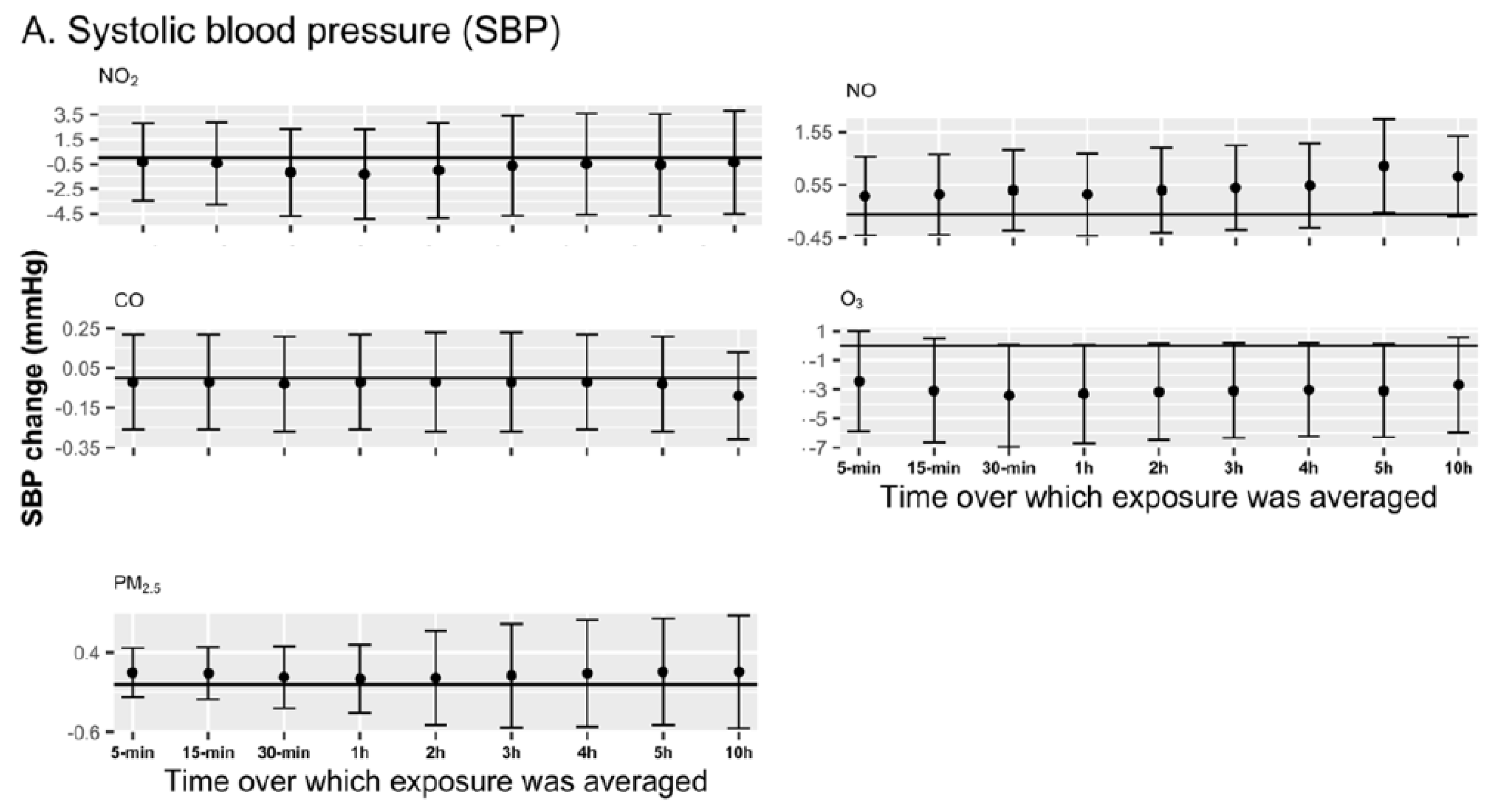
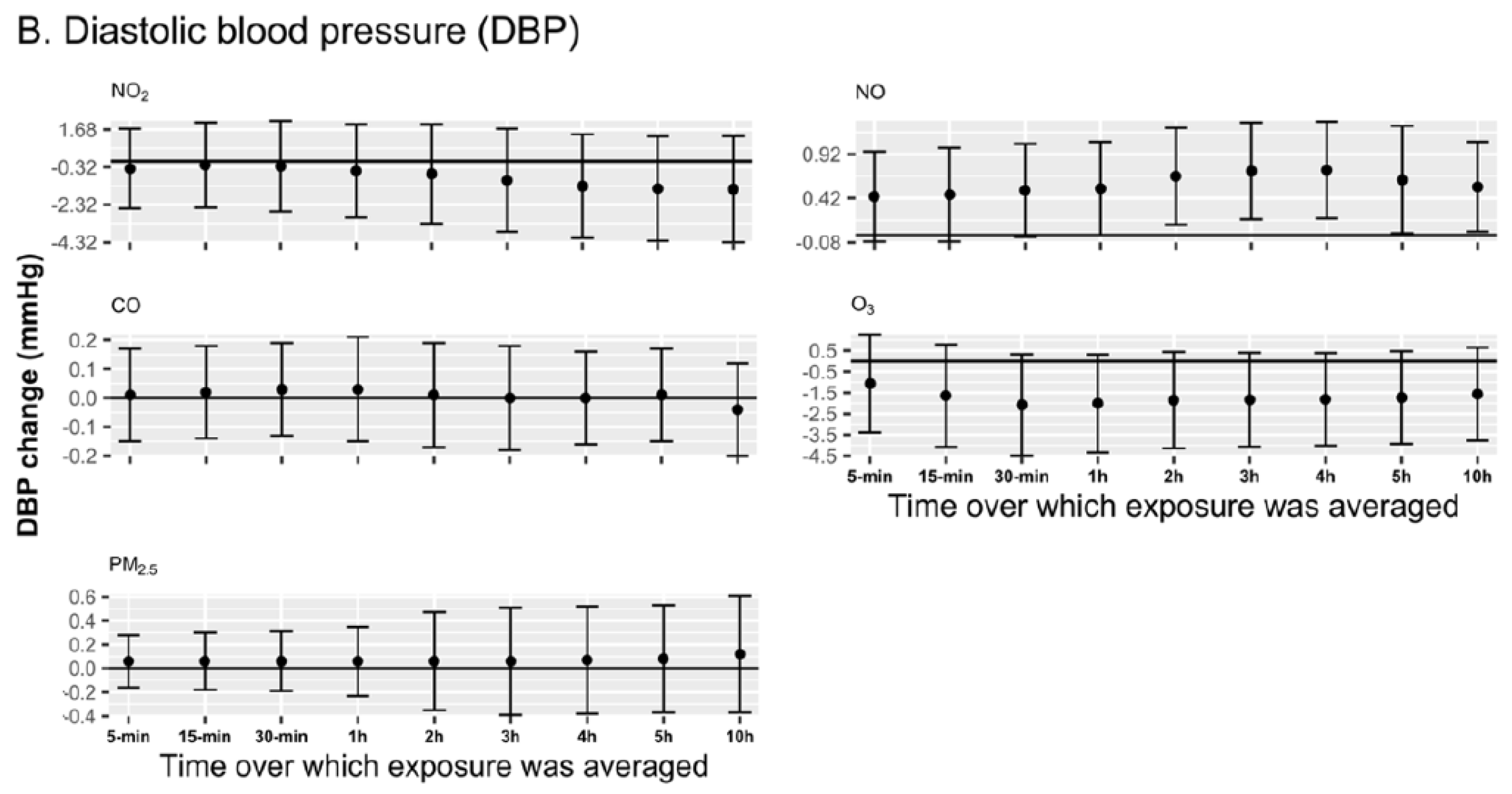
3.4. Sensitivity Analyses
4. Discussion
4.1. Strengths and Limitations
4.2. Conclusion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kannel, W.B. Role of Blood Pressure in Cardiovascular Disease: The Framingham Study. Angiology 1975, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Staplin, N.; de la Sierra, A.; Ruilope, L.M.; Emberson, J.R.; Vinyoles, E.; Gorostidi, M.; Ruiz-Hurtado, G.; Segura, J.; Baigent, C.; Williams, B. Relationship between clinic and ambulatory blood pressure and mortality: An observational cohort study in 59,124 patients. Lancet 2023, 401, 2041–2050. [Google Scholar] [CrossRef]
- Dvonch, J.T.; Kannan, S.; Schulz, A.J.; Keeler, G.J.; Mentz, G.; House, J.; Benjamin, A.; Max, P.; Bard, R.L.; Brook, R.D. Acute Effects of Ambient Particulate Matter on Blood Pressure: Differential Effects Across Urban Communities. Hypertension 2009, 53, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [PubMed]
- Choi, Y.-J.; Kim, S.-H.; Kang, S.-H.; Yoon, C.-H.; Lee, H.-Y.; Youn, T.-J.; Chae, I.-H.; Kim, C.-H. Reconsidering the cut-off diastolic blood pressure for predicting cardiovascular events: A nationwide population-based study from Korea. Eur. Heart J. 2019, 40, 724–731. [Google Scholar] [CrossRef]
- Sanidas, E.; Papadopoulos, D.P.; Grassos, H.; Velliou, M.; Tsioufis, K.; Barbetseas, J.; Papademetriou, V. Air pollution and arterial hypertension. A new risk factor is in the air. J. Am. Soc. Hypertens. 2017, 11, 709–715. [Google Scholar] [CrossRef]
- Brook, R.D.; Weder, A.B.; Rajagopalan, S. “Environmental Hypertensionology” The Effects of Environmental Factors on Blood Pressure in Clinical Practice and Research: Effects of Environmental Factors on BP. J. Clin. Hypertens. 2011, 13, 836–842. [Google Scholar] [CrossRef]
- Mills, N.L.; Donaldson, K.; Hadoke, P.W.; Boon, N.A.; MacNee, W.; Cassee, F.R.; Sandström, T.; Blomberg, A.; Newby, D.E. Adverse cardiovascular effects of air pollution. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 36–44. [Google Scholar] [CrossRef]
- Mills, N.L.; Törnqvist, H.; Robinson, S.D.; Gonzalez, M.C.; Söderberg, S.; Sandström, T.; Blomberg, A.; Newby, D.E.; Donaldson, K. Air Pollution and Atherothrombosis. Inhal. Toxicol. 2007, 19, 81–89. [Google Scholar] [CrossRef]
- Norris, C.; Goldberg, M.S.; Marshall, J.D.; Valois, M.-F.; Pradeep, T.; Narayanswamy, M.; Jain, G.; Sethuraman, K.; Baumgartner, J. A panel study of the acute effects of personal exposure to household air pollution on ambulatory blood pressure in rural Indian women. Environ. Res. 2016, 147, 331–342. [Google Scholar] [CrossRef]
- Delfino, R.J.; Tjoa, T.; Gillen, D.L.; Staimer, N.; Polidori, A.; Arhami, M.; Jamner, L.; Sioutas, C.; Longhurst, J. Traffic-related Air Pollution and Blood Pressure in Elderly Subjects With Coronary Artery Disease. Epidemiology 2010, 21, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sun, Z.; Ruan, Y.; Yan, J.; Mukherjee, B.; Yang, F.; Duan, F.; Sun, L.; Liang, R.; Lian, H.; et al. Personal Black Carbon Exposure Influences Ambulatory Blood Pressure. Hypertension 2014, 63, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Bista, S.; Chatzidiakou, L.; Jones, R.L.; Benmarhnia, T.; Postel-Vinay, N.; Chaix, B. Associations of air pollution mixtures with ambulatory blood pressure: The MobiliSense sensor-based study. Environ. Res. 2023, 227, 115720. [Google Scholar] [CrossRef]
- Stamler, J.; Rose, G.; Stamler, R.; Elliott, P.; Dyer, A.; Marmot, M. INTERSALT study findings. Public health and medical care implications. Hypertension 1989, 14, 570–577. [Google Scholar] [CrossRef]
- Baumgartner, J.; Schauer, J.J.; Ezzati, M.; Lu, L.; Cheng, C.; Patz, J.A.; Bautista, L.E. Indoor Air Pollution and Blood Pressure in Adult Women Living in Rural China. Environ. Health Perspect. 2011, 119, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Xu, Q.-S.; Park, S.-Y.; Kim, J.-H.; Hwang, S.-S.; Lee, K.-H.; Lee, H.-J.; Hong, Y.-C. Seasonal variation of effect of air pollution on blood pressure. J. Epidemiol. Community Health 2007, 61, 314–318. [Google Scholar] [CrossRef]
- Chuang, K.-J.; Yan, Y.-H.; Cheng, T.-J. Effect of Air Pollution on Blood Pressure, Blood Lipids, and Blood Sugar: A Population-Based Approach. J. Occup. Environ. Med. 2010, 52, 258. [Google Scholar] [CrossRef]
- Day, D.B.; Xiang, J.; Mo, J.; Li, F.; Chung, M.; Gong, J.; Weschler, C.J.; Ohman-Strickland, P.A.; Sundell, J.; Weng, W.; et al. Association of Ozone Exposure With Cardiorespiratory Pathophysiologic Mechanisms in Healthy Adults. JAMA Intern. Med. 2017, 177, 1344–1353. [Google Scholar] [CrossRef]
- Zeng, X.-W.; Qian, Z.; Vaughn, M.G.; Nelson, E.J.; Dharmage, S.C.; Bowatte, G.; Perret, J.; Chen, D.-H.; Ma, H.; Lin, S.; et al. Positive association between short-term ambient air pollution exposure and children blood pressure in China–Result from the Seven Northeast Cities (SNEC) study. Environ. Pollut. 2017, 224, 698–705. [Google Scholar] [CrossRef]
- Dons, E.; Int Panis, L.; Van Poppel, M.; Theunis, J.; Willems, H.; Torfs, R.; Wets, G. Impact of time–activity patterns on personal exposure to black carbon. Atmos. Environ. 2011, 45, 3594–3602. [Google Scholar] [CrossRef]
- Kario, K.; Pickering, T.G.; Umeda, Y.; Hoshide, S.; Hoshide, Y.; Morinari, M.; Murata, M.; Kuroda, T.; Schwartz, J.E.; Shimada, K. Morning surge in blood pressure as a predictor of silent and clinical cerebrovascular disease in elderly hypertensives: A prospective study. Circulation 2003, 107, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Keil, A.P.; Buckley, J.P.; O’Brien, K.M.; Ferguson, K.K.; Zhao, S.; White, A.J. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ. Health Perspect. 2020, 128, 047004. [Google Scholar] [CrossRef] [PubMed]
- Bista, S.; Dureau, C.; Chaix, B. Personal exposure to concentrations and inhalation of black carbon according to transport mode use: The MobiliSense sensor-based study. Environ. Int. 2022, 158, 106990. [Google Scholar] [CrossRef]
- Chaix, B.; Bista, S.; Wang, L.; Benmarhnia, T.; Dureau, C.; Duncan, D.T. MobiliSense cohort study protocol: Do air pollution and noise exposure related to transport behaviour have short-term and longer-term health effects in Paris, France? BMJ Open 2022, 12, e048706. [Google Scholar] [CrossRef]
- Amar, J.; Benetos, A.; Blacher, J.; Bobrie, G.; Chamontin, B.; Girerd, X.; Halimi, J.-M.; Herpin, D.; Mounier-Vehier, C.; Mourad, J.-J.; et al. Mesures de la pression artérielle. Méd. Mal. Métab. 2012, 6, 347–349. [Google Scholar] [CrossRef]
- Parati, G.; Stergiou, G.S.; Asmar, R.; Bilo, G.; de Leeuw, P.; Imai, Y.; Kario, K.; Lurbe, E.; Manolis, A.; Mengden, T.; et al. European Society of Hypertension Practice Guidelines for home blood pressure monitoring. J. Hum. Hypertens. 2010, 24, 779–785. [Google Scholar] [CrossRef]
- Chatzidiakou, L.; Krause, A.; Popoola, O.A.M.; Di Antonio, A.; Kellaway, M.; Han, Y.; Squires, F.A.; Wang, T.; Zhang, H.; Wang, Q.; et al. Characterising low-cost sensors in highly portable platforms to quantify personal exposure in diverse environments. Atmos. Meas. Tech. 2019, 12, 4643–4657. [Google Scholar] [CrossRef]
- Metcalf, B.S.; Jeffery, A.N.; Hosking, J.; Voss, L.D.; Sattar, N.; Wilkin, T.J. Objectively Measured Physical Activity and Its Association With Adiponectin and Other Novel Metabolic Markers. Diabetes Care 2009, 32, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Wanner, M.; Probst-Hensch, N.; Kriemler, S.; Meier, F.; Bauman, A.; Martin, B.W. What physical activity surveillance needs: Validity of a single-item questionnaire. Br. J. Sports Med. 2014, 48, 1570–1576. [Google Scholar] [CrossRef]
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Mâsse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sports Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Sagelv, E.H.; Ekelund, U.; Pedersen, S.; Brage, S.; Hansen, B.H.; Johansson, J.; Grimsgaard, S.; Nordström, A.; Horsch, A.; Hopstock, L.A.; et al. Physical activity levels in adults and elderly from triaxial and uniaxial accelerometry. The Tromsø Study. PLoS ONE 2019, 14, e0225670. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport 2011, 14, 411–416. [Google Scholar] [CrossRef]
- Chaix, B.; Benmarhnia, T.; Kestens, Y.; Brondeel, R.; Perchoux, C.; Gerber, P.; Duncan, D.T. Combining sensor tracking with a GPS-based mobility survey to better measure physical activity in trips: Public transport generates walking. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 84. [Google Scholar] [CrossRef]
- Bobbitt, Z. How to Winsorize Data: Definition & Examples. Statology. 2021. Available online: https://www.statology.org/winsorize/ (accessed on 28 May 2025).
- Li, N.; Chen, G.; Liu, F.; Mao, S.; Liu, Y.; Liu, S.; Mao, Z.; Lu, Y.; Wang, C.; Guo, Y.; et al. Associations between long-term exposure to air pollution and blood pressure and effect modifications by behavioral factors. Environ. Res. 2020, 182, 109109. [Google Scholar] [CrossRef] [PubMed]
- Abba, M.S.; Nduka, C.U.; Anjorin, S.; Uthman, O.A. Household Air Pollution and High Blood Pressure: A Secondary Analysis of the 2016 Albania Demographic Health and Survey Dataset. Int. J. Environ. Res. Public Health 2022, 19, 2611. [Google Scholar] [CrossRef] [PubMed]
- Fuks, K.B.; Weinmayr, G.; Basagaña, X.; Gruzieva, O.; Hampel, R.; Oftedal, B.; Sørensen, M.; Wolf, K.; Aamodt, G.; Aasvang, G.M.; et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur. Heart J. 2016, 38, 983–990. [Google Scholar] [CrossRef]
- Bista, S.; Fancello, G.; Zeitouni, K.; Annesi-Maesano, I.; Chaix, B. Relationships between fixed-site ambient measurements of nitrogen dioxide, ozone, and particulate matter and personal exposures in Grand Paris, France: The MobiliSense study. Int. J. Health Geogr. 2025, 24, 5. [Google Scholar] [CrossRef]
- Wu, S.; Deng, F.; Huang, J.; Wang, H.; Shima, M.; Wang, X.; Qin, Y.; Zheng, C.; Wei, H.; Hao, Y.; et al. Blood Pressure Changes and Chemical Constituents of Particulate Air Pollution: Results from the Healthy Volunteer Natural Relocation (HVNR) Study. Environ. Health Perspect. 2013, 121, 66–72. [Google Scholar] [CrossRef]
- Cosselman, K.E.; Krishnan, R.M.; Oron, A.P.; Jansen, K.; Peretz, A.; Sullivan, J.H.; Larson, T.V.; Kaufman, J.D. Blood Pressure Response to Controlled Diesel Exhaust Exposure in Human Subjects. Hypertension 2012, 59, 943–948. [Google Scholar] [CrossRef]
- Hudda, N.; Eliasziw, M.; Hersey, S.O.; Reisner, E.; Brook, R.D.; Zamore, W.; Durant, J.L.; Brugge, D. Effect of Reducing Ambient Traffic-Related Air Pollution on Blood Pressure: A Randomized Crossover Trial. Hypertension 2021, 77, 823–832. [Google Scholar] [CrossRef]
- Ibald-Mulli, A.; Timonen, K.L.; Peters, A.; Heinrich, J.; Wölke, G.; Lanki, T.; Buzorius, G.; Kreyling, W.G.; de Hartog, J.; Hoek, G.; et al. Effects of particulate air pollution on blood pressure and heart rate in subjects with cardiovascular disease: A multicenter approach. Environ. Health Perspect. 2004, 112, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Mirowsky, J.E.; Peltier, R.E.; Lippmann, M.; Thurston, G.; Chen, L.-C.; Neas, L.; Diaz-Sanchez, D.; Laumbach, R.; Carter, J.D.; Gordon, T. Repeated measures of inflammation, blood pressure, and heart rate variability associated with traffic exposures in healthy adults. Environ. Health 2015, 14, 66. [Google Scholar] [CrossRef]
- Weichenthal, S.; Hatzopoulou, M.; Goldberg, M.S. Exposure to traffic-related air pollution during physical activity and acute changes in blood pressure, autonomic and micro-vascular function in women: A cross-over study. Part. Fibre Toxicol. 2014, 11, 70. [Google Scholar] [CrossRef]
- Pun, V.C.; Ho, K. Blood pressure and pulmonary health effects of ozone and black carbon exposure in young adult runners. Sci. Total Environ. 2019, 657, 1–6. [Google Scholar] [CrossRef]
- Strak, M.; Boogaard, H.; Meliefste, K.; Oldenwening, M.; Zuurbier, M.; Brunekreef, B.; Hoek, G. Respiratory health effects of ultrafine and fine particle exposure in cyclists. Occup. Environ. Med. 2010, 67, 118–124. [Google Scholar] [CrossRef]
- Khajavi, A.; Tamehri Zadeh, S.S.; Azizi, F.; Brook, R.D.; Abdi, H.; Zayeri, F.; Hadaegh, F. Impact of short- and long-term exposure to air pollution on blood pressure: A two-decade population-based study in Tehran. Int. J. Hyg. Environ. Health 2021, 234, 113719. [Google Scholar] [CrossRef] [PubMed]
- Urch, B.; Silverman, F.; Corey, P.; Brook, J.R.; Lukic, K.Z.; Rajagopalan, S.; Brook, R.D. Acute Blood Pressure Responses in Healthy Adults During Controlled Air Pollution Exposures. Environ. Health Perspect. 2005, 113, 1052–1055. [Google Scholar] [CrossRef]
- Hoffmann, B.; Luttmann-Gibson, H.; Cohen, A.; Zanobetti, A.; de Souza, C.; Foley, C.; Suh, H.H.; Coull, B.A.; Schwartz, J.; Mittleman, M.; et al. Opposing Effects of Particle Pollution, Ozone, and Ambient Temperature on Arterial Blood Pressure. Environ. Health Perspect. 2012, 120, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Kim, S.-H.; Kang, S.-H.; Kim, S.-Y.; Kim, O.-J.; Yoon, C.-H.; Lee, H.-Y.; Youn, T.-J.; Chae, I.-H.; Kim, C.-H. Short-term effects of air pollution on blood pressure. Sci. Rep. 2019, 9, 20298. [Google Scholar] [CrossRef]
- Brook, R.D.; Bard, R.L.; Burnett, R.T.; Shin, H.H.; Vette, A.; Croghan, C.; Phillips, M.; Rodes, C.; Thornburg, J.; Williams, R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup. Environ. Med. 2011, 68, 224–230. [Google Scholar] [CrossRef]
- Padró-Martínez, L.T.; Patton, A.P.; Trull, J.B.; Zamore, W.; Brugge, D.; Durant, J.L. Mobile monitoring of particle number concentration and other traffic-related air pollutants in a near-highway neighborhood over the course of a year. Atmos. Environ. 2012, 61, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.H.; Van Hee, V.C.; Bergen, S.; Szpiro, A.A.; DeRoo, L.A.; London, S.J.; Marshall, J.D.; Kaufman, J.D.; Sandler, D.P. Long-Term Air Pollution Exposure and Blood Pressure in the Sister Study. Environ. Health Perspect. 2015, 123, 951–958. [Google Scholar] [CrossRef]
- Sérgio Chiarelli, P.; Amador Pereira, L.A.; do Nascimento Saldiva, P.H.; Ferreira Filho, C.; Bueno Garcia, M.L.; Ferreira Braga, A.L.; Conceição Martins, L. The association between air pollution and blood pressure in traffic controllers in Santo André, São Paulo, Brazil. Environ. Res. 2011, 111, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Suh, H.H.; Zanobetti, A. Exposure Error Masks The Relationship Between Traffic-Related Air Pollution and Heart Rate Variability (HRV). J. Occup. Environ. Med. 2010, 52, 685–692. [Google Scholar] [CrossRef]
- Harrabi, I.; Rondeau, V.; Dartigues, J.-F.; Tessier, J.-F.; Filleul, L. Effects of particulate air pollution on systolic blood pressure: A population-based approach. Environ. Res. 2006, 101, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Mead, M.I.; Popoola, O.A.M.; Stewart, G.B.; Landshoff, P.; Calleja, M.; Hayes, M.; Baldovi, J.J.; McLeod, M.W.; Hodgson, T.F.; Dicks, J.; et al. The use of electrochemical sensors for monitoring urban air quality in low-cost, high-density networks. Atmos. Environ. 2013, 70, 186–203. [Google Scholar] [CrossRef]
- Di Antonio, A.; Popoola, O.; Ouyang, B.; Saffell, J.; Jones, R. Developing a Relative Humidity Correction for Low-Cost Sensors Measuring Ambient Particulate Matter. Sensors 2018, 18, 2790. [Google Scholar] [CrossRef]
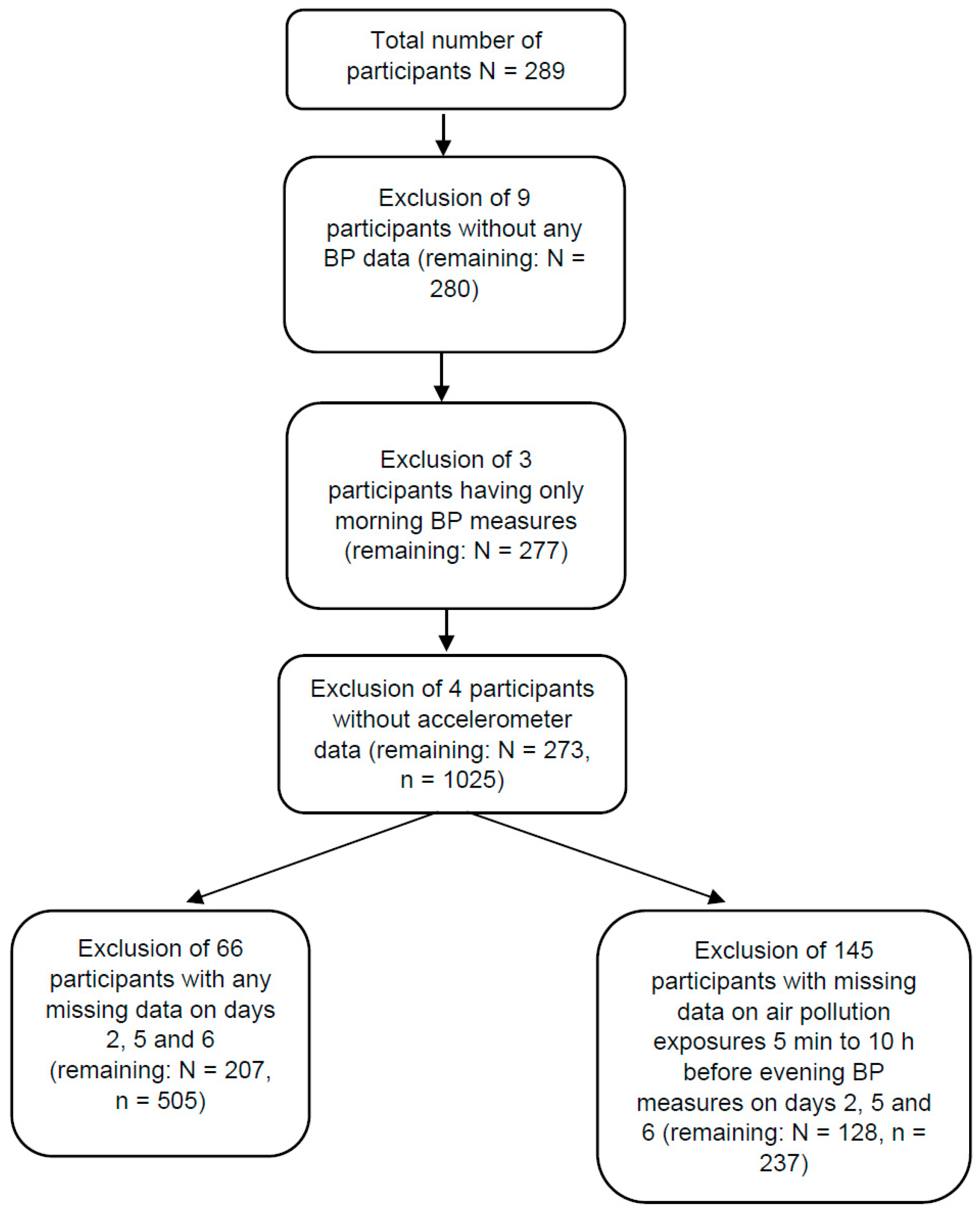
| Personal Characteristics | Mean (Standard Deviation) or N (%) | Median (2.5th Percentile, 97.5th Percentile) |
|---|---|---|
| Age | 50.01 (8.84) | 50 (35, 65) |
| Body mass index (kg/m2) | 25.36 (6.38) | 23.91 (18.64, 39.17) |
| Monthly alcohol consumption (number of times per month) | 8.48 (8.99) | 4.33 (0, 30.42) |
| Sex | ||
| Woman | 118 (57%) | NA |
| Men | 89 (43%) | NA |
| Hypertension | ||
| Yes | 17 (8%) | NA |
| No | 190 (92%) | NA |
| Education level | ||
| Less than baccalauréat | 11 (5%) | NA |
| Baccalauréat equivalent | 50 (24%) | NA |
| Above baccalaureate | 146 (71%) | NA |
| Employment | ||
| Stable (permanent contract) | 141 (68%) | NA |
| Unstable (temporary contract) | 10 (4%) | NA |
| Retired | 26 (13%) | NA |
| Unemployed | 5 (2%) | NA |
| Other | 25 (12%) | NA |
| Residence | ||
| Paris | 49 (24%) | NA |
| Close suburbs | 156 (75%) | NA |
| Far suburbs | 2 (1%) | NA |
| Marital status | ||
| Unmarried | 147 (71%) | NA |
| Married | 69 (29%) | NA |
| Nationality | ||
| French | 201 (97%) | NA |
| Non French | 6 (3%) | NA |
| Blood pressure measurements (mmHg) | ||
| Morning SBP | 115.75 (14.14) | 114 (106, 126) |
| Evening SBP | 117.89 (13.9) | 117 (108, 128) |
| Morning DBP | 73.25 (9.35) | 72 (66, 79) |
| Evening DBP | 73.2 (9.22) | 74 (67, 79) |
| Variables in the Model | Systolic Blood Pressure (SBP) Estimate (95% CI) | Diastolic Blood Pressure (DBP) Estimate (95% CI) |
|---|---|---|
| NO2 | 0.32 (−2.55, 3.19) | −1.13 (−3.12, 0.85) |
| NO | −0.35 (−0.81, 0.10) | −0.07 (−0.38, 0.24) |
| CO | −0.02 (−0.14, 0.11) | −0.09 (−0.18, −0.01) |
| O3 | −0.84 (−2.88, 1.19) | −0.61 (−2.00, 0.78) |
| PM2.5 | 0.04 (−0.20, 0.29) | 0.06 (−0.10, 0.23) |
| Age | −0.06 (−0.22, 0.10) | −0.05 (−0.16, 0.06) |
| Body mass index | −0.12 (−0.30, 0.05) | −0.08 (−0.20, 0.03) |
| Temperature | −0.24 (−0.68, 0.20) | −0.39 (−0.69, −0.09) |
| Relative humidity | −0.28 (−0.45, −0.11) | −0.09 (−0.21, 0.02) |
| Living standard of the residential area | 0.12 (−0.01, 0.25) | 0.06 (−0.03, 0.15) |
| Monthly alcohol drinks | −0.09 (−0.22, 0.04) | −0.07 (−0.16, 0.01) |
| Proportion of time out-of-home | −3.81 (−8.24, 0.61) | −1.01 (−4.10, 2.07) |
| Proportion of time in motorized transport | 3.64 (−11.46, 18.73) | −7.77 (−18.30, 2.76) |
| Percentage of time physically active | −0.12 (−0.29, 0.04) | −0.10 (−0.22, 0.01) |
| Weekend (ref: weekdays) | −0.24 (−2.65, 2.16) | −0.17 (−1.86, 1.52) |
| Male (ref: female) | −0.83 (−3.24, 1.57) | −0.81 (−2.43, 0.81) |
| Residence (ref: Close suburbs) | NA | |
| Far suburbs | 0.05 (−10.67, 10.77) | −2.69 (−9.90, 4.52) |
| Paris | −0.02 (−2.70, 2.65) | −0.57 (−2.37, 1.23) |
| Employment status (ref: others) | NA | |
| Employment (Retired) | 1.70 (−2.89, 6.28) | 0.34 (−1.50, 2.19) |
| Employment (Stable) | 0.39 (−3.22, 4.00) | −1.81 (−5.46, 1.84) |
| Unemployed | 4.36 (−3.25, 11.98) | NA |
| Employment (Unstable) | 1.23 (−4.85, 7.32) | 1.06 (−2.03, 4.14) |
| Education (ref: equivalent to baccalaureat) | NA | NA |
| Education higher than Baccalaureat | −0.21 (−2.95, 2.53) | 1.99 (−3.14, 7.13) |
| Education lower than Baccalaureat | −2.22 (−7.63, 3.19) | −2.45 (−6.55, 1.66) |
| Tertile income (ref: high tertile income) | NA | NA |
| Medium tertile income | 0.67 (−2.05, 3.39) | 1.30 (−0.53, 3.13) |
| Low tertile income | 2.17 (−0.53, 4.87) | 1.28 (−0.54, 3.10) |
| Systolic Blood Pressure | Diastolic Blood Pressure | |||
|---|---|---|---|---|
| Air Pollutants |
Coefficient
β |
Effect of Mixture
ψ (95% CI) |
Coefficient
β |
Effect of Mixture
ψ (95% CI) |
| NO2 | −0.79 | −0.33 (−3.31, 2.65) | −0.27 | −0.53 (−2.66, 1.60) |
| NO | 0.30 | 0.43 | ||
| CO | −0.75 | −1.46 | ||
| O3 | 0.12 | 0.23 | ||
| PM2.5 | 0.78 | 0.53 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekarimunda, L.; Dureau, C.; Chaix, B.; Bista, S. Exposure to Air Pollution and Changes in Resting Blood Pressure from Morning to Evening: The MobiliSense Study. Int. J. Environ. Res. Public Health 2025, 22, 872. https://doi.org/10.3390/ijerph22060872
Sekarimunda L, Dureau C, Chaix B, Bista S. Exposure to Air Pollution and Changes in Resting Blood Pressure from Morning to Evening: The MobiliSense Study. International Journal of Environmental Research and Public Health. 2025; 22(6):872. https://doi.org/10.3390/ijerph22060872
Chicago/Turabian StyleSekarimunda, Lisa, Clelie Dureau, Basile Chaix, and Sanjeev Bista. 2025. "Exposure to Air Pollution and Changes in Resting Blood Pressure from Morning to Evening: The MobiliSense Study" International Journal of Environmental Research and Public Health 22, no. 6: 872. https://doi.org/10.3390/ijerph22060872
APA StyleSekarimunda, L., Dureau, C., Chaix, B., & Bista, S. (2025). Exposure to Air Pollution and Changes in Resting Blood Pressure from Morning to Evening: The MobiliSense Study. International Journal of Environmental Research and Public Health, 22(6), 872. https://doi.org/10.3390/ijerph22060872





