The Impact of a Novel Transfer Process on Patient Bed Days and Length of Stay: A Five-Year Comparative Study at the Mayo Clinic in Rochester and Mankato Quaternary and Tertiary Care Centers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Settings
2.2. Study Population and Design
2.3. Eligibility Criteria
2.4. Parallel Transfer Process Implementation
- Transfer Criteria: Patients eligible for parallel transfer were identified based on an initial assessment at the admitting hospital. Criteria included the patient’s stability, the complexity of the required care, and resource availability at the current facility. Patients requiring specialized services available only at tertiary centers were excluded from parallel transfers.
- Bed Availability and Coordination: Once eligibility was established, bed availability at nearby hospitals of similar acuity was assessed. The admitting hospital coordinated with the receiving facility to confirm the transfer, ensuring continuous care and minimizing the risk of delays.
- Follow-Up Procedures: Following each transfer, a structured follow-up protocol was conducted to assess patient outcomes and address any transfer-related issues. Quality assurance checks and iterative improvements were made based on transfer outcomes, helping refine the process for future cases and ensuring safety and care continuity.
2.5. Parallel Transfer Process
2.6. Dataset Variables and Data Description
2.7. Data Transformations and Normality Testing
2.8. Application of Machine Learning Techniques
- Data Preprocessing: missing values were handled using k-Nearest Neighbors (k-NN) imputation, and outliers were detected and managed using Isolation Forest algorithms.
- Feature Selection: Principal Component Analysis (PCA) was applied to reduce dimensionality and retain the most informative variables.
- Predictive Modeling: Random Forest and Gradient Boosting models were implemented to predict LOS. These algorithms accounted for complex, nonlinear interactions between patient characteristics and outcomes.
- Validation: an 80/20 training–validation split and 10-fold cross-validation were employed to ensure model reliability and minimize overfitting.
- Performance Metrics: predictive accuracy was assessed using the mean squared error (MSE) and R-squared (R2) for continuous outcomes.
2.9. Statistical and Predictive Modeling
- Random Forest and Gradient Boosting: these models were implemented to account for nonlinear data structures and interactions among variables.
- Model Comparison: the predictive performance was evaluated using the mean squared error (MSE) and R-squared (R2) for continuous outcomes.
- Validation Techniques: an 80/20 dataset split was performed for external validation, while 10-fold cross-validation was employed to ensure internal reliability and minimize overfitting.
2.10. Model Validation and Performance Metrics
2.11. Statistical Analysis
2.12. Length of Stay (LOS) Analysis
2.13. Patient Saved Days Calculation
2.14. Outcome Variables
2.15. Use of Generative AI
3. Results
3.1. Baseline Characteristics
3.2. Length of Stay (LOS)
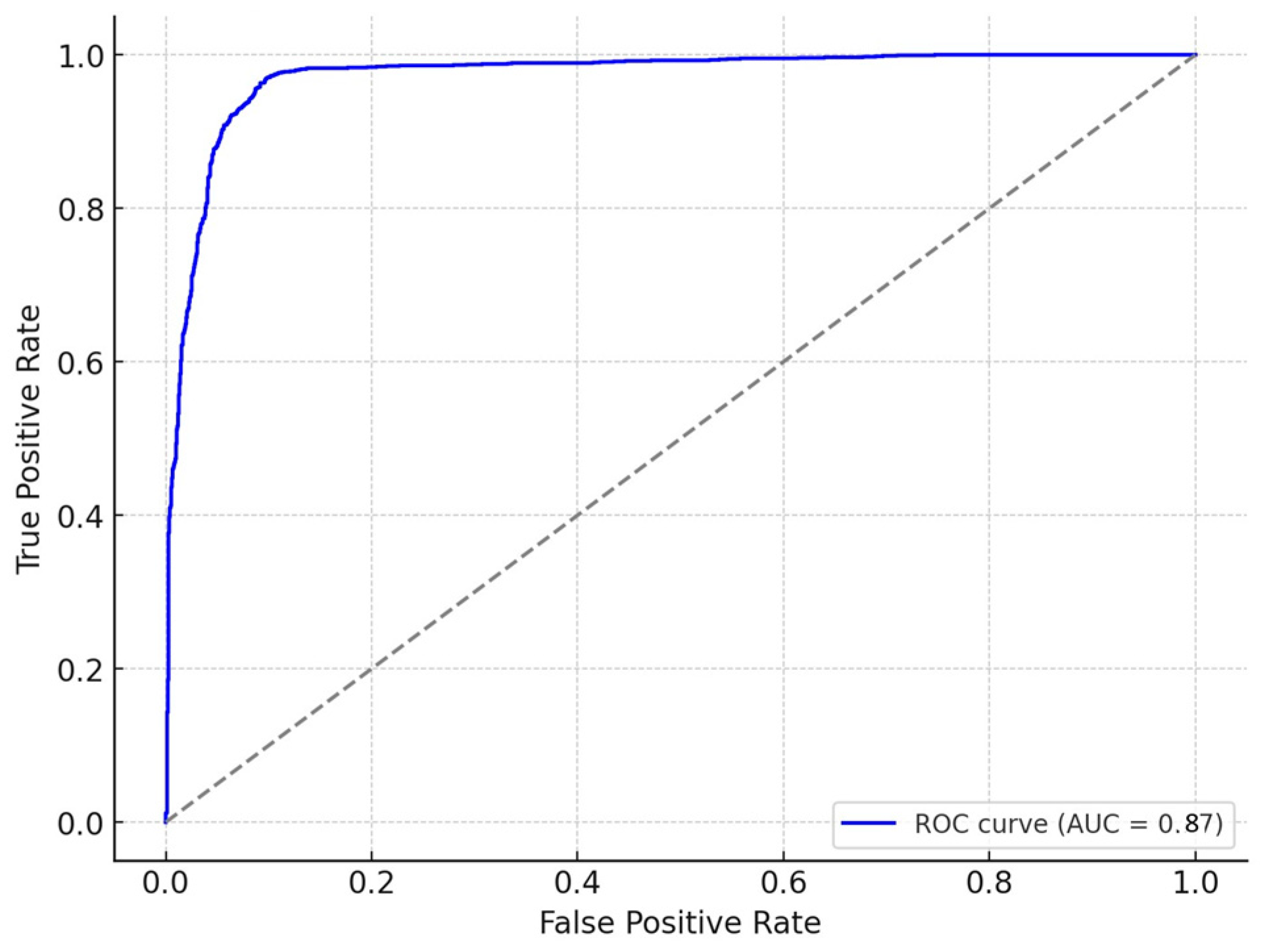
3.3. Justification for Machine Learning Model Choice
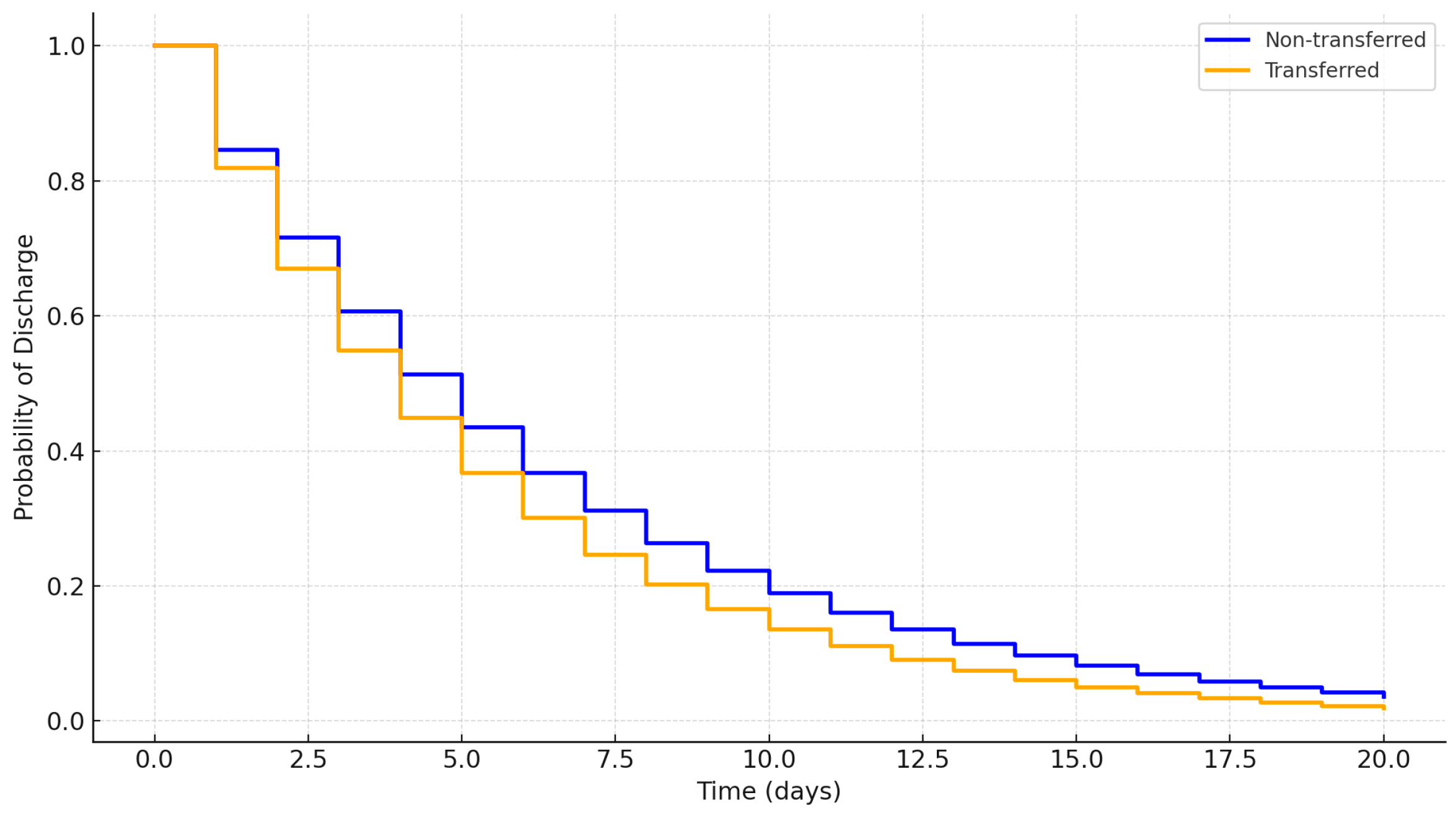
3.4. Kaplan–Meier Survival Analysis
3.5. Clustering Analysis for Patient Subgroups
- 1.
- Cluster A:
- Characteristics: younger patients (<40 years), minimal comorbidities (median Charlson Index = 1), and shorter LOS (<2 days).
- Findings: demonstrated the fastest discharge rates, reducing overall bed occupancy by 20% compared to non-transferred counterparts.
- Statistics: mean LOS difference = −0.8 days (95% CI: −1.2 to −0.4, p < 0.01).
- 2.
- Cluster B:
- Characteristics: middle-aged patients (40–65 years) with moderate comorbidities (median Charlson Index = 3).
- Findings: Showed the most significant resource utilization benefits, defined as a reduction in adjusted LOS and an increase in saved patient days. These patients had an 18% reduction in adjusted LOS and contributed 45% of total saved bed days.
- Statistics:
- ○
- Adjusted LOS reduction: 2.1 days (95% CI: 1.8–2.4, p < 0.001).
- ○
- Saved patient days: median = 3.8 days per patient (95% CI: 3.2–4.4).
- 3.
- Cluster C:
- Characteristics: older patients (>65 years) with high comorbidity scores (median Charlson Index ≥ 6) and longer LOS (>5 days).
- Findings: Experienced limited improvements, with only a 5% reduction in LOS and fewer saved patient days. Transfers were less effective, suggesting the need for alternative care strategies.
- Statistics:
- ○
- LOS reduction: 0.3 days (95% CI: 0.1–0.5, p = 0.03).
- ○
- Saved patient days: median = 1.2 days per patient (95% CI: 0.8–1.6).
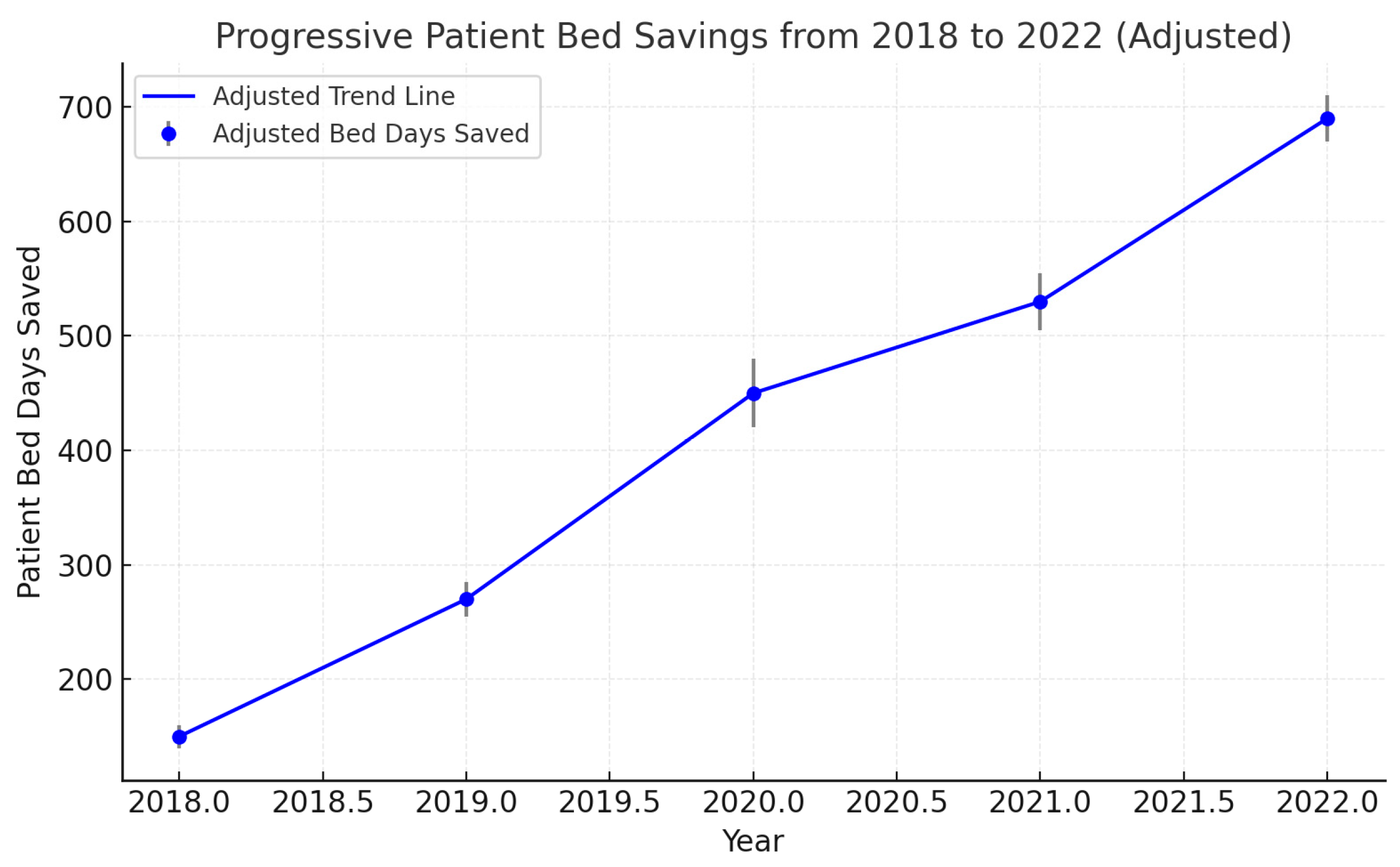
3.6. Saved Patient Days
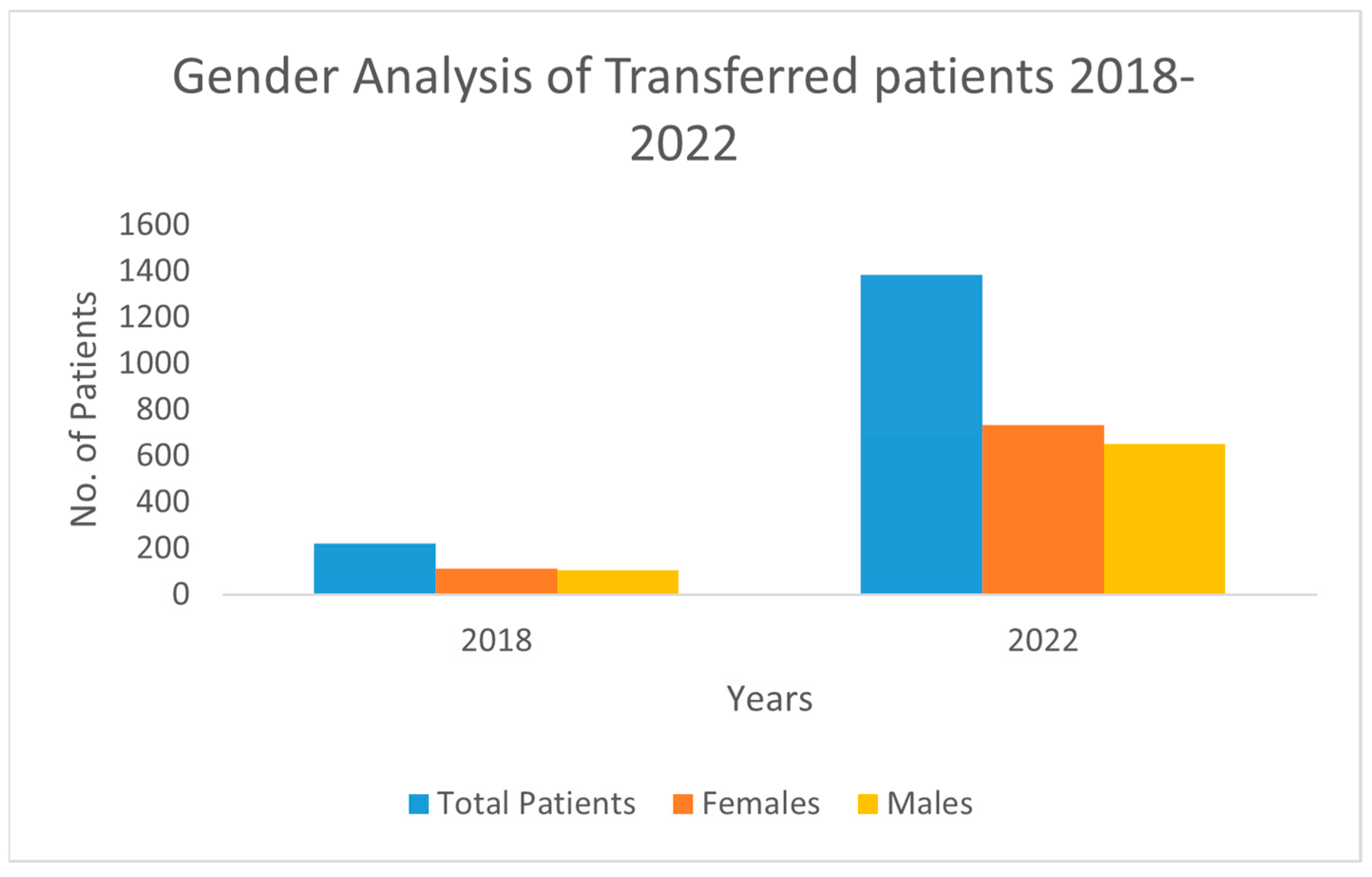
3.7. Gender Analysis
3.8. Predictive Model Results and Subgroup Analysis
4. Discussion
Limitations and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Appropriate Interfacility Patient Transfer. American College of Emergency Physicians. Available online: https://www.acep.org/patient-care/policy-statements/appropriate-interfacility-patient-transfer (accessed on 26 August 2023).
- Mitchell, S.H.; Rigler, J.; Baum, K. Regional Transfer Coordination and Hospital Load Balancing During COVID-19 Surges. JAMA Health Forum 2022, 3, e215048. [Google Scholar] [CrossRef] [PubMed]
- Ligtenberg, J.J.; Arnold, L.G.; Stienstra, Y.; van der Werf, T.S.; Meertens, J.H.; Tulleken, J.E.; Zijlstra, J.G. Quality of interhospital transport of critically ill patients: A prospective audit. Crit. Care 2005, 9, R446–R451. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Hessner, L.; White, A.A.; Davis, K.F.; Herzig, S.J.; Hohmann, S.F. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J. Hosp. Med. 2016, 11, 245–250. [Google Scholar] [CrossRef]
- Hernandez-Boussard, T.; Davies, S.; McDonald, K.; Wang, N.E. Interhospital Facility Transfers in the United States: A Nationwide Outcomes Study. J. Patient Saf. 2017, 13, 187–191. [Google Scholar] [CrossRef]
- Chen, K.C.; Wen, S.H. Impact of interhospital transfer on emergency department timeliness of care and in-hospital outcomes of adult non-trauma patients. Heliyon 2023, 9, e13393. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Zheng, J.; Orav, E.J.; Schnipper, J.L. Inter-hospital transfer and patient outcomes: A retrospective cohort study. BMJ Qual. Saf. 2019, 28, e1. [Google Scholar] [CrossRef]
- Song, J.J.; Lee, S.J.; Song, J.H.; Lee, S.W.; Kim, S.J.; Han, K.S. Effect of Inter-Hospital Transfer on Mortality in Patients Admitted through the Emergency Department. J. Clin. Med. 2024, 13, 4944. [Google Scholar] [CrossRef]
- Russell, P.; Hakendorf, P.; Thompson, C. Inter-hospital lateral transfer does not increase length of stay. Aust. Health Rev. 2015, 39, 400–403. [Google Scholar] [CrossRef]
- Gentimis, T.; Alnaser, A.J.; Durante, A.; Cook, K.; Steele, R. Predicting Hospital Length of Stay Using Neural Networks. Int. J. Big Data Intell. 2019, 6. [Google Scholar] [CrossRef]
- Mahmoudian, Y.; Nemati, A.; Safaei, A.S. A forecasting approach for hospital bed capacity planning using machine learning and deep learning with application to public hospitals. Heal. Anal. 2023, 4. [Google Scholar] [CrossRef]
- Hong, W.S.; Haimovich, A.D.; Taylor, R.A. Predicting hospital admission at emergency department triage using machine learning. PLoS ONE 2018, 13, e0201016. [Google Scholar] [CrossRef] [PubMed]
- Tello, M.; Reich, E.S.; Puckey, J.; Maff, R.; Garcia-Arce, A.; Bhattacharya, B.S.; Feijoo, F. Machine learning based forecast for the prediction of inpatient bed demand. BMC Med. Inform. Decis. Mak. 2022, 22, 55. [Google Scholar] [CrossRef]
- Li, Z.; Chang, J.; Shi, J.; Wang, J. Coordination schemes for resource reallocation and patient transfer in hospital alliance models. Decis. Sci. 2024. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Griffin, K.M.; Karas, M.G.; Ivascu, N.S.; Lief, L. Hospital Preparedness for COVID-19: A Practical Guide from a Critical Care Perspective. Am. J. Respir. Crit. Care Med. 2020, 201, 1337–1344. [Google Scholar] [CrossRef]
- Kadri, S.S.; Sun, J.; Lawandi, A.; Strich, J.R.; Busch, L.M.; Keller, M.; Babiker, A.; Yek, C.; Malik, S.; Krack, J.; et al. Association Between Caseload Surge and COVID-19 Survival in 558 U.S. Hospitals, March to August 2020. Ann. Intern. Med. 2021, 174, 1240–1251. [Google Scholar] [CrossRef]
- Sarzynski, S.H.; Mancera, A.G.; Yek, C.; Rosenthal, N.A.; Kartashov, A.; Hick, J.L.; Mitchell, S.H.; Neupane, M.; Warner, S.; Sun, J.; et al. Trends in Patient Transfers From Overall and Caseload-Strained US Hospitals During the COVID-19 Pandemic. JAMA Netw. Open 2024, 7, e2356174. [Google Scholar] [CrossRef]
- Villarroel, L.; Tams, E.; Smith, L.; Rigler, J.; Wilson, D.; Hu, C.; Glassberg, M.K. Arizona Surge Line: An emergent statewide COVID-19 transfer service with equity as an outcome. Front. Public Heal. 2023, 10, 1028353. [Google Scholar] [CrossRef]
- French, G.; Hulse, M.; Nguyen, D.; Sobotka, K.; Webster, K.; Corman, J.; Aboagye-Nyame, B.; Dion, M.; Johnson, M.; Zalinger, B.; et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic-United States, July 2020–July 2021. Am. J. Transplant. 2022, 22, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Brooten, J.K.; Buckenheimer, A.S.; Hallmark, J.K.; Grey, C.R.; Cline, D.M.; Breznau, C.J.; McQueen, T.S.; Harris, Z.J.; Welsh, D.; Williamson, J.D.; et al. Risky Behavior: Hospital Transfers Associated with Early Mortality and Rates of Goals of Care Discussions. WestJEM 21.2 March Issue 2020, 21, 935–942. [Google Scholar] [CrossRef]
- Kent, J.A.; Patel, V.; Varela, N.A. Gender disparities in health care. Mt. Sinai J. Med. A J. Transl. Pers. Med. 2012, 79, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.F.; Hepworth, J.T. Age and gender differences in health services utilization. Res. Nurs. Health 1996, 19, 323–329. [Google Scholar] [CrossRef]
- Owens, G. Gender Differences in Health Care Expenditures, Resource Utilization, and Quality of Care. J. Manag. Care Pharm. 2008, 14, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Manuel, J.I. Racial/Ethnic and Gender Disparities in Health Care Use and Access. Heal. Serv. Res. 2017, 53, 1407–1429. [Google Scholar] [CrossRef]
- Puentes-Markides, C. Women and access to health care. Soc. Sci. Med. 1992, 35, 619–626. [Google Scholar] [CrossRef]
- van Wijk, C.M.; Van Vliet, K.P.; Kolk, A.M. Gender perspectives and quality of care: Towards appropriate and adequate health care for women. Soc. Sci. Med. 1996, 43, 707–720. [Google Scholar]
- Abella, M.; Hayashi, J.; Martinez, B.; Inouye, M.; Rosander, A.; Kornblith, L.; Elkbuli, A. A National Analysis of Racial and Sex Disparities Among Interhospital Transfers for Emergency General Surgery Patients and Associated Outcomes. J. Surg. Res. 2023, 294, 228–239. [Google Scholar] [CrossRef]
- Fassmer, A.M.; Pulst, A.; Schmiemann, G.; Hoffmann, F. Sex-Specific Differences in Hospital Transfers of Nursing Home Residents: Results from the HOspitalizations and eMERgency Department Visits of Nursing Home Residents (HOMERN) Project. Int. J. Environ. Res. Public Health 2020, 17, 3915. [Google Scholar] [CrossRef]
- Tyler, P.D.; Stone, D.J.; Geisler, B.P.; McLennan, S.; Celi, L.A.; Rush, B. Racial and geographic disparities in inter-hospital intensive care unit transfers. Crit. Care Med. 2018, 46, e76–e80. [Google Scholar] [CrossRef]

| Parameters (N) | Total | 95% CI | p-Value | Annotations |
|---|---|---|---|---|
| Total Emergency Department Visits | 179,066 | - | - | Total visits from 2018–2022; includes all eligible ED encounters. |
| Transferred Visits | 3207 | - | - | Patients transferred to facilities offering equivalent care levels. |
| Non-Transferred Visits | 175,895 | - | - | Patients admitted or observed without inter-facility transfer. |
| Median Length of Stay (LOS) (Days) | 2.5 | 2.4–2.6 | <0.001 | LOS calculated using generalized estimating equations (GEEs) adjusted for confounders. |
| Median LOS—Transfers (Days) | 2.7 | 2.6–2.9 | 0.02 | Adjusted LOS for transferred patients. |
| Median LOS—Non-Transferred (Days) | 2.5 | 2.4–2.6 | Reference | Adjusted LOS for non-transferred patients as the baseline comparator. |
| Transfers—Female (Range) | 113–735 | - | - | Range of annual transfers for females across the study period. |
| Transfers—Male (Range) | 107–651 | - | - | Range of annual transfers for males across the study period. |
| Saved Patient Days (Days) | 598–5237 | - | <0.001 | Defined as the difference between observed and expected LOS based on Diagnosis-Related Group (DRG) standards. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khedr, A.; Hassan, E.; Asim, R.; Khan, M.K.; Duseja, N.; Attallah, N.; Mueller, J.; Newman, J.; Loomis, E.; Bartelt, J.; et al. The Impact of a Novel Transfer Process on Patient Bed Days and Length of Stay: A Five-Year Comparative Study at the Mayo Clinic in Rochester and Mankato Quaternary and Tertiary Care Centers. Int. J. Environ. Res. Public Health 2025, 22, 871. https://doi.org/10.3390/ijerph22060871
Khedr A, Hassan E, Asim R, Khan MK, Duseja N, Attallah N, Mueller J, Newman J, Loomis E, Bartelt J, et al. The Impact of a Novel Transfer Process on Patient Bed Days and Length of Stay: A Five-Year Comparative Study at the Mayo Clinic in Rochester and Mankato Quaternary and Tertiary Care Centers. International Journal of Environmental Research and Public Health. 2025; 22(6):871. https://doi.org/10.3390/ijerph22060871
Chicago/Turabian StyleKhedr, Anwar, Esraa Hassan, Rida Asim, Muhammad Khuzzaim Khan, Nikhil Duseja, Noura Attallah, Jennifer Mueller, Jamie Newman, Erica Loomis, Jennifer Bartelt, and et al. 2025. "The Impact of a Novel Transfer Process on Patient Bed Days and Length of Stay: A Five-Year Comparative Study at the Mayo Clinic in Rochester and Mankato Quaternary and Tertiary Care Centers" International Journal of Environmental Research and Public Health 22, no. 6: 871. https://doi.org/10.3390/ijerph22060871
APA StyleKhedr, A., Hassan, E., Asim, R., Khan, M. K., Duseja, N., Attallah, N., Mueller, J., Newman, J., Loomis, E., Bartelt, J., Khan, S. A., & Bartlett, B. (2025). The Impact of a Novel Transfer Process on Patient Bed Days and Length of Stay: A Five-Year Comparative Study at the Mayo Clinic in Rochester and Mankato Quaternary and Tertiary Care Centers. International Journal of Environmental Research and Public Health, 22(6), 871. https://doi.org/10.3390/ijerph22060871










