Epidemiological Trends in Mesothelioma Mortality in Colombia (1997–2022): A Retrospective National Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Variables and Coding
2.4. Statistical Analysis
2.5. Ethical Considerations
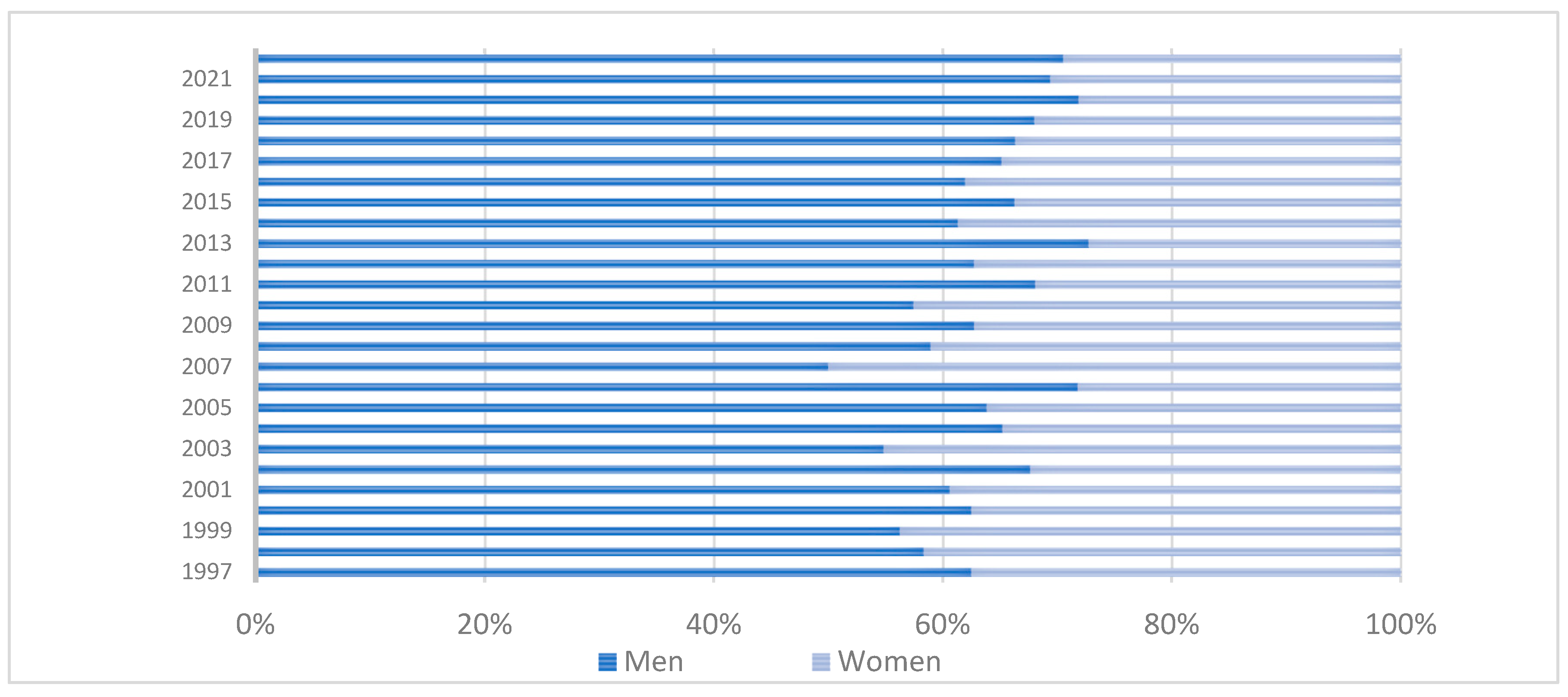
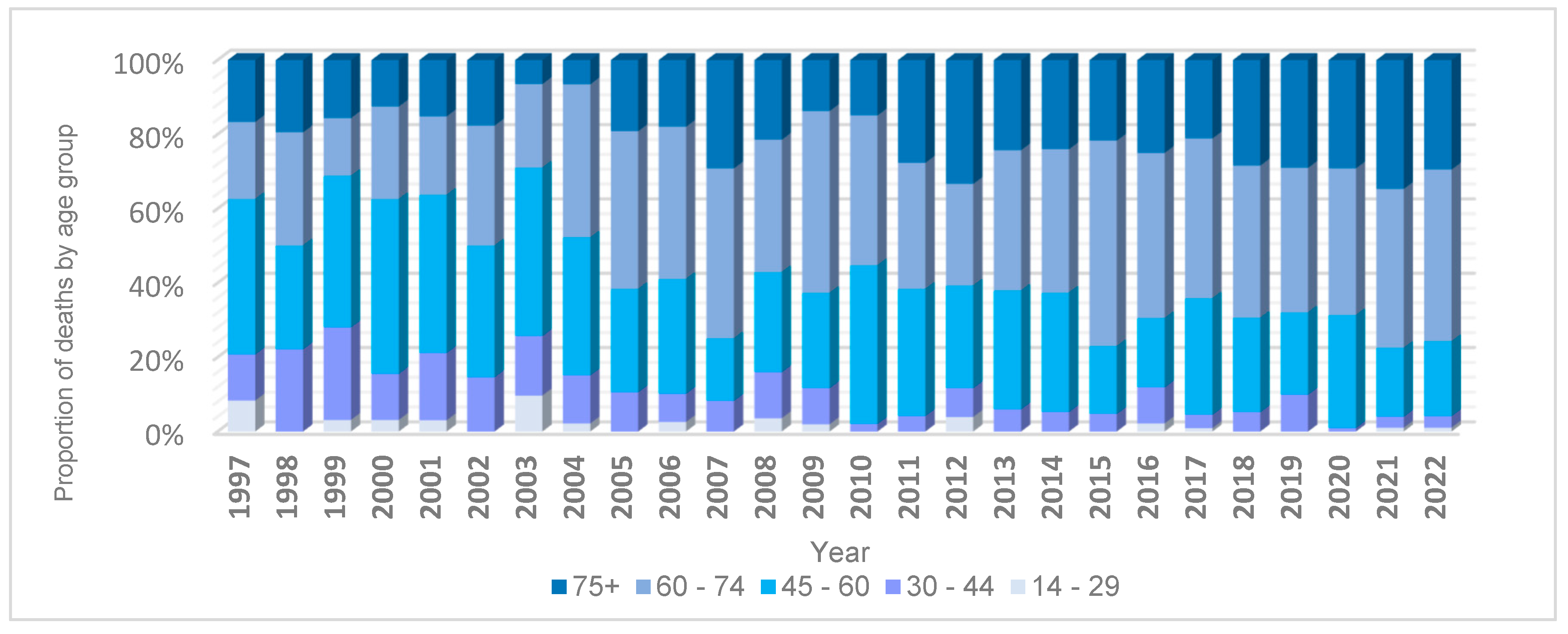
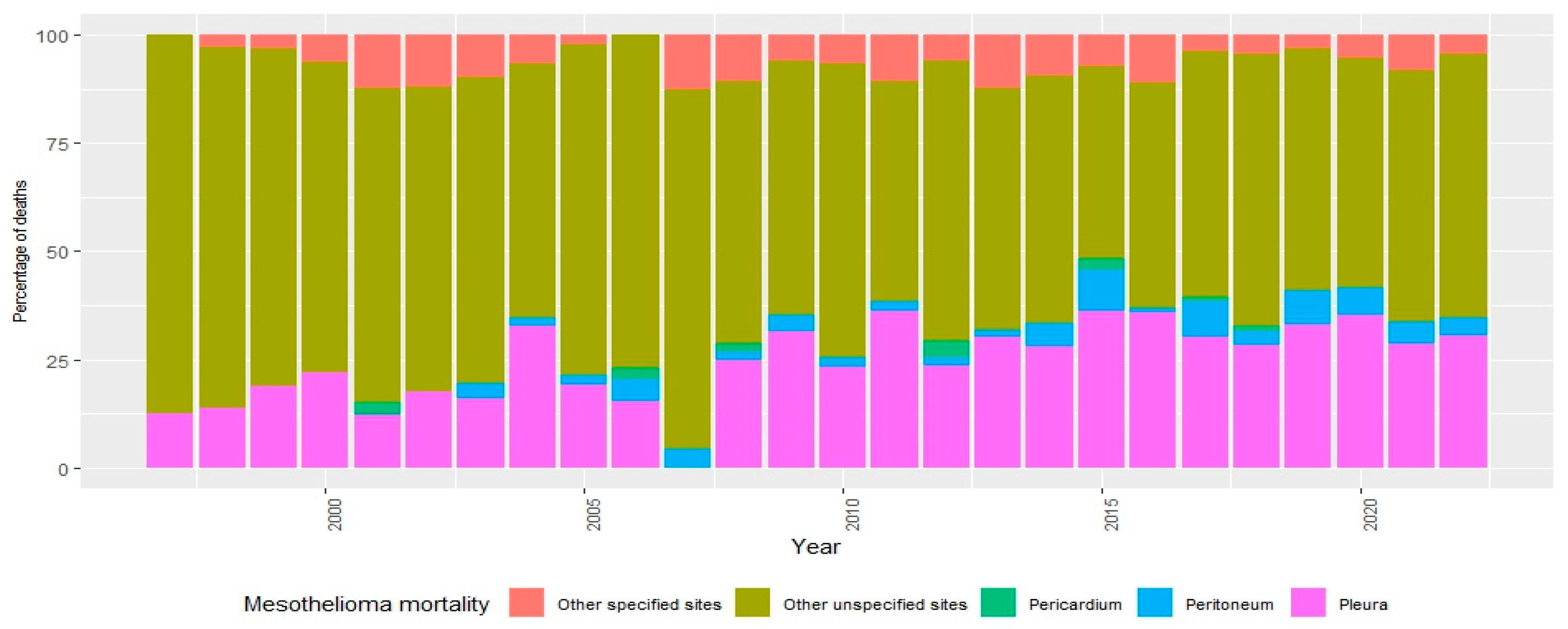
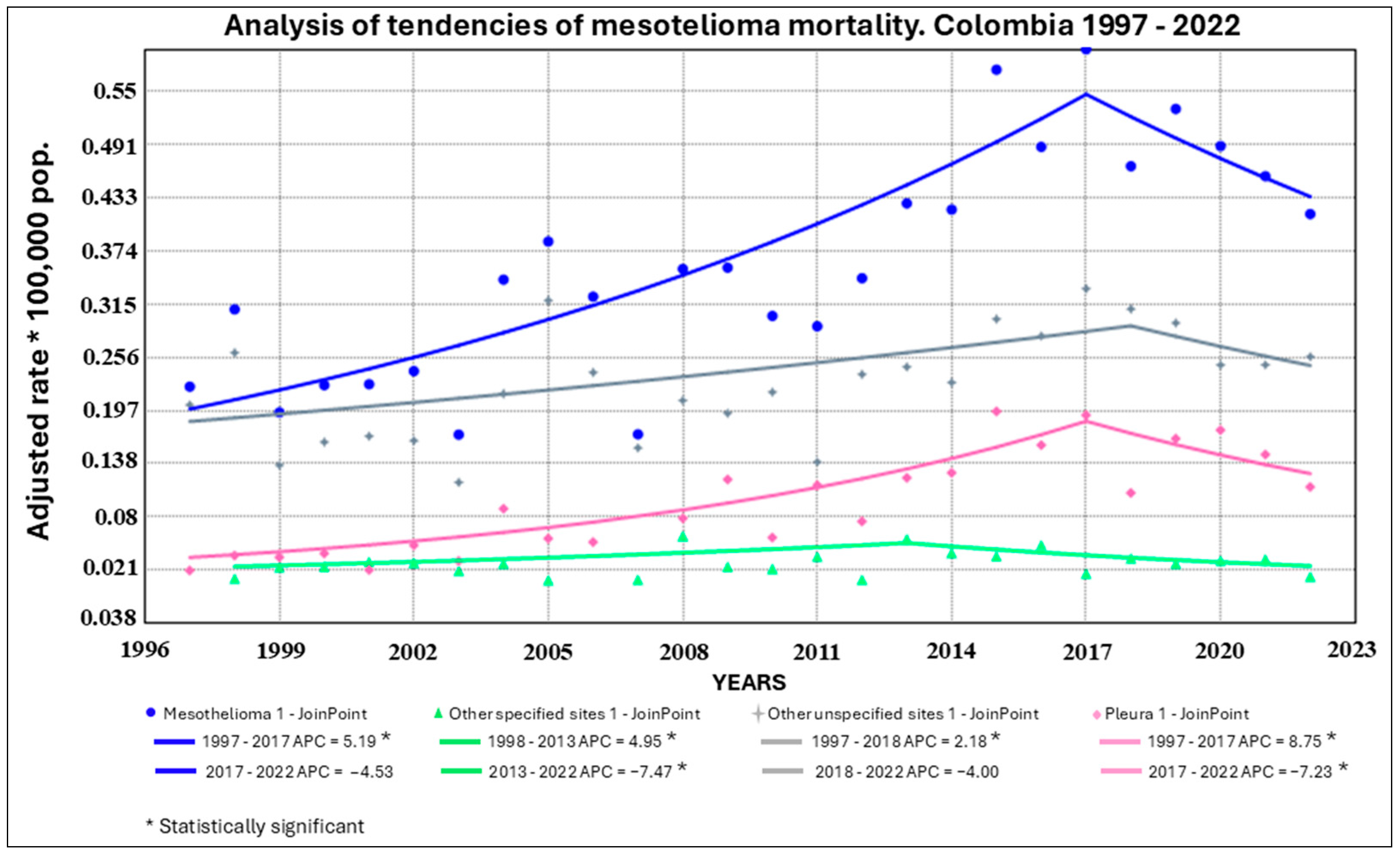
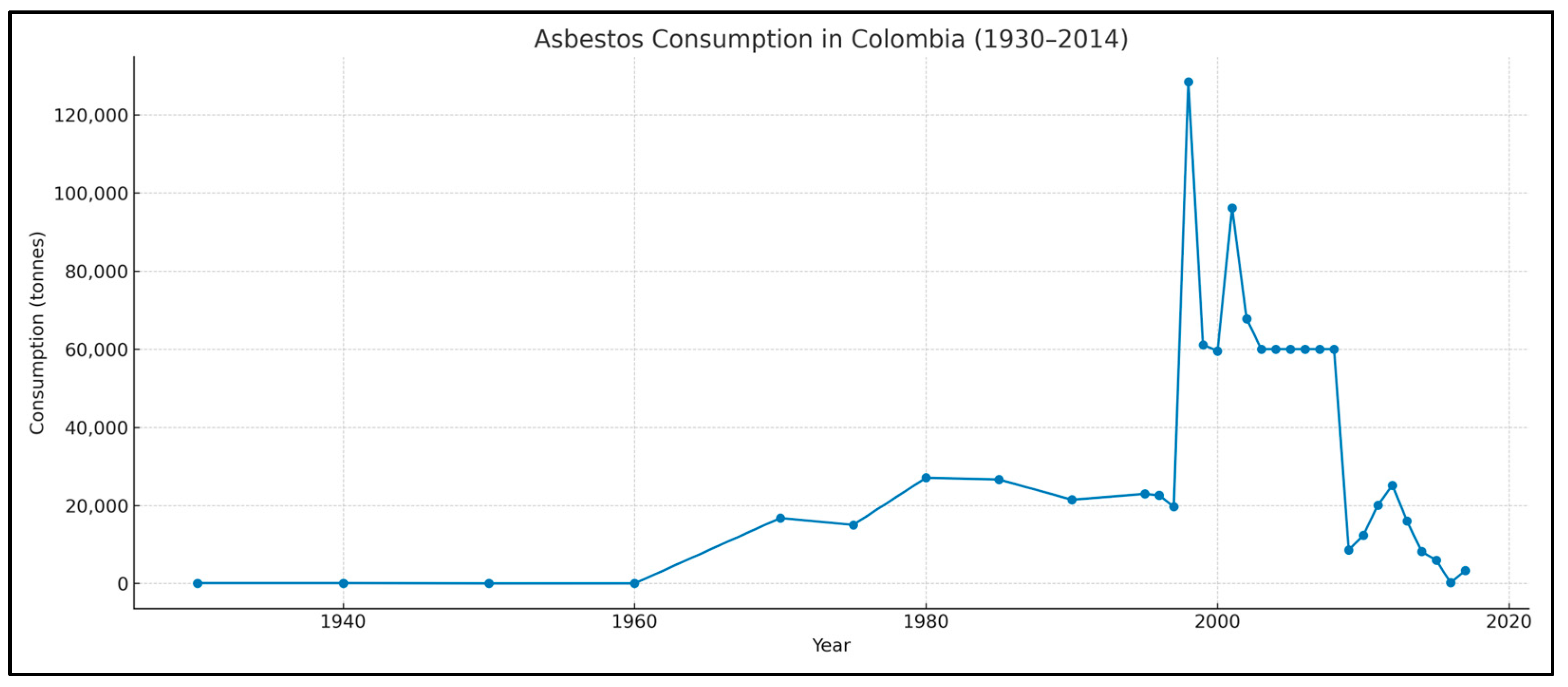
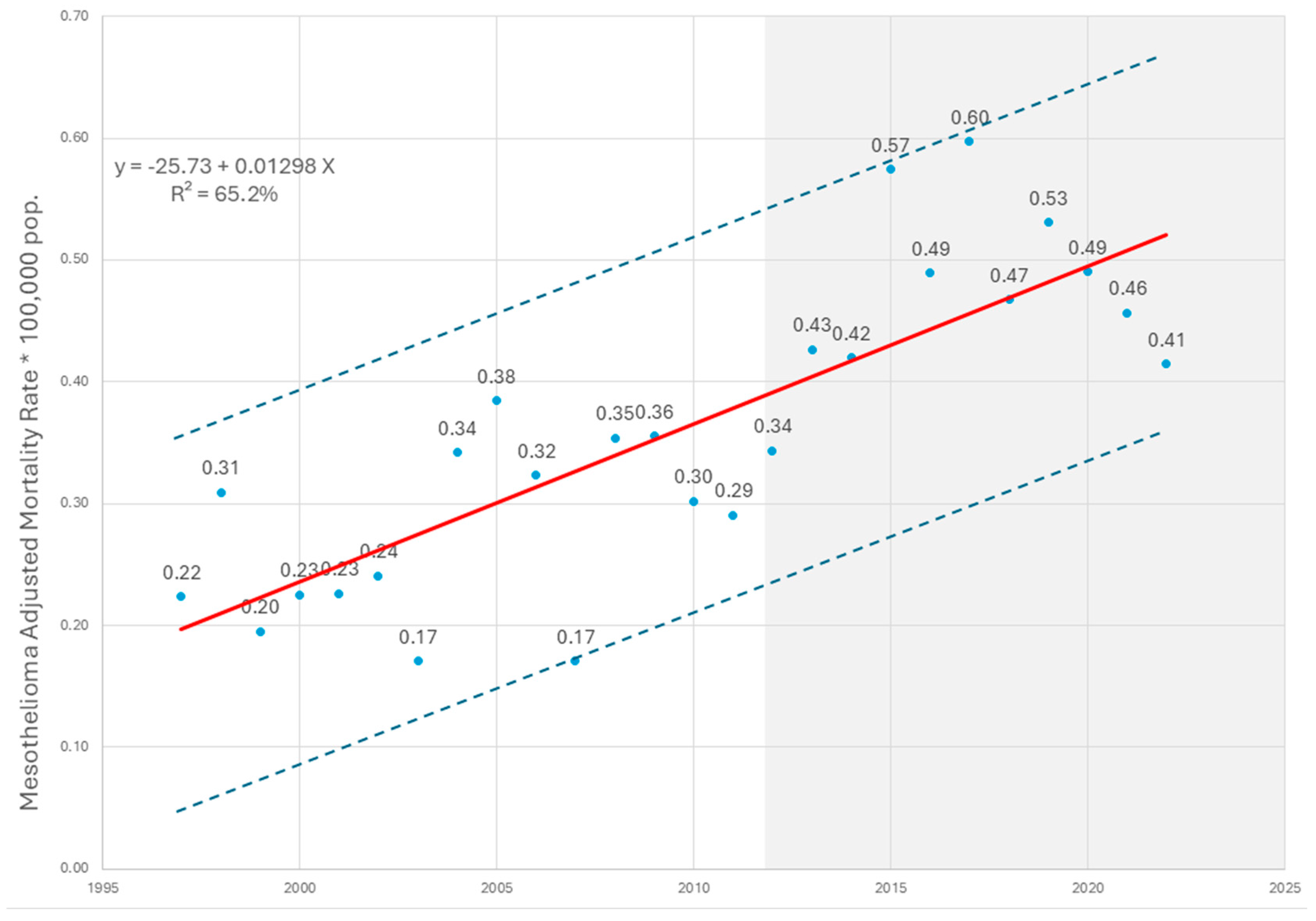
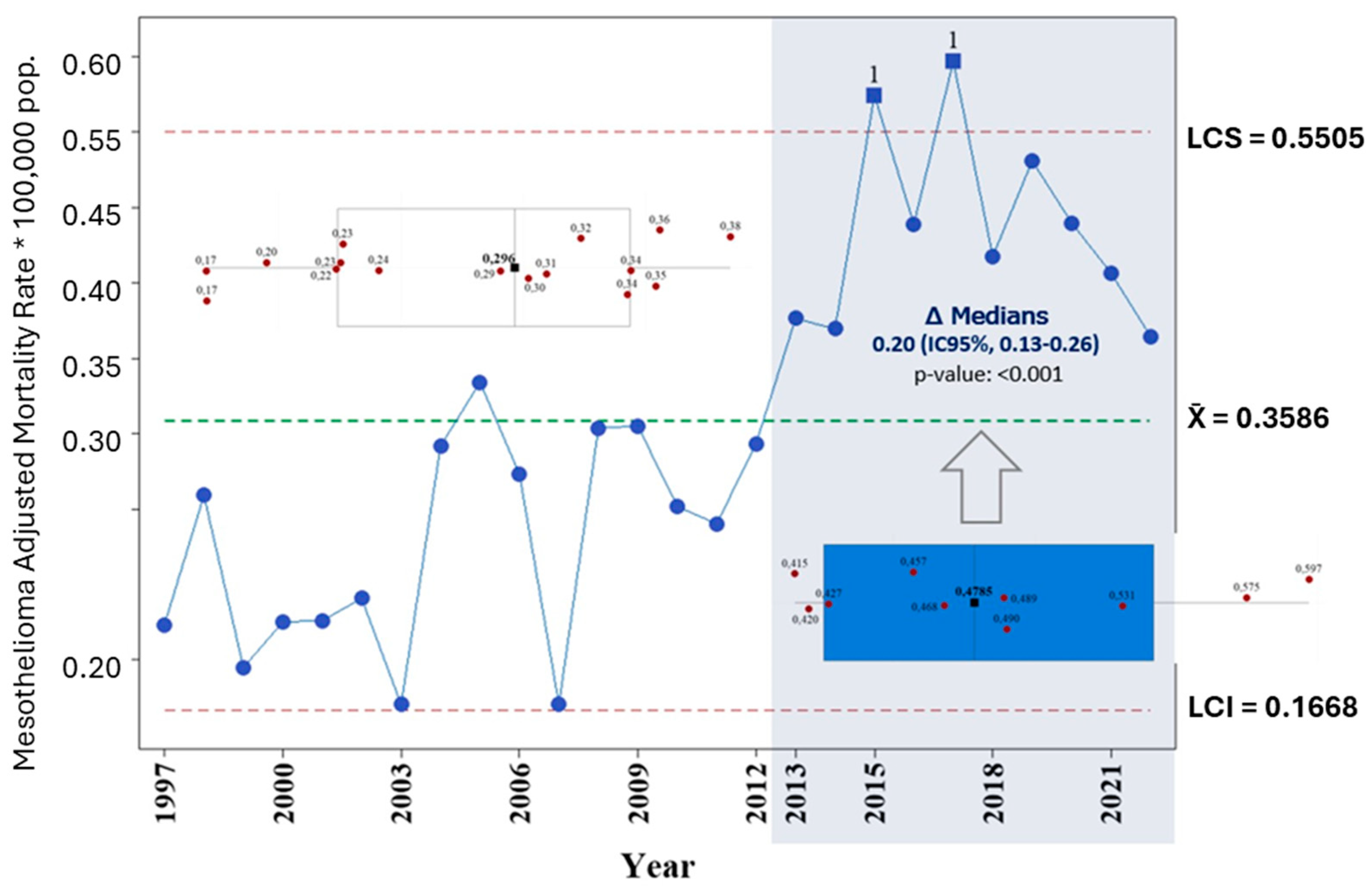
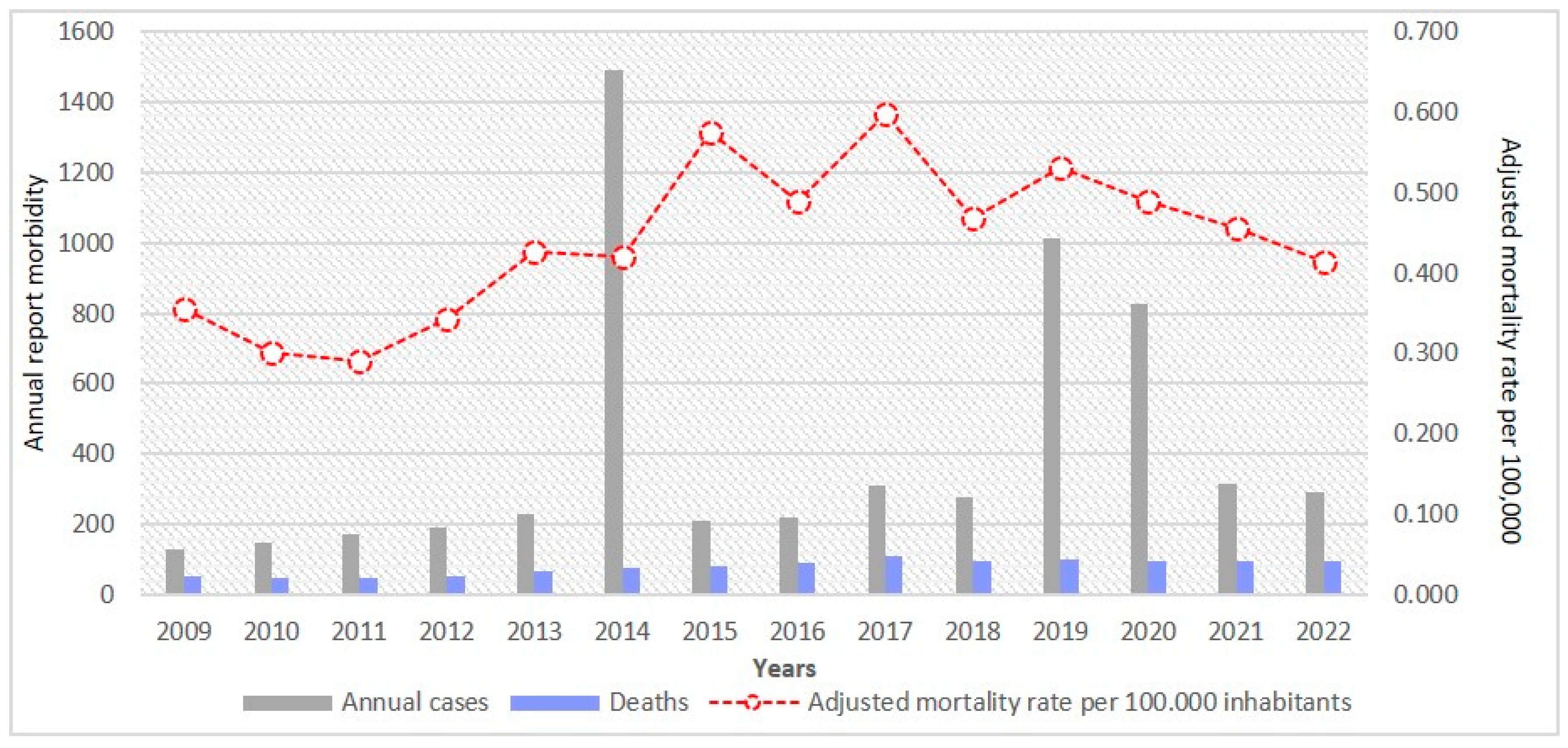
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Malignant Mesothelioma Treatment (PDQ®)-Patient Version. 2023. Available online: https://www.cancer.gov/types/mesothelioma/patient/mesothelioma-treatment-pdq (accessed on 21 March 2025).
- Gómez Trueba, G.; Collado Otero, J.C. Malignant pleural mesothelioma. Update on diagnosis and treatment. Rev. Cuba. Cir. 2020, 59, e831. [Google Scholar]
- Sejben, A.; Pancsa, T.; Tiszlavicz, L.; Furák, J.; Paróczai, D.; Zombori, T. Highlighting the immunohistochemical differences of malignant mesothelioma subtypes via case presentations. Thorac. Cancer 2023, 14, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Patel, T.; Aswal, P. Malignant mesothelioma: A clinicopathological study of 76 cases with emphasis on immunohistochemical evaluation along with review of the literature. Indian J. Pathol. Microbiol. 2021, 64, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Røe, O.D.; Stella, G.M. Malignant pleural mesothelioma: History, controversy and future of a manmade epidemic. Eur. Respir. Rev. 2015, 24, 115–131. [Google Scholar] [CrossRef]
- Furuya, S.; Chimed-Ochir, O.; Takahashi, K.; David, A.; Takala, J. Global asbestos disaster. Int. J. Environ. Res. Public Health 2018, 15, 1000. [Google Scholar] [CrossRef]
- Villamizar, G.; Camero, G. (Eds.) Asbesto en Colombia. Fundamentos para el Debate; Universidad Nacional de Colombia: Bogotá, Colombia, 2019. [Google Scholar]
- Congress of the Republic of Colombia. Law 1968 of 2019. Available online: https://www.minambiente.gov.co/documento-normativa/ley-1968-de-2019/ (accessed on 20 March 2025).
- Baur, X.; Frank, A.L.; Soskolne, C.L.; Oliver, L.C.; Magnani, C. Malignant mesothelioma: Ongoing controversies about its etiology in females. Am. J. Ind. Med. 2021, 64, 543–550. [Google Scholar] [CrossRef]
- Frank, A.L. Mesothelioma and Asbestos. In Toxicology and Risk Assessment; Fan, A.M., Khan, E.M., Alexeeff, G.V., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 289–300. [Google Scholar]
- Baris, Y.I.; Saracci, R.; Simonato, L.; Skidmore, J.W.; Artvinli, M. Malignant mesothelioma and radiological chest abnormalities in two villages in Central Turkey: An epidemiological and environmental investigation. Lancet 1981, 1, 984–987. [Google Scholar] [CrossRef]
- Comba, P.; Granfagna, A.; Paoletti, L. Pleural mesothelioma cases in Biancavilla are related to a new fluoro-edenitic fibrous amphibole. Arch. Environ. Health 2003, 58, 229–232. [Google Scholar] [CrossRef]
- Carbone, M.; Yang, H. BAP1, a new tumor suppressor gene in mesothelioma and other malignancies. Cancer Lett. 2012, 322, 48–57. [Google Scholar] [CrossRef]
- Stayner, L.; Dankovic, D.; Lemen, R. Occupational exposure to chrysotile asbestos and cancer risk: A review of the amphibole hypothesis. Am. J. Public Health 1996, 86, 179–186. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Arsenic Metals Fibres Dusts IARC Monographs on the Evaluation of Carcinogenic Risks to Humans—Volume, 1.0.0.C; World Health Organization: Geneva, Switzerland; International Agency for Research on Cancer: Lyon, France, 2012; Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Arsenic-Metals-Fibres-And-Dusts-2012 (accessed on 21 March 2025).
- Tibaduiza Torres, A.L.; Betancur Romero, J.E.; Silva Aparicio, A.; Rico Mendoza, M.A. Malignant mesothelioma in Colombia: Disease burden, overview, and subnational sociodemographic index, 2015–2020. Pan. Am. J. Public Health 2023, 47, 1. [Google Scholar]
- Marsh, G.M.; Riordan, A.S.; Keeton, K.A.; Benson, S.M. Non-occupational exposure to asbestos and risk of pleural mesothelioma: Review and meta-analysis. Occup. Environ. Med. 2017, 74, 838–846. [Google Scholar] [CrossRef] [PubMed]
- World Labour Organization; World Health Organization. Outline for the Development of National Programmes for the Elimination of Asbestos-Related Diseases; World Labour Organization: Geneva, Switzerland; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Ministry of Health of Colombia. Resolution 8430 of 1993: By Which Scientific, Technical and Administrative Standards for Health Research Are Established; Ministry of Health: Bogotá, Colombia, 1993. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/RESOLUCION-8430-DE-1993.PDF (accessed on 21 March 2025).
- Algranti, E.; Mendonça, E.M.; Carneiro, A.P.; Moreira, B.; de Capitani, E.M. The next asbestos wave: Mortality from mesothelioma in Brazil, 1980-2010. Int. J. Occup. Environ. Health 2015, 21, 190–193. [Google Scholar] [CrossRef]
- Noonan, C.W. Environmental asbestos exposure and risk of mesothelioma. Ann. Transl. Med. 2017, 5, 234. [Google Scholar] [CrossRef]
- Pan, X.L.; Day, H.W.; Wang, W.; Beckett, L.A.; Schenker, M.B. Residential proximity to naturally occurring asbestos and mesothelioma risk in California. Am. J. Respir. Crit. Care Med. 2005, 172, 1019–1025. [Google Scholar] [CrossRef]
- Grundy, G.W.; Miller, R.W. Malignant mesothelioma in childhood: Report of 13 cases. Cancer 1972, 30, 1216–1222. [Google Scholar] [CrossRef]
- Brenner, J.; Sordillo, P.P.; Magill, G.B. Malignant mesothelioma in children: Report of seven cases and review of the literature. Med. Pediatr. Oncol. 1981, 9, 317–325. [Google Scholar] [CrossRef]
- Gordon, R.E.; Fitzgerald, S.; Millette, J. Asbestos in commercial cosmetic talcum powder as a cause of mesothelioma in women. Int. J. Occup. Environ. Health 2014, 20, 318–332. [Google Scholar] [CrossRef]
- Tai, S.-Y.; Wu, J.; Lee, L.J.-H.; Lu, T.-H. Which ICD-9 codes were assigned for malignant mesothelioma in the mortality data in the United States before the ICD-10 was introduced? Am. J. Ind. Med. 2021, 64, 135–142. [Google Scholar] [CrossRef]
- E Hugo, V.; Castaño, A.; Epidemiology Directorate, A.; Team, D.M.; De, T. Excess Mortality from All Causes and COVID-19: Reclassification of Mortality Due to COVID-19; Instituto Nacional de Salud: Bogotá, Colombia, 2022. [Google Scholar]
- World Health Organization. Global Excess Mortality Associated with the COVID-19 Pandemic, January 2020–December 2021; WHO: Geneva, Switzerland, 2022; Available online: https://www.who.int/data/stories/global-excess-deaths-associated-with-covid-19-january-2020-december-2021 (accessed on 31 March 2025).
- Gamble, J.F.; Gibbs, G.W. An evaluation of the risks of lung cancer and mesothelioma from exposure to amphibole cleavage fragments. Regul. Toxicol. Pharmacol. 2008, 52 (Suppl. S1), S154–S186. [Google Scholar] [CrossRef]
- Frank, A.; Monforton, C. Comments on EPA’s Draft Risk Evaluation for Asbestos; Environmental Protection Agency: Parramatta, Australia, 2020. Available online: https://www.regulations.gov/document/EPA-HQ-OPPT-2019-0501-0046 (accessed on 21 March 2025).
- Virta, R.L. Worldwide Asbestos Supply and Consumption Trends from 1900 Through 2003. US Geological Survey Circular 1298; USGS: Reston, VA, USA, 2006. Available online: https://pubs.usgs.gov/circ/2006/1298/ (accessed on 21 March 2025).
- Congress of Colombia. Law 436 of 1998: By Which ILO Convention 162 on the Safe Use of Asbestos Is Adopted; Diario Oficial No. 43.241; Imprenta Nacional de Colombia: Bogotá, Colombia, 19 February 1998. Available online: https://www.alcaldiabogota.gov.co/sisjur/normas/Norma1.jsp?i=37926 (accessed on 31 March 2025).
- Ou, Z.; Li, X.; Cui, J.; Zhu, S.; Feng, K.; Ma, J.; Wu, K.; Chen, Y.; Su, Y.; Tang, S.; et al. Global, regional, and national burden of asbestosis from 1990 to 2019 and the implications for prevention and control. Sci. Total Environ. 2023, 904, 166346. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.A.; Lilis, R.; Daum, S.M.; Fischbein, A.S.; Selikoff, I.J. Household-contact asbestos neoplastic risk. Ann. N. Y. Acad. Sci. 1976, 271, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Inai, K. Pathology of mesothelioma. Environ. Health Prev. Med. 2008, 13, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Frost, G.; Harding, A.H.; Darnton, A.; McElvenny, D.; Morgan, D. Occupational exposure to asbestos and mortality among asbestos removal workers: A Poisson regression analysis. Br. J. Cancer 2008, 99, 822–829. [Google Scholar] [CrossRef]
- Toloza-Pérez, Y.G.; Mesa-Sierra, J.; Malagón-Rojas, J.; Niño-Barrero, Y.F. Impact of the implementation of the Occupational Health and Safety Management System on mortality and years of life potentially lost due to occupational and work-related accidents in Colombia, 2009–2021. J. Natl. Fac. Public Health 2024, 42, e352686. [Google Scholar]
- Congreso de Colombia. Ley 1562 de 2012: Por la cual se Modifica el Sistema de Riesgos Laborales y se Dictan otras Disposiciones en Materia de salud Ocupacional; Diario Oficial No. 48.488; Congreso de Colombia: Bogotá, Colombia, 2012. Available online: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/DIJ/Ley-1562-de-2012.pdf (accessed on 21 March 2025).
- Marinaccio, A.; Binazzi, A.; Bonafede, M.; Corfiati, M.; Di Marzio, D.; Scarselli, A.; Verardo, M.; Mirabelli, D.; Gennaro, V.; Mensi, C.; et al. Malignant mesothelioma due to non-occupational asbestos exposure from the Italian national surveillance system (ReNaM): Epidemiology and public health issues. Occup. Environ. Med. 2015, 72, 648–655. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare (AIHW). Mesothelioma in Australia 2021; Cancer Series no. 139; Cat. no. CAN 145; AIHW: Canberra, Australia, 2022. Available online: https://www.aihw.gov.au/reports/cancer/mesothelioma-in-australia-2021/summary (accessed on 21 March 2025).
- Health and Safety Executive (HSE). Mesothelioma Statistics for Great Britain, 2024; HSE: Bootle, UK, 2023. Available online: https://www.hse.gov.uk/statistics/assets/docs/mesothelioma.pdf (accessed on 21 March 2025).
- Driscoll, T. Asbestos-related disease in Colombia. Presentation at ADDRI/GBD Technical Session. Bogotá, 2023, unpublished presentation.

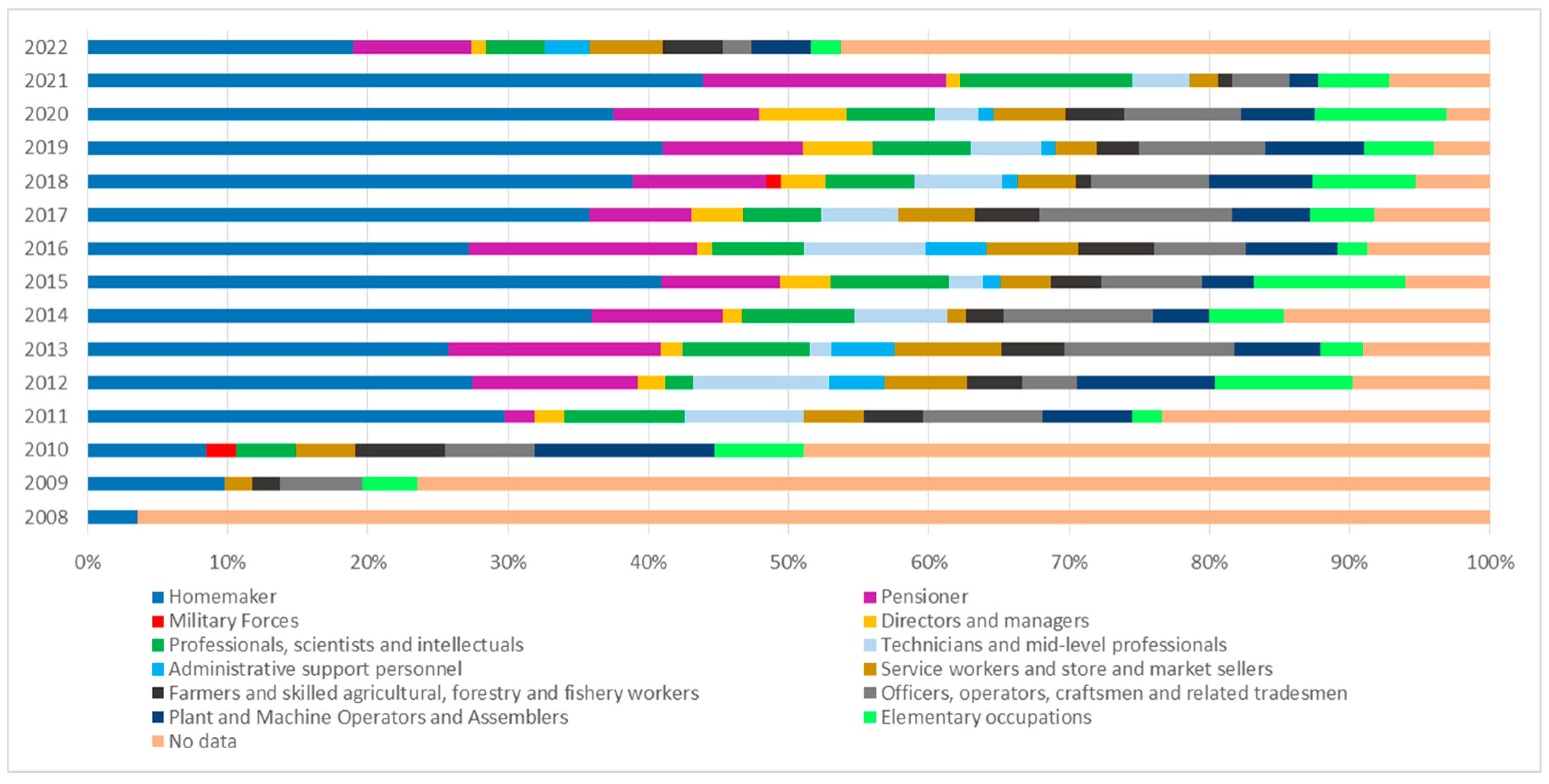

| Occupation | Deaths | Proportion |
|---|---|---|
| Housewife (Para occupational) | 356 | 23.10% |
| Pensioner | 105 | 6.80% |
| Officers, laborers, craftsmen and related trades | 86 | 5.60% |
| Professionals, scientists and intellectuals | 73 | 4.70% |
| Plant and machine operators and assemblers | 61 | 4.00% |
| Elementary occupations | 61 | 4.00% |
| Technicians and mid-level professionals | 49 | 3.20% |
| Service workers and store and market salespersons | 48 | 3.10% |
| Farmers and skilled agricultural, forestry and fishery workers | 39 | 2.50% |
| Directors and Managers | 28 | 1.80% |
| Administrative support personnel | 16 | 1.00% |
| Students | 3 | 0.20% |
| Military Forces | 2 | 0.10% |
| No data | 612 | 39.80% |
| Total | 1539 | 100% |
| Territory | 97 | 98 | 99 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bogotá | 7 | 11 | 9 | 13 | 17 | 17 | 13 | 23 | 21 | 23 | 11 | 22 | 21 | 18 | 21 | 27 | 37 | 34 | 36 | 35 | 40 | 40 | 42 | 49 | 43 | 36 |
| Cundinamarca | 5 | 4 | 4 | 2 | 4 | 6 | 8 | 8 | 3 | 5 | 7 | 11 | 10 | 6 | 8 | 9 | 12 | 14 | 13 | 17 | 15 | 18 | 15 | 13 | 24 | |
| Antioquia | 6 | 4 | 3 | 5 | 5 | 3 | 2 | 1 | 5 | 5 | 5 | 4 | 3 | 3 | 3 | 4 | 6 | 5 | 4 | 4 | 5 | 9 | 7 | 9 | 6 | |
| Valle del Cauca | 1 | 8 | 3 | 2 | 1 | 2 | 1 | 7 | 4 | 1 | 3 | 2 | 1 | 7 | 2 | 1 | 2 | 6 | 7 | 11 | 7 | 11 | 7 | 6 | 7 | 4 |
| Santander | 2 | 4 | 3 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 4 | 4 | 5 | 5 | 5 | 4 | 7 | 2 | |||
| Boyacá | 2 | 1 | 2 | 3 | 1 | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 2 | 5 | 4 | 2 | 2 | 2 | 4 | ||||||
| Nariño | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 7 | 1 | 1 | 1 | ||||||||||
| Tolima | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 2 | 5 | 3 | 2 | 2 | |||||||||||
| Atlántico | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 3 | 2 | 2 | 3 | |||||||||||
| Huila | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 4 | 1 | 1 | 1 | 1 | 3 | 2 | 1 | |||||||||||
| Magdalena | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 3 | 3 | 2 | 2 | 2 | 2 | |||||||||||||
| Bolivar | 1 | 1 | 1 | 3 | 1 | 1 | 1 | 1 | 2 | 4 | 3 | 2 | ||||||||||||||
| Caldas | 1 | 3 | 1 | 1 | 2 | 3 | 1 | 3 | 2 | 1 | 3 | |||||||||||||||
| Meta | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 3 | 2 | 1 | 3 | 1 | ||||||||||||||
| Norte de Santander | 2 | 1 | 2 | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 1 | 1 | ||||||||||||||
| Risaralda | 4 | 1 | 1 | 1 | 2 | 1 | 3 | 1 | 1 | 2 | ||||||||||||||||
| Cauca | 1 | 1 | 3 | 2 | 1 | 1 | 2 | 2 | 1 | |||||||||||||||||
| Quindío | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 1 | |||||||||||||||
| Córdoba | 1 | 1 | 2 | 1 | 2 | 1 | ||||||||||||||||||||
| Cesar | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||
| Casanare | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||||||
| Arauca | 1 | 1 | 1 | 1 | ||||||||||||||||||||||
| Chocó | 1 | 1 | 1 | |||||||||||||||||||||||
| La Guajira | 1 | 2 | ||||||||||||||||||||||||
| Sucre | 1 | 1 | 1 | |||||||||||||||||||||||
| Putumayo | 1 | 1 | 1 | |||||||||||||||||||||||
| Vaupés | 1 | 1 | ||||||||||||||||||||||||
| Caquetá | 1 | |||||||||||||||||||||||||
| Guaviare | 1 | |||||||||||||||||||||||||
| Vichada | 1 | |||||||||||||||||||||||||
| No data | 4 | 1 | 1 | |||||||||||||||||||||||
| 24 | 36 | 32 | 32 | 33 | 34 | 31 | 46 | 47 | 39 | 24 | 56 | 51 | 47 | 47 | 51 | 66 | 75 | 83 | 92 | 109 | 95 | 100 | 96 | 98 | 95 |
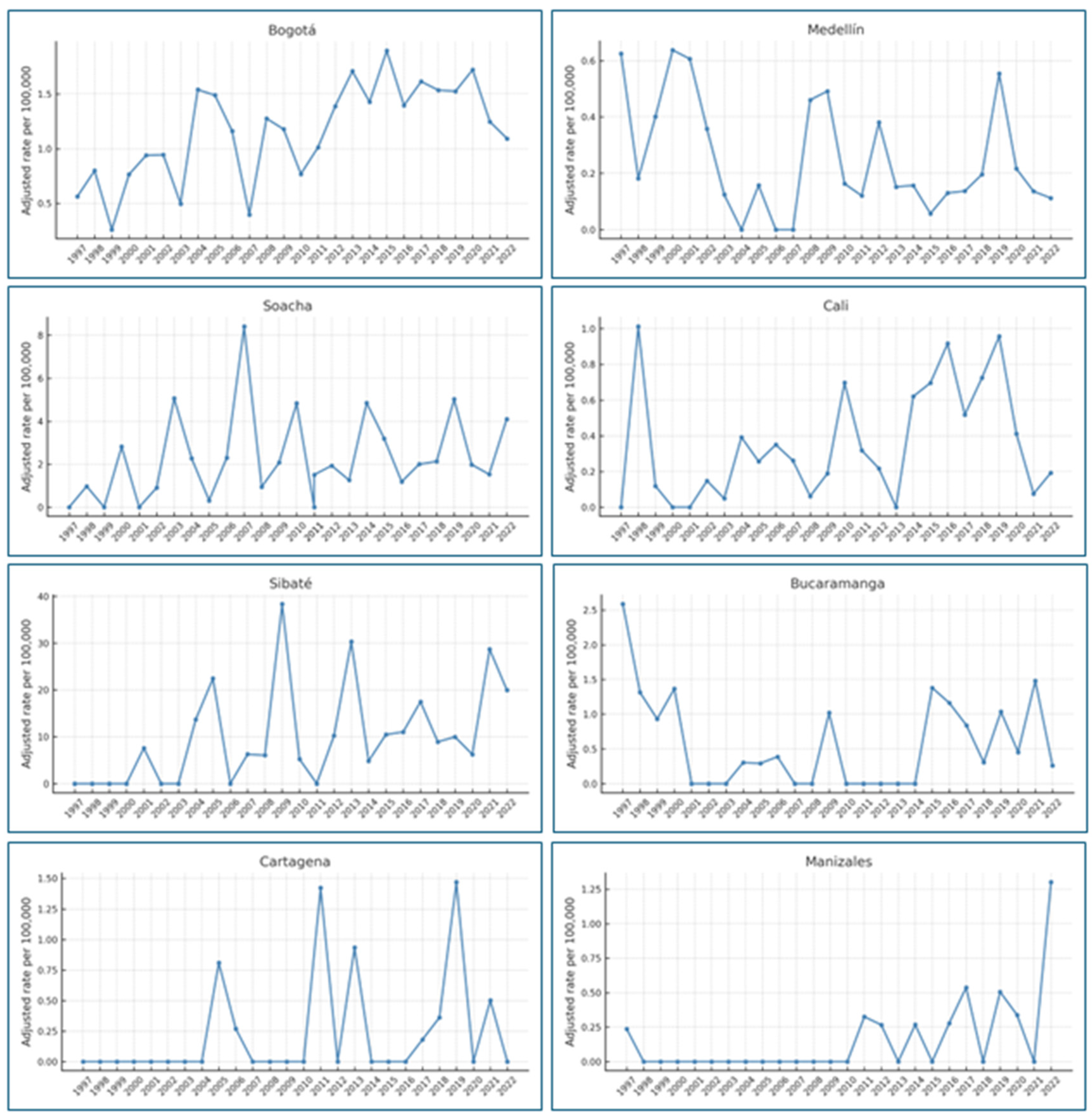
| City | Number of Years with Mortality | Minimum Rate | Maximum Rate |
|---|---|---|---|
| Bogotá | 26 | 0.25 | 1.86 |
| Medellín | 23 | 0.05 | 0.63 |
| Soacha | 23 | 0.30 | 8.41 |
| Cali | 22 | 0.04 | 1.01 |
| Sibaté | 18 | 4.80 | 38.36 |
| Bucaramanga | 16 | 0.25 | 2.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano-Ariza, L.F.; Villamizar, G.; Henríquez-Mendoza, G.; Frank, A.; Camero, G. Epidemiological Trends in Mesothelioma Mortality in Colombia (1997–2022): A Retrospective National Study. Int. J. Environ. Res. Public Health 2025, 22, 787. https://doi.org/10.3390/ijerph22050787
Moyano-Ariza LF, Villamizar G, Henríquez-Mendoza G, Frank A, Camero G. Epidemiological Trends in Mesothelioma Mortality in Colombia (1997–2022): A Retrospective National Study. International Journal of Environmental Research and Public Health. 2025; 22(5):787. https://doi.org/10.3390/ijerph22050787
Chicago/Turabian StyleMoyano-Ariza, Luisa F., Guillermo Villamizar, Giana Henríquez-Mendoza, Arthur Frank, and Gabriel Camero. 2025. "Epidemiological Trends in Mesothelioma Mortality in Colombia (1997–2022): A Retrospective National Study" International Journal of Environmental Research and Public Health 22, no. 5: 787. https://doi.org/10.3390/ijerph22050787
APA StyleMoyano-Ariza, L. F., Villamizar, G., Henríquez-Mendoza, G., Frank, A., & Camero, G. (2025). Epidemiological Trends in Mesothelioma Mortality in Colombia (1997–2022): A Retrospective National Study. International Journal of Environmental Research and Public Health, 22(5), 787. https://doi.org/10.3390/ijerph22050787







