Comparison of Temporomandibular Disorder Signs and Symptoms in CrossFit® Athletes and Sedentary Individuals
Abstract
1. Introduction
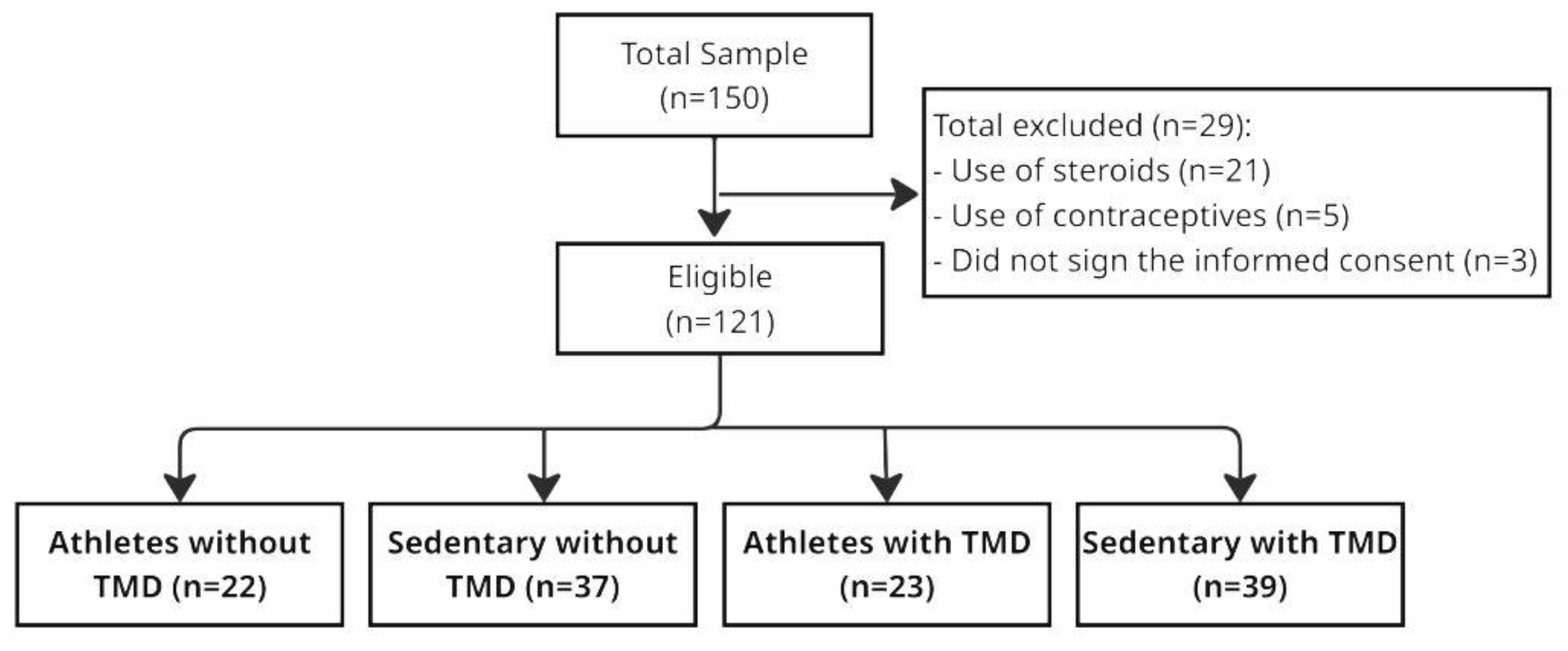
2. Materials and Methods
2.1. Study Design
2.2. Measurements and Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- List, T.; Jensen, R.H. Temporomandibular disorders: Old ideas and new concepts. Cephalalgia 2017, 37, 692–704. [Google Scholar] [CrossRef] [PubMed]
- Leeuw, R.; Klasser, G. Orofacial Pain: Guidelines for Assessment, Diagnosis, and Management; Quintessence: Chicago, IL, USA, 2018; 368p. [Google Scholar]
- Valesan, L.F.; Da-Cas, C.D.; Réus, J.C.; Denardin, A.C.S.; Garanhani, R.R.; Bonotto, D.; Januzzi, E.; de Souza, B.D.M. Prevalence of temporomandibular joint disorders: A systematic review and meta-analysis. Clin. Oral. Investig. 2021, 25, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Bair, E.; Fillingim, R.B.; Gonzalez, Y.; Gordon, S.M.; Lim, P.F.; Ribeiro-Dasilva, M.; Diatchenko, L.; Dubner, R.; Greenspan, J.D.; et al. Clinical orofacial characteristics associated with risk of first-onset TMD: The OPPERA prospective cohort study. Pain 2013, 14, T33–T50. [Google Scholar] [CrossRef]
- Freiwald, H.C.; Schwarzbach, N.P.; Wolowski, A. Impact of sports on temporomandibular dysfunction: A comparison of competitive and recreational female athletes as well as female non-athletes. Clin. Oral. Investig. 2022, 26, 5313–5323. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Abbasi, A.J.; Noorbala, A.A.; Mohebbi, S.Z.; Moharrami, M.; Yekaninejad, M.S. Oral health-related quality of life in patients with temporomandibular disorders: A case-control study considering psychological aspects. Int. J. Dent. Hyg. 2018, 16, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Aguilera, A.; Blanco-Hungría, A.; Biedma-Velázquez, L.; Serrano-Del-Rosal, R.; González-López, L.; Blanco-Aguilera, E.; Segura-Saint-Gerons, R. Application of an oral health-related quality of life questionnaire in primary care patients with orofacial pain and temporomandibular disorders. Med. Oral. Patol. Oral. Cir. Bucal 2014, 19, e127–e135. [Google Scholar] [CrossRef]
- McNeill, C. Management of temporomandibular disorders: Concepts and controversies. J. Prosthet. Dent. 1997, 77, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, A.; Paris-Alemany, A.; López-de-Uralde-Villanueva, I.; La Touche, R. Management of pain in patients with temporomandibular disorder (TMD): Challenges and solutions. J. Pain. Res. 2018, 11, 571–587. [Google Scholar] [CrossRef]
- Messina, G. The role of the styloid apophysis of the temporal bone in the biomechanics of the tongue, mandible, hyoid system: A case study. Eur. J. Transl. Myol. 2020, 30, 8808. [Google Scholar] [CrossRef]
- Talaat, W.M.; Adel, O.I.; Al Bayatti, S. Prevalence of temporomandibular disorders discovered incidentally during routine dental examination using the Research Diagnostic Criteria for Temporomandibular Disorders. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2017, 125, 250–259. [Google Scholar] [CrossRef]
- Meyer, J.; Morrison, J.; Zuniga, J. The benefits and risks of CrossFit: A systematic review. Workplace Health Saf. 2017, 65, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Szeles, P.R.Q.; da Costa, T.S.; da Cunha, R.A.; Hespanhol, L.; Pochini, A.C.; Ramos, L.A.; Cohen, M. CrossFit and the epidemiology of musculoskeletal injuries: A prospective 12-week cohort study. Orthop. J. Sports Med. 2020, 8, 2325967120908884. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, M.F.; Nindl, B.C.; Deuster, P.A.; Baumgartner, N.; Kane, S.F.; Kraemer, W.J.; Sexauer, L.R.; Thompson, W.R.; O’Connor, F.G. Consortium for health and military performance and American College of Sports Medicine consensus paper on extreme conditioning programs in military personnel. Curr. Sport. Med. Rep. 2011, 10, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, H.; Sagar, S.S.; Stenling, A. Fear of failure, psychological stress, and burnout among adolescent athletes competing in high level sport. Scand. J. Med. Sci. Sports 2017, 27, 2091–2102. [Google Scholar] [CrossRef]
- Yang, S.X.; Cheng, S.; Su, D.L. Sports injury and stressor-related disorder in competitive athletes: A systematic review and a new framework. Burns Trauma 2022, 10, tkac017. [Google Scholar] [CrossRef]
- Burrus, M.T.; Werner, B.C.; Starman, J.S.; Gwathmey, F.W.; Carson, E.W.; Wilder, R.P.; Diduch, D.R. Chronic leg pain in athletes. Am. J. Sports Med. 2015, 43, 1538–1547. [Google Scholar] [CrossRef]
- Fett, D.; Trompeter, K.; Platen, P. Back. pain in elite sports: A cross-sectional study on 1114 athletes. PLoS ONE 2017, 12, e0180130. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.C.; Straltsova, H.; Woodgate, M.A.; Kim, K.M.; Lee, J.M.; Lee, J.H.; Gann, J.J. Water ski injuries and chronic pain in collegiate athletes. Int. J. Environ. Res. Public Health 2021, 18, 3939. [Google Scholar] [CrossRef]
- Prasad, S.; Ramanan, D.; Bennani, H.; Paulin, M.; Cannon, R.D.; Palla, S.; Farella, M. Associations among masticatory muscle activity, physical activity and self-reported oral behaviours in adult women. Clin. Oral. Investig. 2021, 25, 5049–5059. [Google Scholar] [CrossRef]
- Starr, C.L.; McGrew, C. TMJ disorders in athletes. Curr. Sport. Med. Rep. 2023, 22, 10–14. [Google Scholar] [CrossRef]
- WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020.
- Pereira, F.J., Jr.; Favilla, E.E.; Dworkin, S.; Huggins, K. Research diagnostic criteria for temporomandibular disorders (RDC/TMD): Formal translation to portuguese. JBC J. Bras. Clin. Odontol. Integr. 2004, 8, 384–395. [Google Scholar]
- Manfredini, D.; Chiappe, G.G.; Bosco, M. Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD) axis I diagnoses in an Italian patient population. J. Oral. Rehabil. 2006, 33, 551–558. [Google Scholar] [CrossRef]
- Bonotto, D.; Namba, E.L.; Veiga, D.M.; Wandembruck, F.; Mussi, F.; Cunali, P.A.; Rosa, E.A.R.; Azevedo-Alanis, L.R. Professional karate-do and mixed martial arts fighters present with a high prevalence of temporomandibular disorders. Dent. Traumatol. 2016, 32, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Ohrbach, R.; Gonzalez, Y.; List, T.; Michelotti, A.; Schiffman, E. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) Clinical Examination Protocol. 2014. Available online: https://www.rdc-tmdinternational.org/ (accessed on 5 August 2019).
- García-Perez, M.A. Use and misuse of corrections for multiple testing. Methods Psychol. 2023, 8, 100120. [Google Scholar] [CrossRef]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: Recommendations of the International RDC/TMD Consortium Network* and Orofacial Pain Special Interest Group. J. Oral. Facial Pain. Headache 2014, 28, 6–27. [Google Scholar] [CrossRef] [PubMed]
- Melo, V.; Monteiro, L.; Orge, C.; Sales, M.; Melo, J.; Rodrigues, B.; Melo, A. Prevalence of temporomandibular disorders in the Brazilian population: A systematic review and meta-analysis. Cranio 2023, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, M.B.; Jensen, T.T. Exercise addiction in CrossFit: Prevalence and psychometric properties of the exercise addiction inventory. Addict. Behav. Rep. 2016, 3, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Ángel Rodríguez, M.; García-Calleja, P.; Terrados, N.; Crespo, I.; Del Valle, M.; Olmedillas, H. Injury in CrossFit®: A systematic review of epidemiology and risk factors. Phys. Sportsmed. 2022, 50, 3–10. [Google Scholar] [CrossRef]
- Poluha, R.L.; Canales, G.T.; Costa, Y.M.; Grossmann, E.; Bonjardim, L.R.; Conti, P.C.R. Temporomandibular joint disc displacement with reduction: A review of mechanisms and clinical presentation. J. Appl. Oral. Sci. 2019, 27, e20180433. [Google Scholar] [CrossRef]
- Freiwald, H.C.; Schwarzbach, N.P.; Wolowski, A. Effects of competitive sports on temporomandibular dysfunction: A literature review. Clin. Oral. Investig. 2021, 25, 55–65. [Google Scholar] [CrossRef]
- Mendoza-Puente, M.; Oliva-Pascual-Vaca, Á.; Rodriguez-Blanco, C.; Heredia-Rizo, A.M.; Torres-Lagares, D.; Ordoñez, F.J. Risk of headache, temporomandibular dysfunction, and local sensitization in male professional boxers: A case-control study. Arch. Phys. Med. Rehabil. 2014, 95, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, D.; Penteado, C.A.; Namba, E.L.; Cunali, P.A.; Rached, R.N.; Azevedo-Alanis, L.R. Prevalence of temporomandibular disorders in rugby players. Gen. Dent. 2019, 67, 72–74. [Google Scholar] [PubMed]
- Willigenburg, N.W.; Borchers, J.R.; Quincy, R.; Kaeding, C.C.; Hewett, T.E. Comparison of injuries in american collegiate football and club rugby: A prospective cohort study. Am. J. Sports Med. 2016, 44, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Hilgenberg-Sydney, P.B.; Wilhelm, J.M.; Pimentel, G.; Petterle, R.; Bonotto, D. Prevalence of temporomandibular disorders and anxiety state levels in ballet dancers a cross-sectional study. J. Dance Med. Sci. 2020, 24, 88–92. [Google Scholar] [CrossRef]
- Saccomanno, S.; Saran, S.; De Luca, M.; Mastrapasqua, R.F.; Raffaelli, L.; Levrini, L. The influence of SARS-CoV-2 pandemic on TMJ disorders, OSAS and BMI. Int. J. Environ. Res. Public Health 2022, 19, 7154. [Google Scholar] [CrossRef] [PubMed]
- Naeije, M.; Veldhuis AHTe Veldhuis ECTe Visscher, C.M.; Lobbezoo, F. Disc displacement within the human temporomandibular joint: A systematic review of a ‘noisy annoyance’. J. Oral. Rehabil. 2013, 40, 139–158. [Google Scholar] [CrossRef]
- Dworkin, S.F.; LeResche, L. Research diagnostic criteria for temporomandibular disorders: Review, criteria, examinations and specifications. J. Craniomandib. Disord. 1992, 6, 301–355. [Google Scholar]
- Dinsdale, A.; Liang, Z.; Thomas, L.; Treleaven, J. Are jaw range of motion, muscle function and proprioception impaired in adults with persistent temporomandibular disorders? A systematic review and meta-analysis. J. Oral. Rehabil. 2020, 47, 1448–1478. [Google Scholar] [CrossRef]
- Senba, E.; Kami, K. A new aspect of chronic pain as a lifestyle-related disease. Neurobiol. Pain 2017, 1, 6–15. [Google Scholar] [CrossRef]
- Kroll, H.R. Exercise therapy for chronic pain. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 263–281. [Google Scholar] [CrossRef]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 1, CD011279. [Google Scholar] [PubMed]
- Sluka, K.A.; Frey-Law, L.; Bement, M.H. Exercise-induced pain and analgesia? Underlying mechanisms and clinical translation. Pain. 2018, 159, S91–S97. [Google Scholar] [CrossRef] [PubMed]
- Borisovskaya, A.; Chmelik, E.; Karnik, A. Exercise and chronic pain. Adv. Exp. Med. Biol. 2020, 1228, 233–253. [Google Scholar] [PubMed]
- Cooper, M.A.; Kluding, P.M.; Wright, D.E. Emerging relationships between exercise, sensory nerves, and neuropathic pain. Front. Neurosci. 2016, 10, 372. [Google Scholar] [CrossRef]
- Naugle, K.M.; Fillingim, R.B.; Riley, J. A meta-analytic review of the hypoalgesic effects of exercise. J. Pain. 2012, 13, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.L. Tissue trauma: The underlying cause of overtraining syndrome? J. Strength. Cond. Res. 2004, 18, 185–193. [Google Scholar] [CrossRef]
- da Rocha, A.L.; Pinto, A.P.; Kohama, E.B.; Pauli, J.R.; de Moura, L.P.; Cintra, D.E.; Ropelle, E.R.; da Silva, A.S.R. The proinflammatory effects of chronic excessive exercise. Cytokine 2019, 119, 57–61. [Google Scholar] [CrossRef]
- Lee, E.; Kim, Y. Effect of university students’ sedentary behavior on stress, anxiety, and depression. Perspect. Psychiatr. Care 2019, 55, 164–169. [Google Scholar] [CrossRef]
- O’hrbach, R.; Dworkin, S.F. The Evolution of TMD Diagnosis: Past, Present, Future. J. Dent. Res. 2016, 95, 1093–1101. [Google Scholar] [CrossRef]
| Variables | Athletes Without TMD | Sedentary Without TMD | Athletes with TMD | Sedentary with TMD | p-Value |
|---|---|---|---|---|---|
| Presence of deviation or deflection | 0.13 | ||||
| Absent | 2 (9.1%) | 5 (13.5%) | 0 (0.0%) | 1 (2.6%) | |
| Present | 20 (90.9%) | 32 (86.5%) | 23 (100.0%) | 38 (97.4%) | |
| Opening noises | <0.001 | ||||
| Absent | 18 (81.8%) | 26 (70.3%) | 5 (21.7%) | 20 (51.3%) | |
| Present | 4 (18.2%) | 11 (29.7%) | 18 (78.3%) | 19 (48.7%) | |
| Closing noises | 0.069 | ||||
| Absent | 19 (86.4%) | 32 (86.5%) | 16 (69.6%) | 25 (64.1%) | |
| Present | 3 (13.6%) | 5 (13.5%) | 7 (30.4%) | 14 (35.9%) | |
| Reciprocal click eliminated during protrusive opening | 0.027 | ||||
| Absent | 22 (100.0%) | 37 (100.0%) | 21 (91.3%) | 33 (84.6%) | |
| Present | 0 (0.0%) | 0 (0.0%) | 2 (8.7%) | 6 (15.4%) | |
| Lateral motion noises | <0.001 | ||||
| Absent | 17 (77.3%) | 35 (94.6%) | 8 (34.8%) | 22 (56.4%) | |
| Present | 5 (22.7%) | 2 (5.4%) | 15 (65.2%) | 17 (43.6%) | |
| Protrusion noises | 0.002 | ||||
| Absent | 21 (95.5%) | 32 (86.5%) | 12 (52.2%) | 29 (74.4%) | |
| Present | 1 (4.5%) | 5 (13.5%) | 11 (47.8%) | 10 (25.6%) | |
| Variables | Athletes Without TMD (n = 22) | Sedentary Without TMD (n = 37) | Athletes with TMD (n = 23) | Sedentary with TMD (n = 39) | p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | Mean (SD) | Median (IQR) | ||
| Unassisted opening without pain (mm) | 41.50 (5.36) a | 41.50 (39.00–45.00) | 32.38 (8.05) b | 32.00 (27.50–35.00) | 40.87 (7.49) a | 39.00 (35.00–48.00) | 34.82 (10.83) b | 35.00 (27.00–44.00) | <0.001 |
| Max. unassisted opening (mm) | 52.91 (6.19) | 52.00 (49.75–55.00) | 49.24 (6.63) | 49.00 (44.50–55.00) | 50.70 (8.76) | 50.00 (45.00–59.00) | 47.28 (11.42) | 50.00 (42.00–55.00) | 0.061 |
| Max. assisted opening (mm) | 55.32 (7.00) | 54.50 (51.00–59.25) | 52.35 (6.04) | 53.00 (48.00–57.50) | 53.39 (8.81) | 53.00 (47.00–62.00) | 50.46 (10.85) | 54.00 (45.00–57.00) | 0.12 |
| Right lateral motion (mm) | 8.68 (1.84) | 9.00 (7.00–10.00) | 8.54 (2.88) | 8.00 (6.50–10.50) | 9.48 (3.19) | 10.00 (8.00–12.00) | 8.59 (3.48) | 8.00 (6.00–10.00) | 0.90 |
| Left lateral motion (mm) | 7.77 (1.54) | 8.00 (7.00–9.00) | 8.03 (2.70) | 8.00 (6.00–10.00) | 7.70 (2.53) | 8.00 (6.00–9.00) | 8.49 (3.56) | 8.00 (6.00–10.00) | 0.84 |
| Protrusion (mm) | 5.77 (2.02) a | 6.00 (4.75–6.00) | 4. 68 (2.44) ab | 3.00 (5.00–6.00) | 5. 48 (3.13) ab | 5.00 (4.00–7.00) | 4.28 (2.96) b | 4.00 (2.00–5.00) | 0.039 |
| Variables | Athletes Without TMD | Sedentary Without TMD | Athletes with TMD | Sedentary with TMD | p-Value |
|---|---|---|---|---|---|
| Report of pain | <0.001 | ||||
| Absent | 22 (100.0%) | 27 (73.0%) | 13 (56.5%) | 6 (15.4%) | |
| Present | 0 (0.0%) | 10 (27.0%) | 10 (43.5%) | 33 (84.6%) | |
| Indication of pain in muscle and/or TMJ by the participant | <0.001 | ||||
| Absent | 22 (100.0%) | 37 (100.0%) | 13 (56.5%) | 3 (7.7%) | |
| Present | 0 (0.0%) | 0 (0.0%) | 10 (43.5%) | 36 (92.3%) | |
| Temporal pain | <0.001 | ||||
| Absent | 22 (100.0%) | 36 (97.3%) | 16 (69.6%) | 13 (33.3%) | |
| Present | 0 (0.0%) | 1 (2.7%) | 7 (30.4%) | 26 (66.7%) | |
| Masseter pain | <0.001 | ||||
| Absent | 21 (95.5%) | 35 (94.6%) | 16 (69.6%) | 4 (10.3%) | |
| Present | 1 (4.5%) | 2 (5.4%) | 7 (30.4%) | 35 (89.7%) | |
| Posterior digastric pain | <0.001 | ||||
| Absent | 20 (90.9%) | 36 (97.3%) | 16 (69.6%) | 14 (35.9%) | |
| Present | 2 (9.1%) | 1 (2.7%) | 7 (30.4%) | 25 (64.1%) | |
| Medial pterygoid pain | <0.001 | ||||
| Absent | 19 (86.4%) | 35 (94.6%) | 17 (73.9%) | 12 (30.8%) | |
| Present | 3 (13.6%) | 2 (5.4%) | 6 (26.1%) | 27 (69.2%) | |
| TMJ pain | <0.001 | ||||
| Absent | 21 (95.5%) | 36 (97.3%) | 18 (78.3%) | 12 (30.8%) | |
| Present | 1 (4.5%) | 1 (2.7%) | 5 (21.7%) | 27 (69.2%) | |
| Temporal tendon pain | <0.001 | ||||
| Absent | 19 (86.4%) | 34 (91.9%) | 14 (60.9%) | 13 (33.3%) | |
| Present | 3 (13.6%) | 3 (8.1%) | 9 (39.1%) | 26 (66.7%) |
| Variables | OR (95%CI) | p-Value |
|---|---|---|
| Report of pain | ||
| Sedentary vs. athletes | 9.53 (3.16–28.78) | <0.001 |
| With TMD vs. without TMD | 19.24 (6.63–55.85) | <0.001 |
| Indication of pain in muscle and/or TMJ by the participant | ||
| Sedentary vs. athletes | 15.60 (3.70–65.69) | <0.001 |
| With TMD vs. without TMD | - | - |
| Presence of deviation or deflection | ||
| Sedentary vs. athletes | 0.53 (0.10–2.81) | 0.45 |
| With TMD vs. without TMD | 8.29 (0.98–69.77) | 0.052 |
| Opening noises | ||
| Sedentary vs. athletes | 0.64 (0.29–1.43) | 0.28 |
| With TMD vs. without TMD | 4.41 (2.02–9.64) | <0.001 |
| Closing noises | ||
| Sedentary vs. athletes | 1.17 (0.48–2.89) | 0.73 |
| With TMD vs. without TMD | 3.27 (1.31–8.14) | 0.011 |
| Reciprocal click eliminated during protrusive opening | ||
| Sedentary vs. athletes | 1.91 (0.35–10.36) | 0.45 |
| With TMD vs. without TMD | - | - |
| Lateral motion noises | ||
| Sedentary vs. athletes | 0.33 (0.13–0.82) | 0.016 |
| With TMD vs. without TMD | 8.98 (3.38–23.85) | <0.001 |
| Protrusion noises | ||
| Sedentary vs. athletes | 0.65 (0.26–1.62) | 0.35 |
| With TMD vs. without TMD | 4.58 (1.69–12.43) | 0.003 |
| Temporal pain | ||
| Sedentary vs. athletes | 4.74 (1.58–14.20) | 0.005 |
| With TMD vs. without TMD | 82.53 (10.42–653.92) | <0.001 |
| Masseter pain | ||
| Sedentary vs. athletes | 13.51 (3.88–47.07) | <0.001 |
| With TMD vs. without TMD | 83.64 (19.11–366.15) | <0.001 |
| Posterior digastric pain | ||
| Sedentary vs. athletes | 2.64 (0.97–7.18) | 0.057 |
| With TMD vs. without TMD | 21.74 (6.02–78.52) | <0.001 |
| Medial pterygoid pain | ||
| Sedentary vs. athletes | 3.19 (1.20–8.51) | 0.021 |
| With TMD vs. without TMD | 13.85 (4.72–40.60) | <0.001 |
| TMJ pain | ||
| Sedentary vs. athletes | 6.04 (1.97–18.55) | 0.002 |
| With TMD vs. without TMD | 39.64 (8.47–185.43) | <0.001 |
| Temporal tendon pain | ||
| Sedentary vs. athletes | 1.98 (0.79–4.99) | 0.15 |
| With TMD vs. without TMD | 11.99 (4.43–32.47) | <0.001 |
| Variables | (95%CI) * | p-Value |
|---|---|---|
| Unassisted opening without pain | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.12 (−0.17; −0.07) | <0.001 |
| Athletes with TMD | −0.01 (−0.05; 0.03) | 0.62 |
| Sedentary with TMD | −0.10 (−0.16; −0.03) | 0.005 |
| Max. unassisted opening | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.03 (−0.06; −0.003) | 0.033 § |
| Athletes with TMD | −0.02 (−0.06; 0.02) | 0.25 |
| Sedentary with TMD | −0.06 (−0.12; −0.01) | 0.032 § |
| Max. assisted opening | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.02 (−0.05; 0.004) | 0.097 |
| Athletes with TMD | −0.02 (−0.06; 0.02) | 0.35 |
| Sedentary with TMD | −0.05 (−0.10; 0.001) | 0.055 |
| Right lateral motion | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.02 (−0.09; 0.05) | 0.55 |
| Athletes with TMD | 0.003 (−0.11; 0.11) | 0.96 |
| Sedentary with TMD | −0.02 (−0.09; 0.05) | 0.53 |
| Left lateral motion | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.003 (−0.07; 0.07) | 0.94 |
| Athletes with TMD | −0.03 (−0.13; 0.07) | 0.54 |
| Sedentary with TMD | 0.01 (−0.07; 0.09) | 0.83 |
| Protrusion | ||
| Athletes without TMD | Ref. | |
| Sedentary without TMD | −0.10 (−0.19; −0.01) | 0.040 § |
| Athletes with TMD | −0.05 (−0.17; 0.06) | 0.36 |
| Sedentary with TMD | −0.17 (−0.31; −0.04) | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, A.P.V.B.; Cardoso, R.L.F.; de Andrade, C.C.F.V.; de Oliveira, J.M.D.; D’Arce, M.B.F.; Ribeiro, A.B.; de Arruda, C.N.F.; Ribeiro de Andrade, J.S.; Miarka, B.; Badaró, M.M. Comparison of Temporomandibular Disorder Signs and Symptoms in CrossFit® Athletes and Sedentary Individuals. Int. J. Environ. Res. Public Health 2025, 22, 785. https://doi.org/10.3390/ijerph22050785
Martins APVB, Cardoso RLF, de Andrade CCFV, de Oliveira JMD, D’Arce MBF, Ribeiro AB, de Arruda CNF, Ribeiro de Andrade JS, Miarka B, Badaró MM. Comparison of Temporomandibular Disorder Signs and Symptoms in CrossFit® Athletes and Sedentary Individuals. International Journal of Environmental Research and Public Health. 2025; 22(5):785. https://doi.org/10.3390/ijerph22050785
Chicago/Turabian StyleMartins, Ana Paula Varela Brown, Ranele Luiza Ferreira Cardoso, Caio César Ferreira Versiani de Andrade, Júlia Meller Dias de Oliveira, Maria Beatriz Freitas D’Arce, Adriana Barbosa Ribeiro, Carolina Noronha Ferraz de Arruda, Juliana Silva Ribeiro de Andrade, Bianca Miarka, and Maurício Malheiros Badaró. 2025. "Comparison of Temporomandibular Disorder Signs and Symptoms in CrossFit® Athletes and Sedentary Individuals" International Journal of Environmental Research and Public Health 22, no. 5: 785. https://doi.org/10.3390/ijerph22050785
APA StyleMartins, A. P. V. B., Cardoso, R. L. F., de Andrade, C. C. F. V., de Oliveira, J. M. D., D’Arce, M. B. F., Ribeiro, A. B., de Arruda, C. N. F., Ribeiro de Andrade, J. S., Miarka, B., & Badaró, M. M. (2025). Comparison of Temporomandibular Disorder Signs and Symptoms in CrossFit® Athletes and Sedentary Individuals. International Journal of Environmental Research and Public Health, 22(5), 785. https://doi.org/10.3390/ijerph22050785







