Multimorbidity Patterns and Functioning Associations Among Adults in a Local South African Setting: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Setting, and Recruitment
2.2. Sample Size Considerations
2.3. Health Conditions and Multimorbidity
2.4. Functioning Outcomes
2.5. Sociodemographic and Lifestyle-Related Variables
2.6. Procedures
2.7. Statistical Analysis
2.7.1. Exploratory Factor Analysis (Pattern Analysis)
2.7.2. Associations with Functioning Outcomes
2.8. Ethical Considerations
3. Results
3.1. Participant Characteristics
3.2. Multimorbidity Patterns
3.3. Associations Between Multimorbidity Patterns and Functioning
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5STS | Five-times sit-to-stand test |
| ACE | Angiotensin-converting enzyme |
| ACR | Albumin to creatinine ratio |
| ABET | Adult basic education training |
| ALP | Alkaline phosphatase |
| ALT | Alanine aminotransferase |
| ART | Antiretroviral therapy |
| AST | Aspartate aminotransferase |
| BMI | Body mass index |
| CHCs | Community health centres |
| CI | Confidence interval |
| COPD | Chronic obstructive pulmonary disease |
| CES-D-10 | Centre for Epidemiologic Studies Short Depression Scale 10 |
| EFA | Exploratory factor analysis |
| eGFR | Estimated glomerular filtration rate |
| GGT | Gamma-glutamyl transpeptidase |
| HbA1c | Glycated haemoglobin |
| HDL | High-density lipoprotein cholesterol |
| HICs | High-income countries |
| HIV | Human immunodeficiency virus |
| HGS | Handgrip strength |
| HREC | Health Research Ethics Committee |
| KMO | Kaiser–Meyer–Olkin |
| LDL | Low-density lipoprotein cholesterol |
| LMICs | Low and middle income countries |
| SD | Standard deviation |
| STEP | Step test and exercise prescription tool |
| VO2max | Maximum oxygen consumption |
| WHODAS 2.0 | World Health Organization Disability Assessment Schedule 2.0 |
| ZAR | South African Rand |
Appendix A
| Health Condition | Diagnostic Criteria | Notes |
|---|---|---|
| Diabetes (type 1 or 2) |
| |
| Kidney disease |
| |
| Liver disease |
| |
| Anaemia |
| |
| Heart disease (chronic heart disease (CHD) or coronary artery disease (CAD)) |
| Self-reported diagnosis of heart disease, coronary artery disease, or having had a heart attack were all considered positive indications. |
| High blood cholesterol |
| |
| HIV |
| Confirmed via second rapid test from different manufacturer or HIV ELISA for incongruent results |
| Hypertension |
| Measured three times on the left arm with 2–5 min intervals; participant seated upright with uncrossed legs and arm at heart level. |
| Obesity |
| |
| Arthritis * | Self-reported diagnosis of arthritis (osteo- or rheumatoid arthritis) or use of specific medication such as disease-modifying antirheumatic drugs (DMARDs) | |
| Chronic lung disease * |
| |
| Depression |
| |
| Cataracts * |
| |
| Stroke * |
| |
| Cancer * |
|
References
- Xu, X.; Mishra, G.D.; Jones, M. Evidence on multimorbidity from definition to intervention: An overview of systematic reviews. Ageing Res. Rev. 2017, 37, 53–68. [Google Scholar] [CrossRef]
- Nguyen, H.; Manolova, G.; Daskalopoulou, C.; Vitoratou, S.; Prince, M.; Prina, A.M. Prevalence of multimorbidity in community settings: A systematic review and meta-analysis of observational studies. J. Comorb. 2019, 9, 2235042X19870934. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.T.; Hamid, F.; Pati, S.; Atun, R.; Millett, C. Impact of noncommunicable disease multimorbidity on healthcare utilisation and out-of-pocket expenditures in middle-income countries: Cross sectional analysis. PLoS ONE 2015, 10, e0127199. [Google Scholar] [CrossRef] [PubMed]
- Arokiasamy, P.; Uttamacharya, U.; Jain, K.; Biritwum, R.B.; Yawson, A.E.; Wu, F.; Guo, Y.; Maximova, T.; Espinoza, B.M.; Salinas Rodríguez, A.; et al. The impact of multimorbidity on adult physical and mental health in low- and middle-income countries: What does the study on global ageing and adult health (SAGE) reveal? BMC Med. 2015, 13, 178. [Google Scholar] [CrossRef]
- Jackson, C.A.; Jones, M.; Tooth, L.; Mishra, G.D.; Byles, J.; Dobson, A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing 2015, 44, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.K.; Tawiah, R.; Sawyer, M.; Scuffham, P. Patterns of multimorbid health conditions: A systematic review of analytical methods and comparison analysis. Int. J. Epidemiol. 2018, 47, 1687–1704. [Google Scholar] [CrossRef]
- Sukumaran, L.; Winston, A.; Sabin, C.A. Understanding the conditions included in data-driven patterns of multimorbidity: A scoping review. Eur. J. Public Health 2024, 34, 35–43. [Google Scholar] [CrossRef]
- Prados-Torres, A.; Calderón-Larrañaga, A.; Hancco-Saavedra, J.; Poblador-Plou, B.; Van Den Akker, M. Multimorbidity patterns: A systematic review. J. Clin. Epidemiol. 2014, 67, 254–266. [Google Scholar] [CrossRef]
- Busija, L.; Lim, K.; Szoeke, C.; Sanders, K.M.; McCabe, M.P. Do replicable profiles of multimorbidity exist? Systematic review and synthesis. Eur. J. Epidemiol. 2019, 34, 1025–1053. [Google Scholar] [CrossRef]
- Pati, S.; Swain, S.; Metsemakers, J.; Knottnerus, J.A.; van den Akker, M. Pattern and severity of multimorbidity among patients attending primary care settings in Odisha, India. PLoS ONE 2017, 12, e0183966. [Google Scholar] [CrossRef]
- Roomaney, R.A.; van Wyk, B.; Cois, A.; Pillay van-Wyk, V. Multimorbidity patterns in South Africa: A latent class analysis. Front. Public Health 2023, 10, 1082587. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.; Mercer, S.W.; Norbury, M.; Watt, G.; Wyke, S.; Guthrie, B. Epidemiology of multimorbidity and implications for health care, research, and medical education: A cross-sectional study. Lancet 2012, 380, 37–43. [Google Scholar] [CrossRef]
- Afshar, S.; Roderick, P.J.; Kowal, P.; Dimitrov, B.D.; Hill, A.G. Multimorbidity and the inequalities of global ageing: A cross-sectional study of 28 countries using the World Health Surveys. BMC Public Health 2015, 15, 776. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.S.; Meng, X.; Cao, G.Y.; Huang, Z.T.; Chen, Z.S.; Han, L.; Wang, K.; Su, H.X.; Luo, Y.; Hu, Y.; et al. Associations between multimorbidity and physical performance in older Chinese adults. Int. J. Environ. Res. Public Health 2020, 17, 4546. [Google Scholar] [CrossRef]
- Berner, K.; Nizeyimana, E.; Bedada, D.T.; Louw, Q.A. Multimorbidity patterns and function among adults in low- and middle-income countries: A scoping review. BMJ Open 2025, 15, e096522. [Google Scholar] [CrossRef]
- Strijdom, H.; De Boever, P.; Walzl, G.; Essop, M.F.; Nawrot, T.S.; Webster, I.; Westcott, C.; Mashele, N.; Everson, F.; Malherbe, S.T.; et al. Cardiovascular risk and endothelial function in people living with HIV/AIDS: Design of the multi-site, longitudinal EndoAfrica study in the Western Cape Province of South Africa. BMC Infect. Dis. 2017, 17, 41. [Google Scholar] [CrossRef]
- Watkins, M.W.; Watkins, M.W. Exploratory factor analysis: A guide to best practice. J. Black Psychol. 2018, 44, 219–246. [Google Scholar] [CrossRef]
- Costello, A.B.; Osborne, J. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Pract. Assess. Res. Eval. 2005, 10, 1–9. [Google Scholar] [CrossRef]
- de Winter, J.C.F.; Dodou, D.; Wieringa, P.A. Exploratory factor analysis with small sample sizes. Multivar. Behav. Res. 2009, 44, 147–181. [Google Scholar] [CrossRef]
- Baron, E.C.; Davies, T.; Lund, C. Validation of the 10-item Centre for Epidemiological Studies Depression Scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry 2017, 17, 6. [Google Scholar] [CrossRef]
- Cheung, C.-L.; Nguyen, U.-S.D.T.; Au, E.; Tan, K.C.B.; Kung, A.W.C. Association of handgrip strength with chronic diseases and multimorbidity: A cross-sectional study. Age 2012, 35, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, Y.; Su, Q.; Wang, J.; Zeng, H.; Chen, Y.; Zhou, J.; Wang, Y. Association between muscle strength and cardiometabolic multimorbidity risk among middle-aged and older Chinese adults: A nationwide longitudinal cohort study. BMC Public Health 2024, 24, 2012. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A review of the measurement of grip strength in clinical and epidemiological studies: Towards a standardised approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Shove, M.E.; Barreca, S.R.; Masters, L.M. Five-repetition sit-to-stand test performance by community-dwelling adults: A preliminary investigation of times, determinants, and relationship with self-reported physical performance. Isokinet. Exerc. Sci. 2007, 15, 77–81. [Google Scholar] [CrossRef]
- Albalwi, A.A.; Alharbi, A.A. Optimal procedure and characteristics in using five times sit to stand test among older adults: A systematic review. Medicine 2023, 102, e34160. [Google Scholar] [CrossRef]
- Knight, E.; Stuckey, M.I.; Petrella, R.J. Validation of the Step Test and Exercise Prescription tool for adults. Can. J. Diabetes 2014, 38, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Stuckey, M.; Knight, E.; Petrella, R.J. The Step Test and Exercise Prescription Tool in Primary Care: A Critical Review. Crit. Rev. Phys. Rehab. Med. 2012, 24, 109–123. [Google Scholar] [CrossRef]
- Katri, L.; Mikhail, S.; Niina, K.; Esa, B.; Katri, L.; Mikhail, S.; Niina, K.; Esa, B. Psychometric properties of 12-item self-administered World Health Organization disability assessment schedule 2.0 (WHODAS 2.0) among general population and people with non-acute physical causes of disability—Systematic review. Disabil. Rehabil. 2022, 43, 789–794. [Google Scholar] [CrossRef]
- Roso-Llorach, A.; Violán, C.; Foguet-Boreu, Q.; Rodriguez-Blanco, T.; Pons-Vigués, M.; Pujol-Ribera, E.; Valderas, J.M. Comparative analysis of methods for identifying multimorbidity patterns: A study of ‘real-world’ data. BMJ Open 2018, 8, 18986. [Google Scholar] [CrossRef]
- Prados-Torres, A.; Poblador-Plou, B.; Calderón-Larrañaga, A.; Gimeno-Feliu, L.A.; González-Rubio, F.; Poncel-Falcó, A.; Sicras-Mainar, A.; Alcalá-Nalvaiz, J.T. Multimorbidity patterns in primary care: Interactions among chronic diseases using factor analysis. PLoS ONE 2012, 7, e32190. [Google Scholar] [CrossRef]
- Cimarras-Otal, C.; Calderón-Larrañaga, A.; Poblador-Plou, B.; González-Rubio, F.; Gimeno-Feliu, L.A.; Arjol-Serrano, J.L.; Prados-Torres, A. Association between physical activity, multimorbidity, self-rated health and functional limitation in the Spanish population. BMC Public Health 2014, 14, 1170. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, A.; Chavis, M.; Watkins, J.; Wilson, T. The five-times-sit-to-stand test: Validity, reliability and detectable change in older females. Aging Clin. Exp. Res. 2012, 24, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Arocha Rodulfo, J.I. Approach to the cardiometabolic continuum. Narrative description. Clin. Investig. Arterioscler. 2020, 33, 158–167. [Google Scholar] [CrossRef]
- Rabe, K.; Hurst, J.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Gómez-Olivé, F.X.; Manne-Goehler, J.; Wade, A.N.; Tollman, S.; Gaziano, T.A.; Salomon, J.A. Multimorbidity and care for hypertension, diabetes and HIV among older adults in rural South Africa. Bull. World Health Organ. 2019, 97, 10–23. [Google Scholar] [CrossRef]
- Roomaney, R.A.; van Wyk, B.; Cois, A.; Pillay-van Wyk, V. Multimorbidity patterns in a national HIV survey of South African youth and adults. Front. Public Health 2022, 10, 862993. [Google Scholar] [CrossRef]
- Williams, C.; Kamau, F.M.; Everson, F.; Kgokane, B.; De Boever, P.; Goswami, N.; Webster, I.; Strijdom, H. HIV and antiretroviral therapy are independently associated with cardiometabolic variables and cardiac electrical activity in adults from the Western Cape region of South Africa. J. Clin. Med. 2021, 10, 4112. [Google Scholar] [CrossRef]
- Lebina, L.; Malatji, T.; Nabeemeeah, F.; Motsomi, K.; Siwelana, T.; Hlongwane, K.; Martinson, N. The prevalence of multimorbidity in virally suppressed HIV-positive patients in Limpopo. South. Afr. J. HIV Med. 2023, 24, 1495. [Google Scholar] [CrossRef]
- Oystacher, T.; Blasco, D.; He, E.; Huang, D.; Schear, R.; McGoldrick, D.; Link, B.; Yang, L.H. Understanding stigma as a barrier to accessing cancer treatment in South Africa: Implications for public health campaigns. Pan Afr. Med. J. 2018, 29, 1–12. [Google Scholar] [CrossRef]
- Singh, E.; Underwood, J.; Nattey, C.; Babb, C.; Sengayi, M.; Kellett, P. South African National Cancer Registry: Effect of withheld data from private health systems on cancer incidence estimates. S. Afr. Med. J. 2015, 105, 107–109. [Google Scholar] [CrossRef]
- Chabalala, T.V.; Maimela, E.; Mashaba, R.G.; Ntimana, C.B.; Netshapapame, T.S.; Mphasha, M.H.; Chabalala, T.V.; Maimela, E.; Mashaba, R.G.; Ntimana, C.B.; et al. Factors that contribute to women’s reluctance to undergo cervical cancer screening in clinics of Limpopo Province, South Africa. BMC Public Health 2025, 25, 1303. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Gálvez, J.; Ortega-Martín, E.; Carretero-Bravo, J.; Pérez-Muñoz, C.; Suárez-Lledó, V.; Ramos-Fiol, B. Social determinants of multimorbidity patterns: A systematic review. Front. Public Health 2023, 11, 1081518. [Google Scholar] [CrossRef]
- Siconolfi, S.; Garber, C.; Lasater, T.; Carleton, R. A simple, valid step test for estimating maximal oxygen uptake in epidemiologic studies. Am. J. Epidemiol. 1985, 121, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Sun, S.; Tian, H.; Li, S.; Wu, Y. Sex Differences in the Associations of Handgrip Strength and Asymmetry With Multimorbidity: Evidence From the English Longitudinal Study of Ageing. J. Am. Med. Dir. Assoc. 2022, 23, 493–498. [Google Scholar] [CrossRef]
- Calderón-Larrañaga, A.; Vetrano, D.L.; Ferrucci, L.; Mercer, S.W.; Marengoni, A.; Onder, G.; Eriksdotter, M.; Fratiglioni, L. Multimorbidity and functional impairment-bidirectional interplay, synergistic effects and common pathways. J. Intern. Med. 2019, 285, 255–271. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Yang, S.; Chen, Y.; Cao, G.; Xu, R.; Jia, X.; Hou, L.; Li, J.; Bi, C.; Wang, X. Multimorbidity patterns and associations with gait, balance and lower extremity muscle function in the elderly: A cross-sectional study in Northwest China. Int. J. Gen. Med. 2023, 16, 3179–3192. [Google Scholar] [CrossRef]
- Wilk, P.; Ruiz-Castell, M.; Stranges, S.; Bohn, T.; Fagherazzi, G.; Nicholson, K.; Moran, V.; Makovski, T.; Pi Alperin, M.; Zeegers, M.; et al. Relationship between multimorbidity, functional limitation, and quality of life among middle-aged and older adults: Findings from the longitudinal analysis of the 2013–2020 Survey of Health, Ageing, and Retirement in Europe (SHARE). Qual. Life Res. 2024, 33, 169–181. [Google Scholar] [CrossRef]
- Ruiz-Baena, J.; Moreno-Juste, A.; Poblador-Plou, B.; Castillo-Jimena, M.; Calderón-Larrañaga, A.; Lozano-Hernández, C.; Gimeno-Miguel, A.; Gimeno-Feliú, L. Influence of social determinants of health on quality of life in patients with multimorbidity and polypharmacy. PLoS ONE 2024, 19, e0297702. [Google Scholar] [CrossRef]

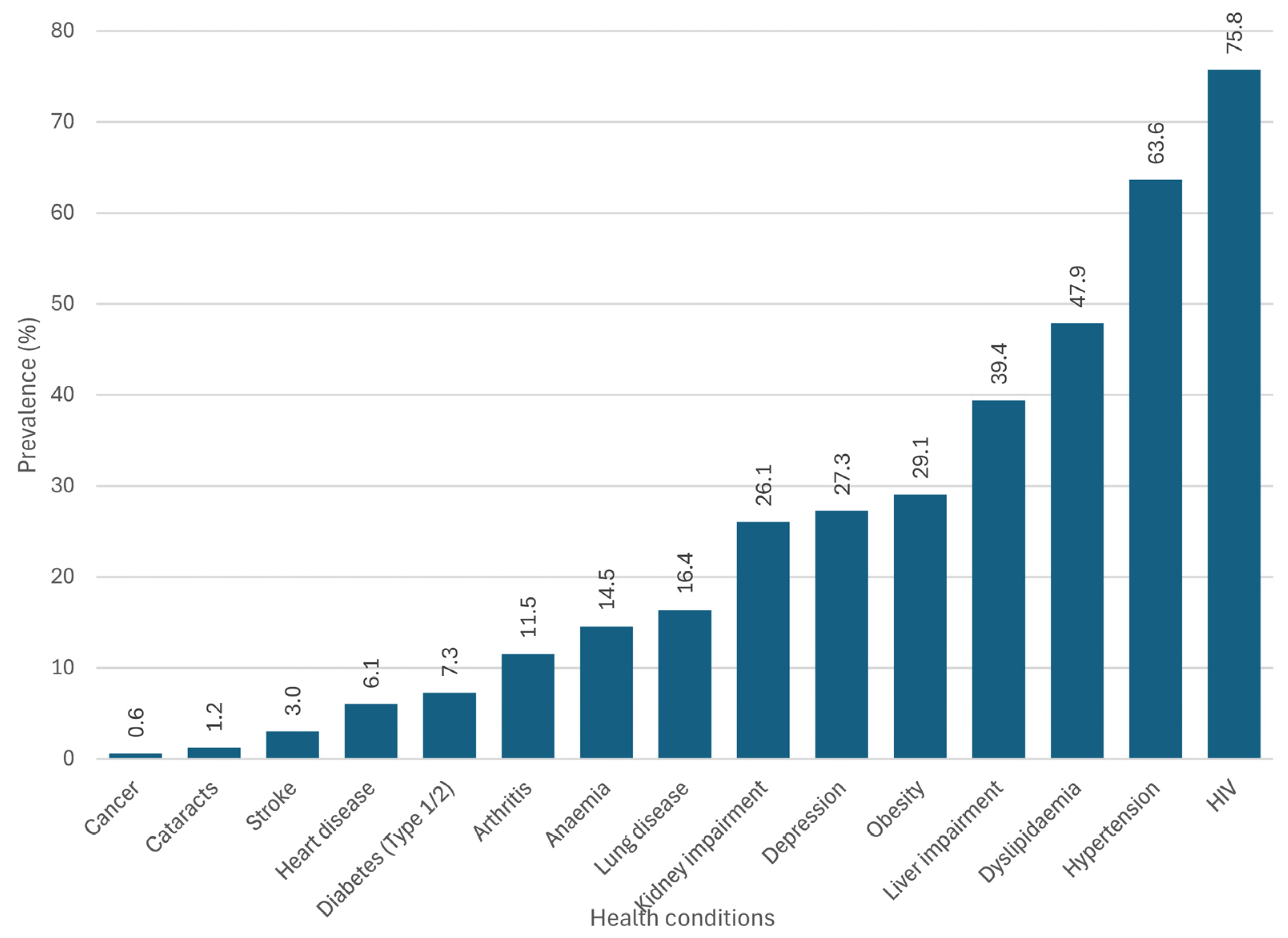
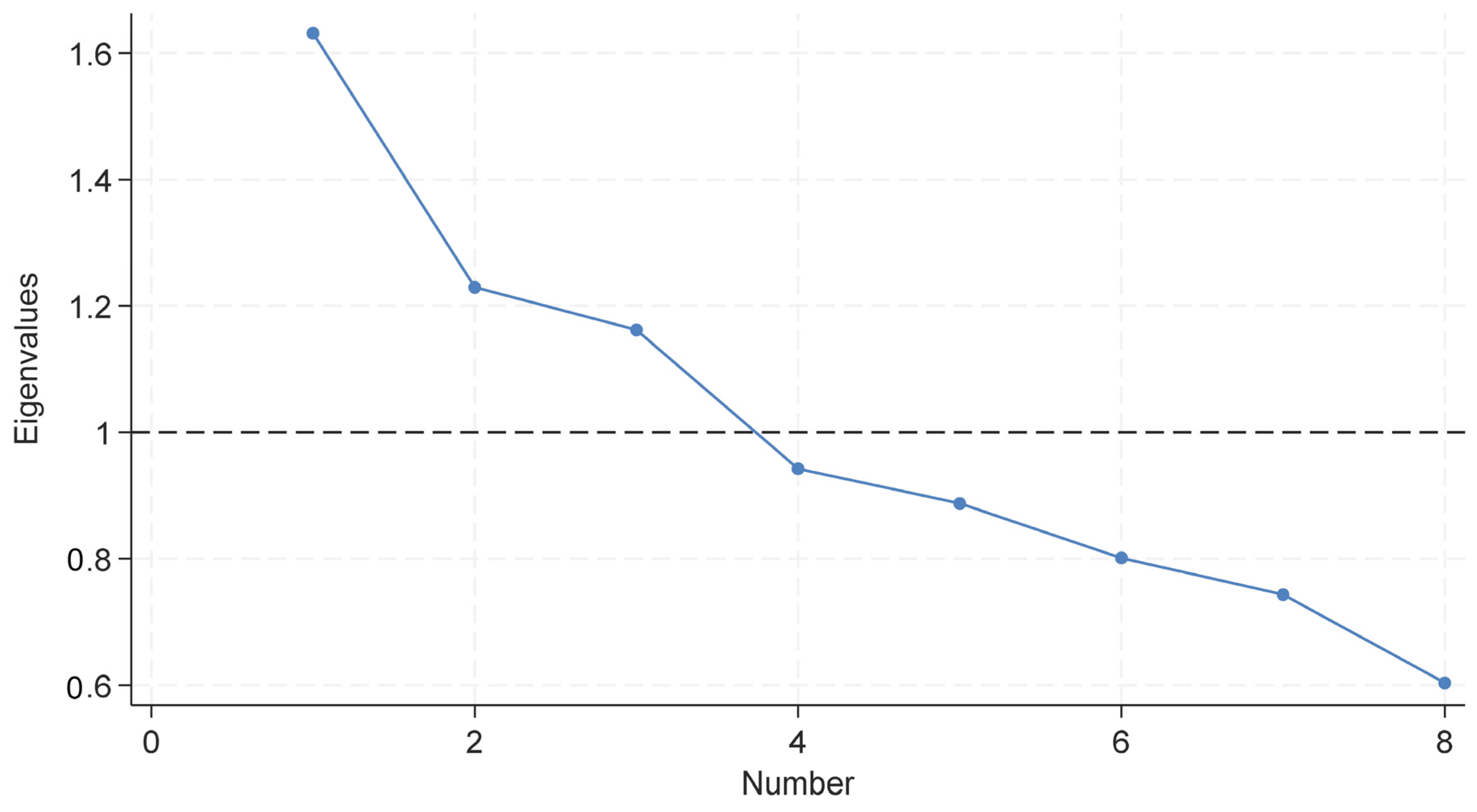
| Characteristic | Total | Women (n = 109) | Men (n = 56) | p-Value |
|---|---|---|---|---|
| Age in years, mean (SD) | 41.58 (9.99) | 40.71 (10.06) | 43.27 (9.71) | 0.119 |
| Education level, n (%) | ||||
| None | 11 (6.67%) | 8 (7.34%) | 3 (5.36%) | |
| Primary school | 67 (40.61%) | 48 (44.04%) | 19 (33.93%) | |
| High school | 72 (43.64%) | 45 (41.28%) | 27 (48.21%) | |
| ABET (adult basic education training) | 1 (0.61%) | 0 (0.00%) | 1 (0.92%) | 0.451 |
| College/University/Other tertiary institution | 14 (8.48%) | 7 (6.42%) | 7 (12.50%) | |
| Unemployed, n (%) | 93 (56.36%) | 64 (58.72%) | 29 (51.79%) | 0.395 |
| Monthly household income, n (%) | ||||
| <1000 ZAR | 32 (19.39%) | 20 (18.35%) | 12 (21.43%) | |
| ≥1000 ZAR to <5000 ZAR | 88 (53.33%) | 64 (58.71%) | 24 (42.86%) | 0.126 |
| ≥5000 ZAR | 45 (27.27%) | 25 (22.94%) | 20 (35.71%) | |
| Current or former smoker, n (%) | 104 (63.03%) | 68 (62.39%) | 36 (64.29%) | 0.811 |
| Alcohol consumption in last 12 months, n (%) | 86 (52.12%) | 58 (50.00%) | 28 (53.21%) | 0.696 |
| Functioning | ||||
| WHODAS-12 percentage score, median (range) | 8.33 (0.00–79.17) | 10.42 (0.00–66.67) | 6.25 (0.00–79.16) | 0.213 |
| No limitation (0–4%), n (%) | 51 (31.10%) | 32 (29.36%) | 19 (34.55%) | |
| Mild limitations (5–24%), n (%) | 72 (43.90%) | 48 (44.04%) | 24 (43.64%) | |
| Moderate (25–49%), n (%) | 23 (20.12%) | 33 (21.10%) | 10 (18.18%) | 0.865 |
| Severe (50–95%), n (%) | 8 (4.88%) | 6 (5.50%) | 2 (3.64%) | |
| Extreme/Cannot do (96–100%), n (%) | 0 (0.00%) | 0 (0.00%) | 0 (0.00%) | |
| Chair rise time in seconds, mean (SD) | 9.17 (2.88) | 9.25 (3.19) | 9.02 (2.20) | 0.650 |
| STEP VO2max, mean (SD) | 42.4 (17.1) | 39.1 (16.9) | 49.1 (15.7) | 0.000 |
| Handgrip strength in kilograms, mean (SD) | 27.78 (7.62) | 27.57 (7.82) | 28.18 (7.26) | 0.318 |
| Normalised handgrip strength, mean (SD) | 0.43 (0.15) | 0.44 (0.15) | 0.44 (0.14) | 0.495 |
| Health Condition | Factor | ||
|---|---|---|---|
| HIV Pattern (Pattern 1) | Hypertension Pattern (Pattern 2) | Cardiovascular Pattern (Pattern 3) | |
| Stroke | 0.0109 | −0.0027 | 0.3019 |
| Obesity | 0.3518 | 0.1562 | −0.1075 |
| Lung disease | −0.0553 | 0.2651 | −0.1381 |
| Heart disease | 0.2295 | −0.0034 | 0.2845 |
| HIV | −0.5211 | 0.0840 | 0.0130 |
| Hypertension | 0.1866 | −0.3855 | 0.0268 |
| Hypercholesteraemia | 0.3844 | −0.1044 | 0.2503 |
| Anaemia | −0.0834 | 0.3178 | 0.0688 |
| Pattern | Unadjusted Analysis | Adjusted Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Step Test VO2max | Normalised Handgrip Strength | Chair Rise Time | WHODAS-12 Impairment Level | Step Test VO2max 1 | Normalised Handgrip Strength 2 | Chair Rise Time 2,3 | WHODAS-12 Impairment Level 4 | |
| Pattern 1 (HIV-cholesterol-obesity) | ||||||||
| β (95% CI) | −6.60 (−9.59, −3.60) *** | −0.10 (−0.14, −0.07) *** | −0.30 (−1.06, 0.45) | Mild: −0.11 (−0.68, 0.46) | −6.41 (−9.45, −3.36) *** | −0.11 (−0.14, −0.07) *** | −0.40 (−1.17, 0.38) | Mild: 0.05 (−0.55, 0.64) |
| Moderate: −0.67 (−1.46, 0.12) | Moderate: −0.54 (−1.35, 0.26) | |||||||
| Severe: −0.95 (−2.48, 0.59) | Severe: −1.75 (−3.68, 0.19) † | |||||||
| Pattern 2 (hypertension-anaemia-lung disease) | ||||||||
| β (95% CI) | −2.36 (−5.95, 1.22) | −0.04 (−0.08, 0.005) † | −0.13 (−1.04, 0.78) | Mild: 0.85 (0.07, 1.64) * | −2.41 (−6.09, 1.28) | −0.05 (−0.09, −0.003) * | −0.18 (−1.12, 0.76) | Mild: 1.12 (0.28, 1.97) ** |
| Moderate: 1.30 (0.41, 1.48) * | Moderate: 1.48 (0.53, 2.43) ** | |||||||
| Severe: 1.69 (0.54, 2. 84) * | Severe: 1.50 (0.00, 3.01) † | |||||||
| Pattern 3 (stroke-heart disease-cholesterol) | ||||||||
| β (95% CI) | −0.15 (−4.13, 3.83) | 0.04 (−0.004, 0.09) † | −0.54 (−1.55, 0.47) | Mild: −0.95 (−2.48, 0.59) | −0.15 (−4.14, 3.85) | 0.04 (−0.01, 0.09) † | −0.60 (−1.63, 0.42) | Mild: 1.14 (0.06, 2.21) * |
| Moderate: 1.27 (−0.16, 2.70) | Moderate: 1.66 (0.47, 2.84) ** | |||||||
| Severe: 1.90 (0.39, 3.43) * | Severe: 2.16 (0.32, 4.01) * | |||||||
| Model Statistics | ||||||||
| R2 | 0.118 5 | 0.183 5 | 0.014 5 | 0.058 6 | 0.124 5 | 0.211 5 | 0.026 5 | 0.117 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berner, K.; Bedada, D.T.; Strijdom, H.; Webster, I.; Louw, Q. Multimorbidity Patterns and Functioning Associations Among Adults in a Local South African Setting: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2025, 22, 780. https://doi.org/10.3390/ijerph22050780
Berner K, Bedada DT, Strijdom H, Webster I, Louw Q. Multimorbidity Patterns and Functioning Associations Among Adults in a Local South African Setting: A Cross-Sectional Study. International Journal of Environmental Research and Public Health. 2025; 22(5):780. https://doi.org/10.3390/ijerph22050780
Chicago/Turabian StyleBerner, Karina, Diribsa Tsegaye Bedada, Hans Strijdom, Ingrid Webster, and Quinette Louw. 2025. "Multimorbidity Patterns and Functioning Associations Among Adults in a Local South African Setting: A Cross-Sectional Study" International Journal of Environmental Research and Public Health 22, no. 5: 780. https://doi.org/10.3390/ijerph22050780
APA StyleBerner, K., Bedada, D. T., Strijdom, H., Webster, I., & Louw, Q. (2025). Multimorbidity Patterns and Functioning Associations Among Adults in a Local South African Setting: A Cross-Sectional Study. International Journal of Environmental Research and Public Health, 22(5), 780. https://doi.org/10.3390/ijerph22050780






