Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Animals
2.3. Experimental Design
2.4. Determination of Sperm Parameters
2.4.1. Epididymal Sperm Count
2.4.2. Sperm Mobility
2.4.3. Sperm Viability
2.4.4. Sperm Morphology
2.5. Measurement of Cadmium and Zinc Levels in the Testicles
2.6. Assessment of Testosterone
2.7. Histopathological Analysis
2.8. Statistical Analysis
3. Results
3.1. Toxic Effect of Cadmium on Rat Testes at Three Time Points
3.1.1. Body Weight, Testicular and Epididymal Weights, and Testicular Dimensions
3.1.2. Analyses of Sperm Parameters
3.1.3. Testosterone Assay
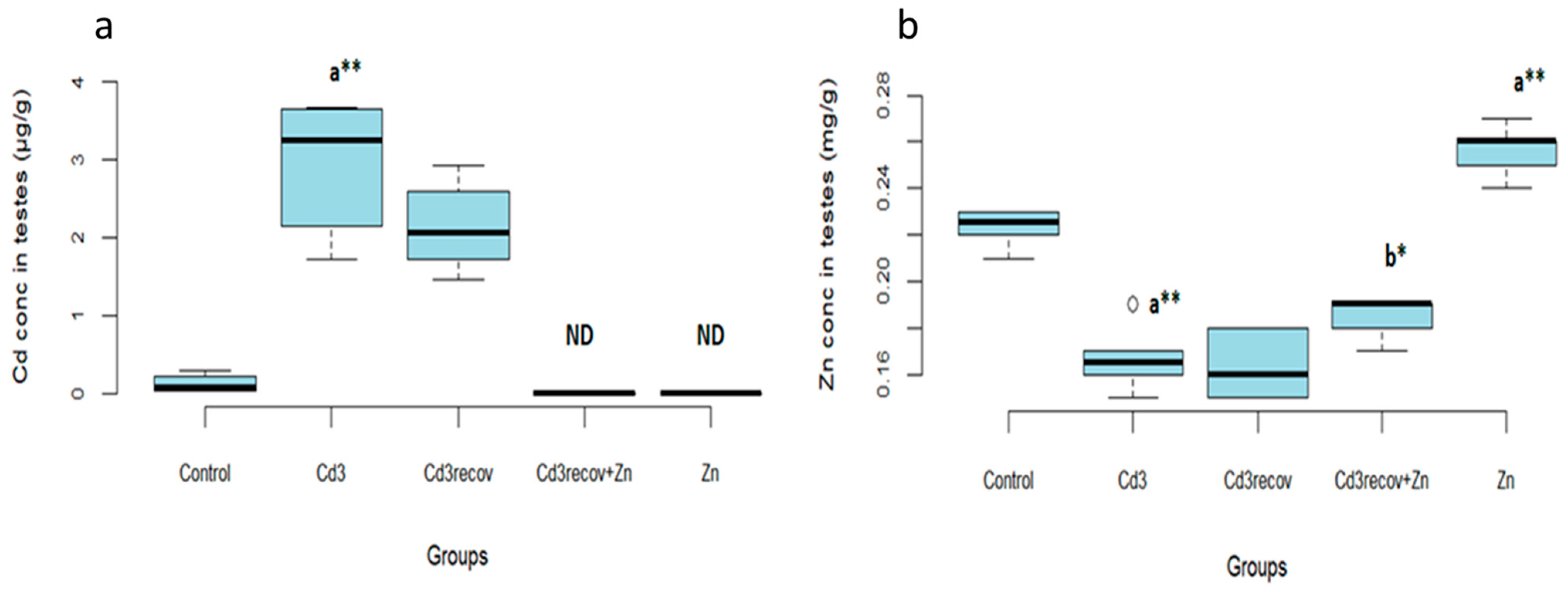
3.1.4. Cadmium and Zinc Levels in the Testes
3.1.5. Testicular Histology
3.2. Study of the Reversibility of Cadmium Toxicity
3.2.1. Body Weight, Testicular and Epididymal Weights, and Testicular Dimensions
3.2.2. Analyses of Sperm Parameters
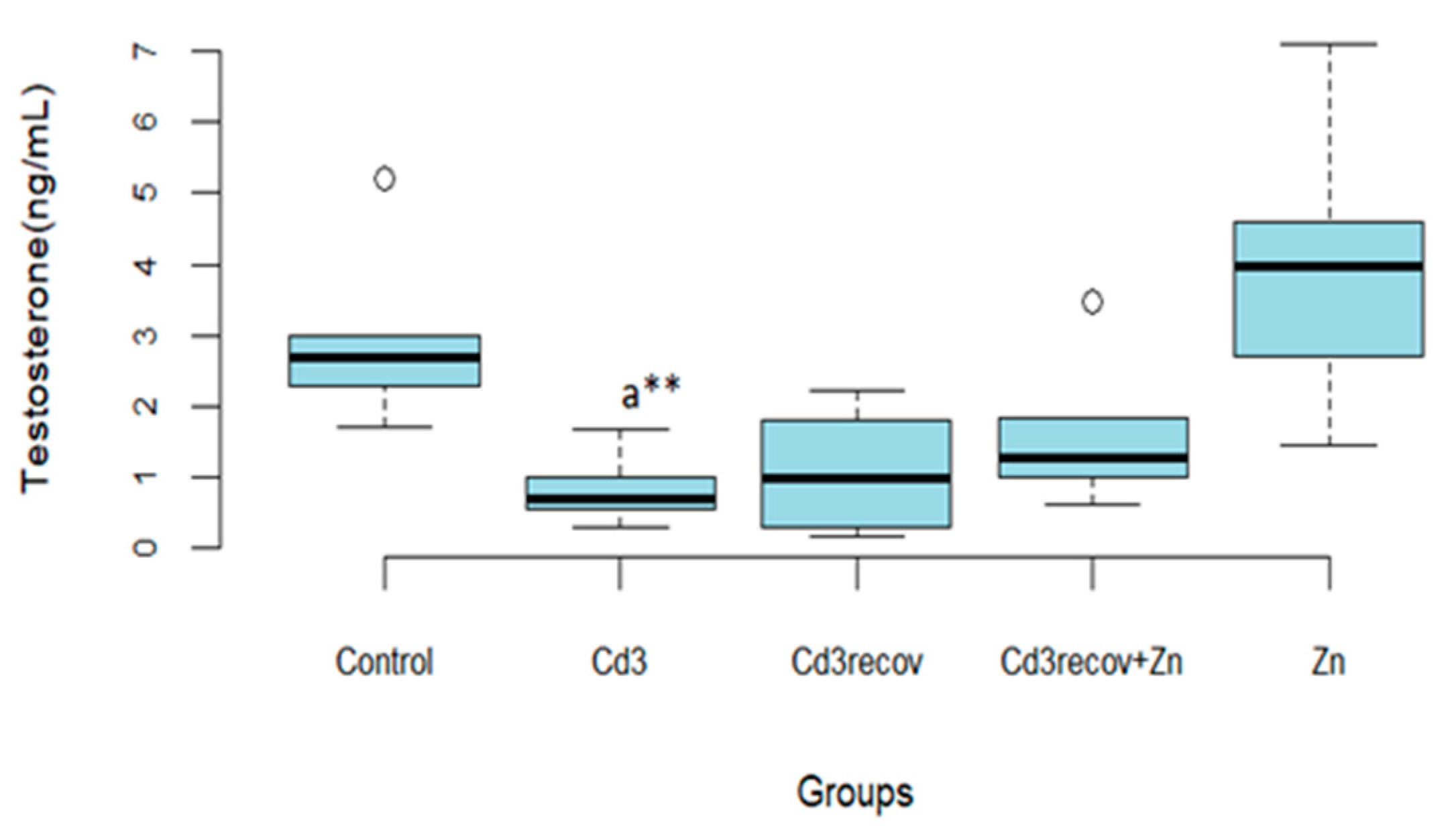
3.2.3. Testosterone Assay
3.2.4. Cadmium and Zinc Levels in the Testes
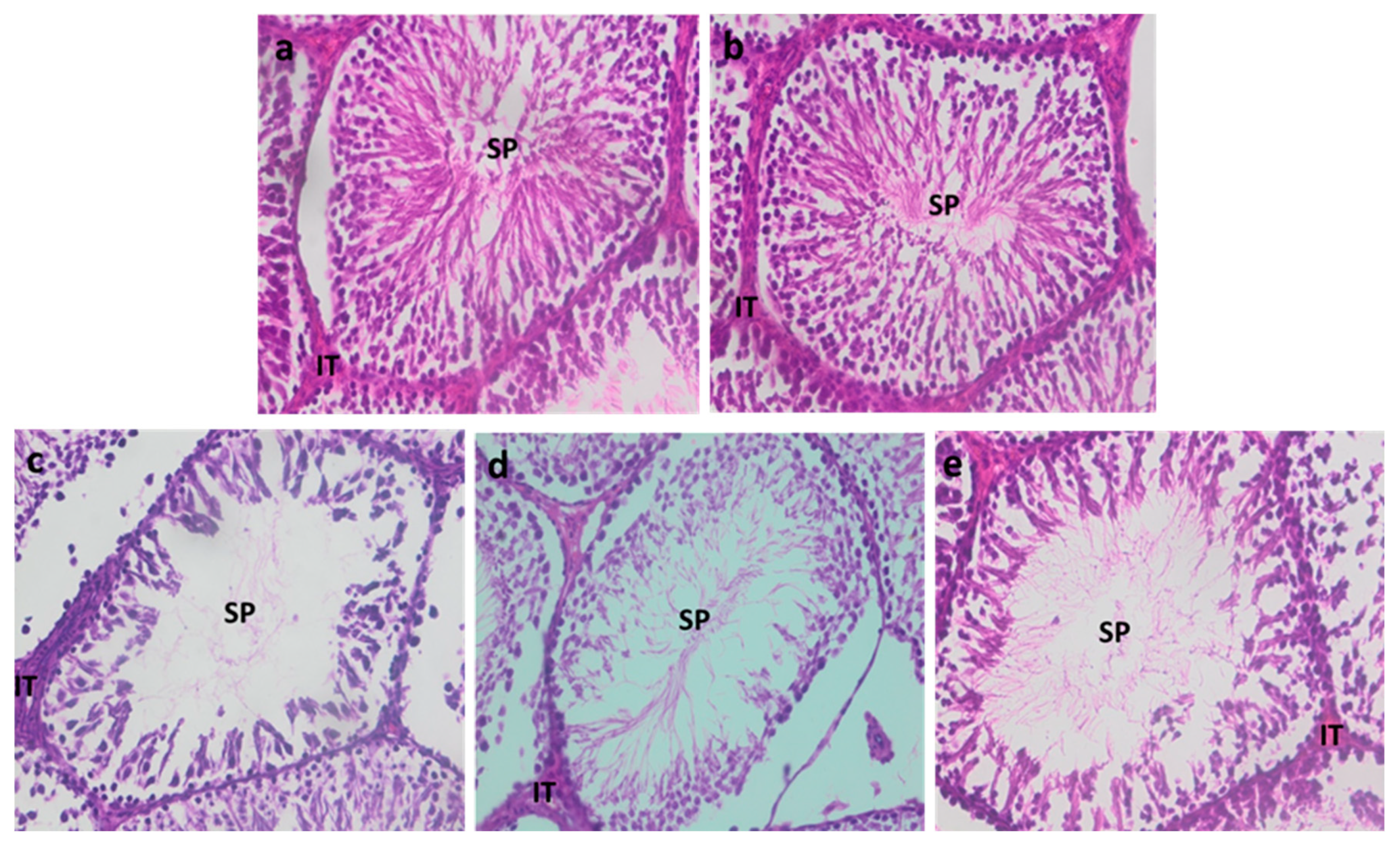
3.2.5. Testicular Histology
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamothe, S.; Kerlan, V.; Christin-Maitre, S. Qualité du sperme et fertilité: Rôle de l’environnement et de la santé. Ann. D’endocrinologie 2018, 79, S1–S9. [Google Scholar] [CrossRef] [PubMed]
- Leaver, R.B. Male Infertility: An Overview of Causes and Treatment Options. Br. J. Nurs. 2016, 25, S35–S40. [Google Scholar] [CrossRef]
- Bendayan, M.; Alter, L.; Swierkowski-Blanchard, N.; Caceres-Sanchez, L.; Selva, J.; Robin, G.; Boitrelle, F. Toxiques, mode de vie, environnement: Quels impacts sur la fertilité masculine? Gynécologie Obs. Fertil. Sénologie 2018, 46, 47–56. [Google Scholar] [CrossRef]
- Genchi, G.; Sinicropi, M.S.; Lauria, G.; Carocci, A.; Catalano, A. The Effects of Cadmium Toxicity. Int. J. Environ. Res. Public Health 2020, 17, 3782. [Google Scholar] [CrossRef]
- Adamkovicova, M.; Toman, R.; Martiniakova, M.; Omelka, R.; Babosova, R.; Krajcovicova, V.; Grosskopf, B.; Massanyi, P. Sperm Motility and Morphology Changes in Rats Exposed to Cadmium and Diazinon. Reprod. Biol. Endocrinol. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, S.; Li, S. Relationship between Cadmium Content in Semen and Male Infertility: A Meta-Analysis. Environ. Sci. Pollut. Res. Int. 2019, 26, 1947–1953. [Google Scholar] [CrossRef]
- Ganguly, K.; Levänen, B.; Palmberg, L.; Åkesson, A.; Lindén, A. Cadmium in Tobacco Smokers: A Neglected Link to Lung Disease? Eur. Respir. Rev. 2018, 27, 170122. [Google Scholar] [CrossRef] [PubMed]
- Zečević, N.; Kocić, J.; Perović, M.; Stojsavljević, A. Detrimental Effects of Cadmium on Male Infertility: A Review. Ecotoxicol. Environ. Saf. 2025, 290, 117623. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Panchal, H.; Saraf, P. Cadmium as a Testicular Toxicant: A Review. J. Appl. Toxicol. 2021, 41, 105–117. [Google Scholar] [CrossRef]
- Si, M.; Lang, J. The Roles of Metallothioneins in Carcinogenesis. J. Hematol. Oncol. 2018, 11, 107. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, J.K.; Siwach, A.; Sachdeva, D.; Sachdeva, S.N. Revisiting Cadmium-Induced Toxicity in the Male Reproductive System: An Update. Arch. Toxicol. 2024, 98, 3619–3639. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Li, X.; Ge, R.-S. Toxicological Effects of Cadmium on Mammalian Testis. Front. Genet. 2020, 11, 527. [Google Scholar] [CrossRef]
- Corpuz-Hilsabeck, M.; Culty, M. Impact of Endocrine Disrupting Chemicals and Pharmaceuticals on Sertoli Cell Development and Functions. Front. Endocrinol. 2023, 14, 1095894. [Google Scholar] [CrossRef]
- Sun, J.; Yu, G.; Zhang, Y.; Liu, X.; Du, C.; Wang, L.; Li, Z.; Wang, C. Heavy Metal Level in Human Semen with Different Fertility: A Meta-Analysis. Biol. Trace Elem. Res. 2017, 176, 27–36. [Google Scholar] [CrossRef]
- Wagner, H.; Cheng, J.W.; Ko, E.Y. Role of Reactive Oxygen Species in Male Infertility: An Updated Review of Literature. Arab. J. Urol. 2018, 16, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Majzoub, A.; Agarwal, A. Oxidative Stress and Sperm Function: A Systematic Review on Evaluation and Management. Arab. J. Urol. 2019, 17, 87–97. [Google Scholar] [CrossRef]
- Kaltsas, A. Oxidative Stress and Male Infertility: The Protective Role of Antioxidants. Medicina 2023, 59, 1769. [Google Scholar] [CrossRef]
- Fallah, A.; Mohammad-Hasani, A.; Colagar, A.H. Zinc Is an Essential Element for Male Fertility: A Review of Zn Roles in Men’s Health, Germination, Sperm Quality, and Fertilization. J. Reprod. Infertil. 2018, 19, 69–81. [Google Scholar]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective Roles of Onion and Garlic Extracts on Cadmium-Induced Changes in Sperm Characteristics and Testicular Oxidative Damage in Rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef]
- Omu, A.E.; Al-Azemi, M.K.; Al-Maghrebi, M.; Mathew, C.T.; Omu, F.E.; Kehinde, E.O.; Anim, J.T.; Oriowo, M.A.; Memon, A. Molecular Basis for the Effects of Zinc Deficiency on Spermatogenesis: An Experimental Study in the Sprague-Dawley Rat Model. Indian J. Urol. 2015, 31, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Stelmashook, E.V.; Alexandrova, O.P.; Genrikhs, E.E.; Novikova, S.V.; Salmina, A.B.; Isaev, N.K. Effect of Zinc and Copper Ions on Cadmium-Induced Toxicity in Rat Cultured Cortical Neurons. J. Trace Elem. Med. Biol. 2022, 73, 127012. [Google Scholar] [CrossRef]
- Lane, E.A.; Canty, M.J.; More, S.J. Cadmium Exposure and Consequence for the Health and Productivity of Farmed Ruminants. Res. Vet. Sci. 2015, 101, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Babaknejad, N.; Bahrami, S.; Moshtaghie, A.A.; Nayeri, H.; Rajabi, P.; Iranpour, F.G. Cadmium Testicular Toxicity in Male Wistar Rats: Protective Roles of Zinc and Magnesium. Biol. Trace Elem. Res. 2018, 185, 106–115. [Google Scholar] [CrossRef]
- Jacquillet, G.; Barbier, O.; Cougnon, M.; Tauc, M.; Namorado, M.C.; Martin, D.; Reyes, J.L.; Poujeol, P. Zinc Protects Renal Function during Cadmium Intoxication in the Rat. Am. J. Physiol. Renal Physiol. 2006, 290, F127–F137. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, H.; Lin, S.; Wang, K.; Wang, H.; Liu, Z. Protective Effect of Naringenin against Cadmium-Induced Testicular Toxicity in Male SD Rats. J. Inorg. Biochem. 2021, 214, 111310. [Google Scholar] [CrossRef]
- Lahimer, M.; Montjean, D.; Cabry, R.; Capelle, S.; Lefranc, E.; Bach, V.; Ajina, M.; Ben Ali, H.; Khorsi-Cauet, H.; Benkhalifa, M. Paternal Age Matters: Association with Sperm Criteria’s- Spermatozoa DNA Integrity and Methylation Profile. J. Clin. Med. 2023, 12, 4928. [Google Scholar] [CrossRef] [PubMed]
- Nwokocha, C.R.; Owu, D.U.; Nwokocha, M.I.; Ufearo, C.S.; Iwuala, M.O.E. Comparative Study on the Efficacy of Allium Sativum (Garlic) in Reducing Some Heavy Metal Accumulation in Liver of Wistar Rats. Food Chem. Toxicol. 2012, 50, 222–226. [Google Scholar] [CrossRef]
- Anjum, M.R.; Madhu, P.; Reddy, K.P.; Reddy, P.S. The Protective Effects of Zinc in Lead-Induced Testicular and Epididymal Toxicity in Wistar Rats. Toxicol. Ind. Health 2017, 33, 265–276. [Google Scholar] [CrossRef]
- Nna, V.U.; Ujah, G.A.; Mohamed, M.; Etim, K.B.; Igba, B.O.; Augustine, E.R.; Osim, E.E. Cadmium Chloride-Induced Testicular Toxicity in Male Wistar Rats; Prophylactic Effect of Quercetin, and Assessment of Testicular Recovery Following Cadmium Chloride Withdrawal. Biomed. Pharmacother. 2017, 94, 109–123. [Google Scholar] [CrossRef]
- Eriyamremu, G.E.; Asagba, S.O.; Onyeneke, E.C.; Adaikpoh, M.A. Changes in Carboxypeptidase A, Dipeptidase and Na+/K+ ATPase Activities in the Intestine of Rats Orally Exposed to Different Doses of Cadmium. Biometals 2005, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Szuster-Ciesielska, A.; Stachura, A.; Słotwińska, M.; Kamińska, T.; Śnieżko, R.; Paduch, R.; Abramczyk, D.; Filar, J.; Kandefer-Szerszeń, M. The Inhibitory Effect of Zinc on Cadmium-Induced Cell Apoptosis and Reactive Oxygen Species (ROS) Production in Cell Cultures. Toxicology 2000, 145, 159–171. [Google Scholar] [CrossRef]
- Yavasoglu, A.; Karaaslan, M.A.; Uyanikgil, Y.; Sayim, F.; Ates, U.; Yavasoglu, N.U.K. Toxic Effects of Anatoxin-a on Testes and Sperm Counts of Male Mice. Exp. Toxicol. Pathol. 2008, 60, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Owoeye, O.; Aiyegbusi, M.A.; Dagunduro, J.O.; Daramola, Y.M.; Farombi, E.O. Kolaviron Protects against Benzo[a]Pyrene-Induced Functional Alterations along the Brain-Pituitary-Gonadal Axis in Male Rats. Environ. Toxicol. Pharmacol. 2015, 40, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Nemmiche, S. Oxidative Signaling Response to Cadmium Exposure. Toxicol. Sci. 2017, 156, 4–10. [Google Scholar] [CrossRef]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Evidence of Melatonin Ameliorative Effects on the Blood-Testis Barrier and Sperm Quality Alterations Induced by Cadmium in the Rat Testis. Ecotoxicol. Environ. Saf. 2021, 226, 112878. [Google Scholar] [CrossRef]
- de Angelis, C.; Galdiero, M.; Pivonello, C.; Salzano, C.; Gianfrilli, D.; Piscitelli, P.; Lenzi, A.; Colao, A.; Pivonello, R. The Environment and Male Reproduction: The Effect of Cadmium Exposure on Reproductive Function and Its Implication in Fertility. Reprod. Toxicol. 2017, 73, 105–127. [Google Scholar] [CrossRef]
- Venditti, M.; Ben Rhouma, M.; Romano, M.Z.; Messaoudi, I.; Reiter, R.J.; Minucci, S. Altered Expression of DAAM1 and PREP Induced by Cadmium Toxicity Is Counteracted by Melatonin in the Rat Testis. Genes 2021, 12, 1016. [Google Scholar] [CrossRef]
- Ng, T.B.; Liu, W.K. Toxic Effect of Heavy Metals on Cells Isolated from the Rat Adrenal and Testis. Vitr. Cell. Dev. Biol. 1990, 26, 24–28. [Google Scholar] [CrossRef]
- Yang, J.-M.; Arnush, M.; Chen, Q.-Y.; Wu, X.-D.; Pang, B.; Jiang, X.-Z. Cadmium-Induced Damage to Primary Cultures of Rat Leydig Cells. Reprod. Toxicol. 2003, 17, 553–560. [Google Scholar] [CrossRef]
- Lafuente, A.; Márquez, N.; Pérez-Lorenzo, M.; Pazo, D.; Esquifino, A.I. Pubertal and Postpubertal Cadmium Exposure Differentially Affects the Hypothalamic–Pituitary–Testicular Axis Function in the Rat. Food Chem. Toxicol. 2000, 38, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Calderoni, A.M.; Oliveros, L.; Jahn, G.; Anton, R.; Luco, J.; Giménez, M.S. Alterations in the Lipid Content of Pituitary Gland and Serum Prolactin and Growth Hormone in Cadmium Treated Rats. Biometals 2005, 18, 213–220. [Google Scholar] [CrossRef]
- Miler, E.A.; Nudler, S.I.; Quinteros, F.A.; Cabilla, J.P.; Ronchetti, S.A.; Duvilanski, B.H. Cadmium Induced-Oxidative Stress in Pituitary Gland Is Reversed by Removing the Contamination Source. Hum. Exp. Toxicol. 2010, 29, 873–880. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Discovery of Human Zinc Deficiency: Its Impact on Human Health and Disease. Adv. Nutr. 2013, 4, 176–190. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Adebayo, O.L.; Otuechere, C.A.; Iserhienrhien, B.O.; Badejo, T.A. Selenium and Rutin Alone or in Combination Do Not Have Stronger Protective Effects than Their Separate Effects against Cadmium-Induced Renal Damage. Pharm. Biol. 2016, 54, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Bonda, E.; Włostowski, T.; Krasowska, A. Testicular Toxicity Induced by Dietary Cadmium Is Associated with Decreased Testicular Zinc and Increased Hepatic and Renal Metallothionein and Zinc in the Bank Vole (Clethrionomys glareolus). Biometals 2004, 17, 615–624. [Google Scholar] [CrossRef]
- Maret, W. The Redox Biology of Redox-Inert Zinc Ions. Free Radic. Biol. Med. 2019, 134, 311–326. [Google Scholar] [CrossRef]
- Marreiro, D.d.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; de Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef]
- AL-BADER, A.; OMU, A.E.; DASHTI, H. Chronic Cadmium Toxicity to Sperm of Heavy Cigarette Smokers: Immunomodulation by Zinc. Arch. Androl. 1999, 43, 135–140. [Google Scholar] [CrossRef]
- Sankako, M.K.; Garcia, P.C.; Piffer, R.C.; Dallaqua, B.; Damasceno, D.C.; Pereira, O.C.M. Possible Mechanism by Which Zinc Protects the Testicular Function of Rats Exposed to Cigarette Smoke. Pharmacol. Rep. 2012, 64, 1537–1546. [Google Scholar] [CrossRef]
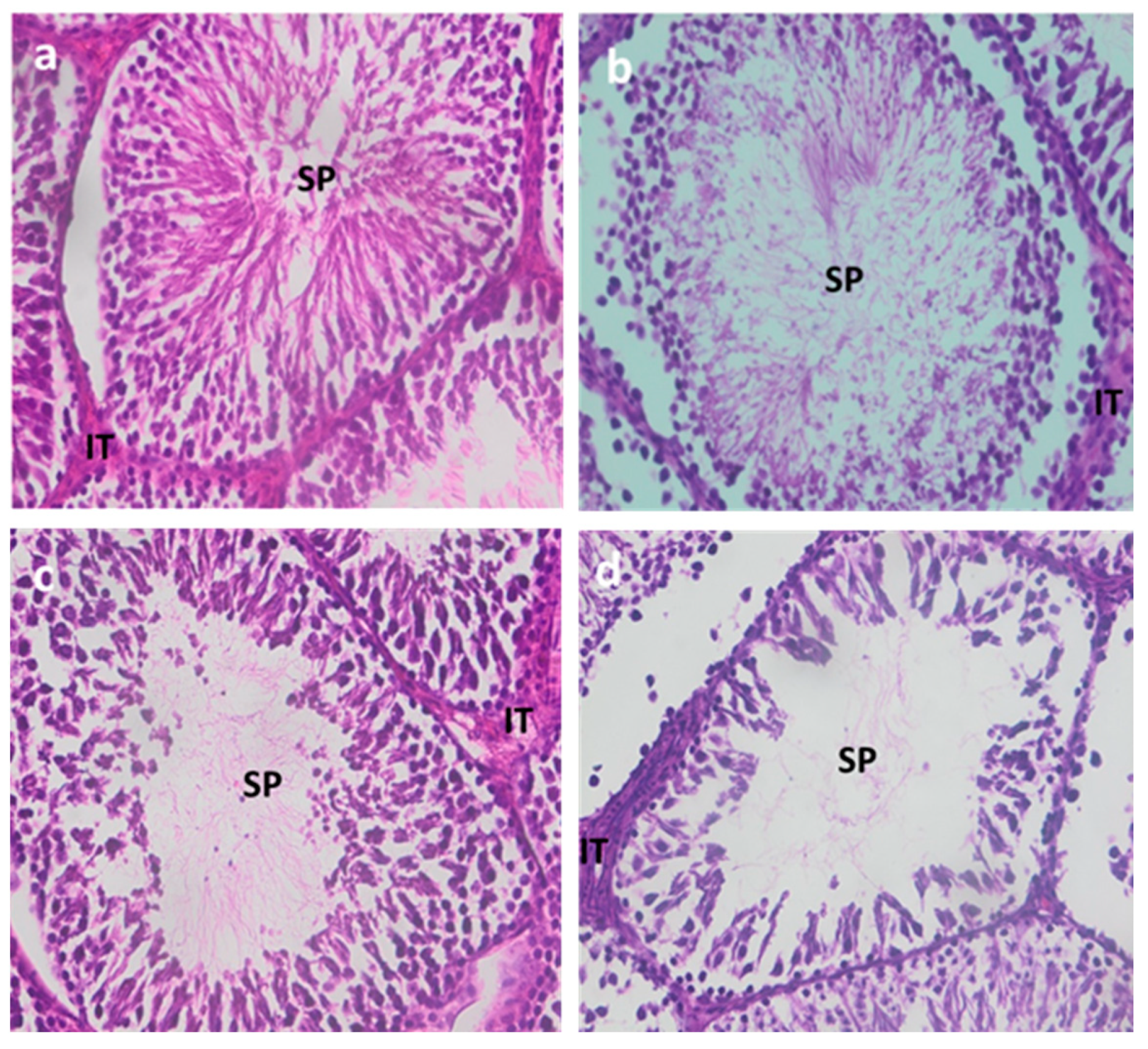

| Control | Cd1 | Cd2 | Cd3 | ||
|---|---|---|---|---|---|
| Final body weight | 307.0 ± 12.36 | 264.0 ± 43.76 * | 253.3 ± 14.15 ** | 252.8 ± 30.72 ** | |
| Testis | Weight | 1.79 ± 0.07 | 1.73 ± 0.06 | 1.66 ± 0.10 * | 1.60 ± 0.12 ** |
| Length | 2.21 ± 0.11 | 2.11 ± 0.04 | 2.10 ± 0.06 | 2.08 ± 0.07 * | |
| Width | 1.2 ± 0.06 | 1.16 ± 0.10 | 1.05 ± 0.08 ** | 1.05 ± 0.05 ** | |
| Depth | 1.05 ± 0.05 | 1 ± 0 * | 0.95 ± 0.08 * | 0.91 ± 0.11 * | |
| Epididymis | 1.18 ± 0.08 | 1.04 ± 0.07 * | 0.89 ± 0.1 ** | 0.77 ± 0.07 ** | |
| Control | Cd1 | Cd2 | Cd3 | |
|---|---|---|---|---|
| Count (×106) | 101.5 ± 4.42 | 72.53 ± 3.87 ** | 58.13 ± 4.25 ** | 35.2 ± 5.72 ** |
| MotilityA + B (%) | 81.67 ± 3.07 | 70.17 ± 1.16 ** | 54.83 ± 3.43 ** | 33.67 ± 2.25 ** |
| Immotile | 14.33 ± 1.86 | 28.0 ± 1.41 ** | 44.5 ± 3.08 ** | 65 ± 2.28 ** |
| Viability (%) | 85.67 ± 1.63 | 72 ± 1.41 ** | 59.33 ± 3.98 ** | 39.33 ± 2.5 ** |
| Morphology | ||||
| Normal | 87.17 ± 1.83 | 74.5 ± 2.07 ** | 61.33 ± 3.32 ** | 49.33 ± 5.57 ** |
| Abnormal | 12.83 ± 1.83 | 25.5 ± 2.07 ** | 38.67 ± 3.32 ** | 50.67 ± 5.57 ** |
| Control | Cd1 | Cd2 | Cd3 | |
|---|---|---|---|---|
| Testosterone (ng/mL) | 2.92 ± 1.21 | 1.91 ± 0.91 | 1.39 ± 0.70 * | 0.80 ± 0.49 ** |
| Control | Cd1 | Cd2 | Cd3 | |
|---|---|---|---|---|
| Cd conc in testes (µg/g) | 0.12 ± 0.10 | 1.36 ± 0.46 ** | 1.87 ± 0.49 ** | 2.94 ± 0.84 ** |
| Zn conc in testes (mg/g) | 0.22 ± 0.008 | 0.19 ± 0.007 ** | 0.18 ± 0.00 ** | 0.16 ± 0.01 ** |
| Control | Cd3 | Cd3recov | Cd3recov + Zn | Zn | ||
|---|---|---|---|---|---|---|
| Final body weight | 307.0 ± 12.36 | 252.8 ± 30.72 a* | 299 ± 27.72 | 302.5 ± 17.71 b* | 297.3 ± 7.14 | |
| Testis | Weight | 1.79 ± 0.07 | 1.60 ± 0.12 a** | 1.68 ± 0.12 | 1.70 ± 0.05 | 1.77 ± 0.04 |
| Length | 2.21 ± 0.11 | 2.08 ± 0.07 a* | 2.10 ± 0.08 | 2.11 ± 0.09 | 2.2 ± 0 | |
| Width | 1.2 ± 0.06 | 1.05 ± 0.05 a** | 1.16 ± 0.05 b** | 1.16 ± 0.05 b** | 1.28 ± 0.07 | |
| Depth | 1.05 ± 0.05 | 0.91 ± 0.11 a* | 1.01 ± 0.04 | 1 ± 0 b** | 1.06 ± 0.05 | |
| Epididymis | 1.18 ± 0.08 | 0.77 ± 0.07 a** | 0.88 ± 0.08 | 0.9 ± 0.07 | 1.12 ± 0.08 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Benbella, J.; Housbane, S.; Kadil, Y.; Kabbali, F.; Ghicha, I.; Bazhar, H.; Bousselham, F.; Banid, A.; Hammani, O.; Louanjli, N.; et al. Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation. Int. J. Environ. Res. Public Health 2025, 22, 454. https://doi.org/10.3390/ijerph22030454
Ait Benbella J, Housbane S, Kadil Y, Kabbali F, Ghicha I, Bazhar H, Bousselham F, Banid A, Hammani O, Louanjli N, et al. Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation. International Journal of Environmental Research and Public Health. 2025; 22(3):454. https://doi.org/10.3390/ijerph22030454
Chicago/Turabian StyleAit Benbella, Jihane, Samy Housbane, Youness Kadil, Fatimaezzahra Kabbali, Ikram Ghicha, Hasnaa Bazhar, Fatiha Bousselham, Afaf Banid, Othmane Hammani, Noureddine Louanjli, and et al. 2025. "Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation" International Journal of Environmental Research and Public Health 22, no. 3: 454. https://doi.org/10.3390/ijerph22030454
APA StyleAit Benbella, J., Housbane, S., Kadil, Y., Kabbali, F., Ghicha, I., Bazhar, H., Bousselham, F., Banid, A., Hammani, O., Louanjli, N., Karkouri, M., Mellouki, A., Filali, H., & Aboutaieb, R. (2025). Evaluation of the Reversibility of Cadmium-Induced Testicular Toxicity Following Recovery Alone or with Zinc Supplementation. International Journal of Environmental Research and Public Health, 22(3), 454. https://doi.org/10.3390/ijerph22030454






