Impact of COVID-19 on the HIV Treatment Outcomes Among Men Who Have Sex with Men in South Africa After the Implementation of a Differentiated Service Delivery Model: An Interrupted Time Series Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Population and Area
2.2.1. KwaZulu-Natal (eThekwini and UMgungundlovu Districts)
2.2.2. Gauteng (Tshwane and Ekurhuleni Districts)
2.2.3. Mpumalanga (Ehlanzeni District)
2.3. Data Source
Description of the DSD Model
2.4. Research Measures
2.5. Validity and Quality Assessment
2.6. Statistical Analysis
2.7. Ethics Approval
3. Results
Characteristics of the Study Population
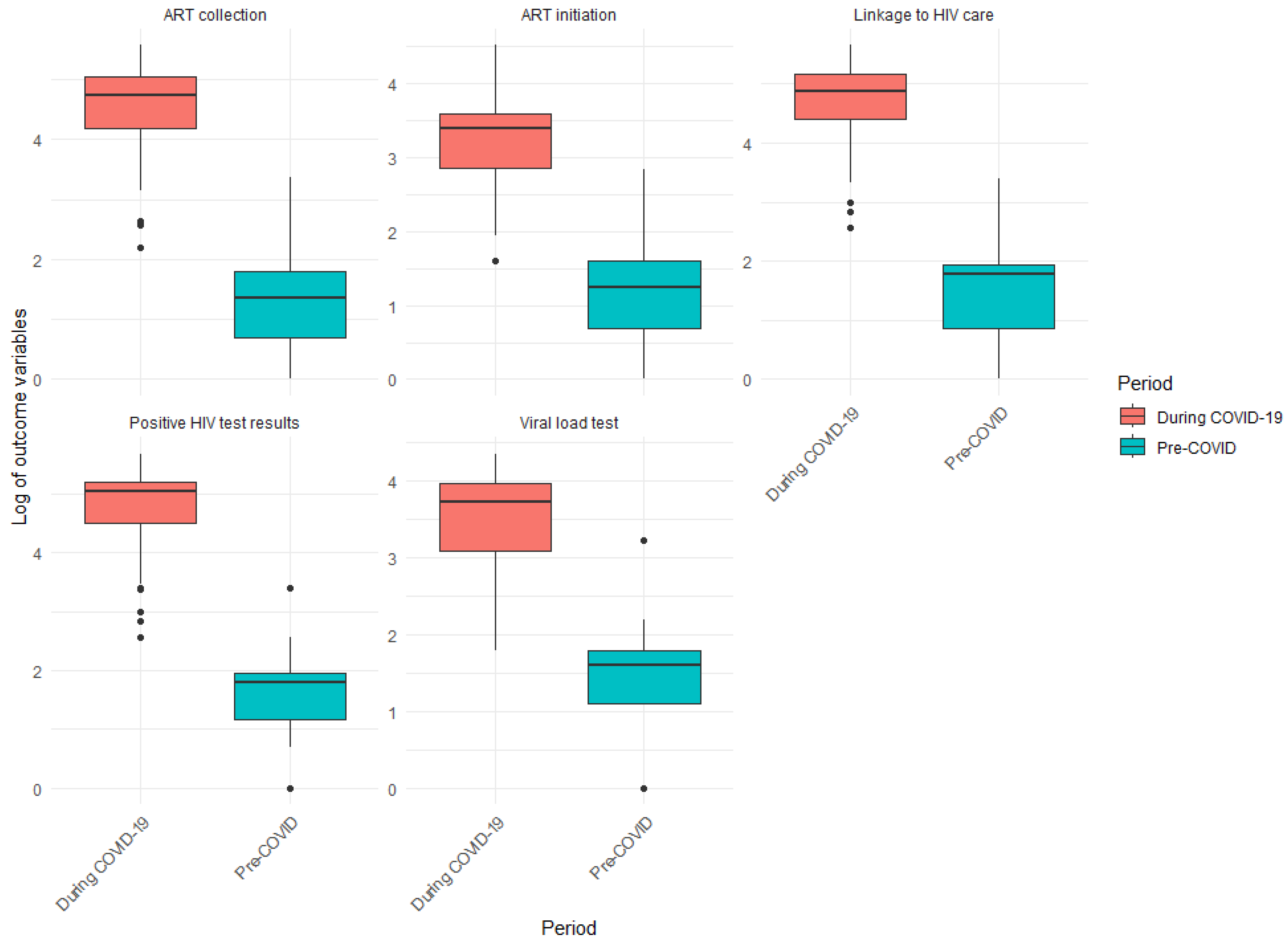
- Positive HIV tests
- Linkage to HIV care
- ART initiation
- ART collection
- Viral load tests
4. Discussion
4.1. Practical Implications
4.2. Strengths and Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Declaration
Abbreviations
| MSM | Men who have sex with men |
| COVID-19 | Coronavirus disease 2019 |
| DSD | Differentiated Service Delivery Model |
| ART | Antiretroviral Therapy |
| PLHIV | People living with HIV |
| KPs | Key populations |
| FSW | Female sex workers |
| PWUD | People who use drugs |
| PWID | People who inject drugs |
| TG | Transgender |
| SSA | Sub-Saharan Africa |
| CD4 | Cluster of differentiation 4 |
| STI | Sexually transmitted infection |
| NGO | Non-government organization |
| CDC | Centres for Disease Control and Prevention |
| NDoH | National Department of Health |
| SMS | Short message service |
References
- Johnson, L.F.; Dorrington, R.E. Thembisa Version 4.6: A Model for Evaluating the Impact of HIV/AIDS in South Africa. 2023. Available online: https://www.thembisa.org/content/downloadPage/Thembisa4_6report (accessed on 2 October 2023).
- World Health Organization. Consolidated Guidelines on HIV Prevention, Diagnosis, Treatment and Care for Key Populations; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- UNAIDS. HIV Service Disruptions in 2020. 2020. Available online: https://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2020/may/20200511_PR_HIV_modelling (accessed on 25 July 2021).
- UNAIDS. Worldwide, More than Half of New HIV Infections Now Among Key Populations and Their Sexual Partners. 2019. Available online: https://www.unaids.org/en/resources/presscentre/featurestories/2019/november/20191105_key-populations (accessed on 2 August 2022).
- Stone, J.; Mukandavire, C.; Boily, M.C.; Fraser, H.; Mishra, S.; Schwartz, S.; Rao, A.; Looker, K.J.; Quaife, M.; Terris-Prestholt, F.; et al. Estimating the contribution of key populations towards HIV transmission in South Africa. J. Int. AIDS Soc. 2021, 24, e25650. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Morales, G.J.; Kilonzo, T.M.; Dayton, R.L.; Musundi, R.T.; Mbole, J.M.; Malaba, S.J.; Ogwang, B.E.; Isac, S.K.; Moses, S.; et al. Can a national government implement a violence prevention and response strategy for key populations in a criminalized setting? A case study from Kenya. J. Int. AIDS Soc. 2018, 21, e25122. [Google Scholar] [CrossRef]
- Babel, R.A.; Wang, P.; Alessi, E.J.; Raymond, H.F.; Wei, C. Stigma, HIV risk, and access to HIV prevention and treatment services among men who have sex with men (MSM) in the United States: A scoping review. AIDS Behav. 2021, 25, 3574–3604. [Google Scholar] [CrossRef] [PubMed]
- Sandfort, T.G.; Knox, J.; Collier, K.L.; Lane, T.; Reddy, V. HIV testing practices of South African township MSM in the era of expanded access to ART. AIDS Behav. 2015, 19, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Maleke, K.; Daniels, J.; Lane, T.; Struthers, H.; McIntyre, J.; Coates, T. How social stigma sustains the HIV treatment gap for MSM in Mpumalanga, South Africa. Glob. Health Promot. 2019, 26, 6–13. [Google Scholar] [CrossRef]
- Duby, Z.; Nkosi, B.; Scheibe, A.; Brown, B.; Bekker, L.G. ‘Scared of going to the clinic’: Contextualising healthcare access for men who have sex with men, female sex workers and people who use drugs in two South African cities. S. Afr. J. HIV Med. 2018, 19, 1–8. [Google Scholar] [CrossRef]
- Ravele, T.T.; Seretlo, R.J.; Mokgatle, M.M.; Seretlo, R.; Ravele, T.T.; Seretlo, R.J. We are treated differently: Experiences of men who have sex with men in South African clinics. S. Afr. Fam. Pract. 2025, 67, a6050. [Google Scholar] [CrossRef]
- Caballero-Hoyos, R.; Monárrez-Espino, J.; Ramírez-Ortíz, M.G.; Cárdenas-Medina, F.M. Factors associated with unprotected anal sex among men who have sex with men in Mexico. Infect. Dis. Rep. 2022, 14, 547–557. [Google Scholar] [CrossRef]
- Malefo, M.A.; Ayo-Yusuf, O.; Mokgatle, M.M. Risk factors for sexually transmitted infections among men who have sex with men. Afr. J. Prim. Health Care Fam. Med. 2023, 15, 4080. [Google Scholar] [CrossRef]
- Quinn, K.G.; Voisin, D.R. ART adherence among men who have sex with men living with HIV: Key challenges and opportunities. Curr. HIV/AIDS Rep. 2020, 17, 290–300. [Google Scholar] [CrossRef]
- Graham, S.M.; Mugo, P.; Gichuru, E.; Thiong’o, O.; Macharia, M.; Okuku, H.S.; van der Elst, E.; Price, M.A.; Muraguri, N.; Sanders, E.J. Adherence to Antiretroviral Therapy and Clinical Outcomes Among Young Adults Reporting High-Risk Sexual Behavior, Including Men Who Have Sex with Men, in Coastal Kenya. AIDS Behav. 2013, 17, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yuan, T.; Wang, J.; Fitzpatrick, T.; Li, Q.; Li, P.; Tang, X.; Xu, G.; Chen, D.; Liang, B.; et al. Sex differences in HIV treatment outcomes and adherence by exposure groups among adults in Guangdong, China: A retrospective observational cohort study. EClinicalMedicine 2020, 22, 100351. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Silhol, R.; Knight, J.; Phaswana-Mafuya, R.; Diouf, D.; Wang, L.; Schwartz, S.; Boily, M.C.; Baral, S. Estimating the epidemic consequences of HIV prevention gaps among key populations. J. Int. AIDS Soc. 2021, 24, e25739. [Google Scholar] [CrossRef] [PubMed]
- da Silva Junior, F.J.; de Souza Monteiro, C.F.; Costa, A.P.; Campos, L.R.; Miranda, P.I.; de Souza Monteiro, T.A.; Lima, R.A.; Lopes-Junior, L.C. Impact of COVID-19 pandemic on mental health of young people and adults: A systematic review protocol of observational studies. BMJ Open 2020, 10, e039426. [Google Scholar] [CrossRef]
- Mirzaei, H.; Moradi, Y.; Abbaszadeh, S.; Nasiri, N.; Mehmandoost, S.; Khezri, M.; Tavakoli, F.; Sharifi, H. The impact of COVID-19 on disruptions of HIV-related services: A rapid review. Med. J. Islam. Repub. Iran 2022, 36, 734–742. [Google Scholar] [CrossRef]
- Dorward, J.; Khubone, T.; Gate, K.; Ngobese, H.; Sookrajh, Y.; Mkhize, S.; Jeewa, A.; Bottomley, C.; Lewis, L.; Baisley, K.; et al. The impact of the COVID-19 lockdown on HIV care in 65 South African primary care clinics: An interrupted time series analysis. Lancet HIV 2021, 8, e158–e165. [Google Scholar] [CrossRef]
- Jardim, C.G.R.; Zamani, R.; Akrami, M. Evaluating the impact of the COVID-19 pandemic on accessing HIV services in South Africa: A systematic review. Int. J. Environ. Res. Public Health 2022, 19, 11899. [Google Scholar] [CrossRef]
- Benade, M.; Long, L.; Rosen, S.; Meyer-Rath, G.; Tucker, J.M.; Miot, J. Reduction in initiations of HIV treatment in South Africa during the COVID pandemic. BMC Health Serv. 2022, 22, 428. [Google Scholar] [CrossRef]
- World Health Organization. Maintaining Essential Health Services: Operational Guidance for the COVID-19 Context; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-essential_health_services-2020.2 (accessed on 22 October 2020).
- Pierre, G.; Uwineza, A.; Dzinamarira, T. Attendance to HIV antiretroviral collection clinic appointments during COVID-19 lockdown. A single center study in Kigali, Rwanda. AIDS Behav. 2020, 24, 3299–3301. [Google Scholar] [CrossRef]
- Chanda-Kapata, P.; Ntoumi, F.; Kapata, N.; Lungu, P.; Mucheleng’anga, L.A.; Chakaya, J.; Tembo, J.; Himwaze, C.; Ansumana, R.; Asogun, D.; et al. Tuberculosis, HIV/AIDS and malaria health services in sub-Saharan Africa–a situation analysis of the disruptions and impact of the COVID-19 pandemic. Int. J. Infect. Dis. 2022, 124, S41–S46. [Google Scholar] [CrossRef]
- Long, L.; Kuchukhidze, S.; Pascoe, S.; Nichols, B.E.; Fox, M.P.; Cele, R.; Govathson, C.; Huber, A.N.; Flynn, D.; Rosen, S. Retention in care and viral suppression in differentiated service delivery models for HIV treatment delivery in sub-Saharan Africa: A rapid systematic review. J. Int. AIDS Soc. 2020, 23, e25640. [Google Scholar] [CrossRef]
- UNAIDS. UNAIDS DATA 2021. 2021. Available online: https://www.unaids.org/sites/default/files/media_asset/JC3032_AIDS_Data_book_2021_En.pdf (accessed on 15 May 2022).
- Pan American Health Organization and World Health Organization. Key Populations. 2022. Available online: https://www.paho.org/en/topics/key-populations (accessed on 31 July 2022).
- Statistics South Africa. 2024 Mid-Year Population Estimates. 2024. Available online: https://www.statssa.gov.za/?page_id=1854&PPN=P0302&SCH=73952 (accessed on 24 February 2025).
- Municipalities of South Africa. Municipalities. 2024. Available online: https://municipalities.co.za/ (accessed on 23 February 2025).
- Human Sciences Research Council. KwaZulu-Natal Reports Second-Highest HIV Prevalence Rate in South Africa. 2024. Available online: https://hsrc.ac.za/press-releases/phsb/kwazulu-natal-reports-second-highest-hiv-prevalence-rate-in-south africa/#:~:text=The%20survey%20further%20revealed%20that,mirror%20those%20for%20ART%20coverage (accessed on 21 February 2025).
- Human Sciences Research Council. SABSSM VI Provincial Dialogue: Gauteng Province Media Pack. 2024. Available online: https://hsrc.ac.za/special-projects/sabssm-survey-series/sabssm-vi-provincial-dialogue-gauteng-province-media-pack/ (accessed on 21 February 2025).
- Human Sciences Research Council. Mpumalanga Province Grapples with Highest HIV Prevalence Rate in SA. 2024. Available online: https://hsrc.ac.za/press-releases/phsb/mpumalanga-province-grapples-with-highest-hiv-prevalence-rate-in-sa/#:~:text=Viral%20load%20suppression%20(VLS),to%2085.6%25%20in%20Gert%20Sibande (accessed on 21 February 2025).
- South African National Aids Council. National Strategic Plan for HIV, TB, and STIs 2023–2028. 2023. Available online: https://sanac.org.za/wp-content/uploads/2023/05/SANAC-NSP-2023-2028-Web-Version.pdf (accessed on 6 August 2024).
- Bello, B.; Ndagurwa, P.; Omogiate, S.; Luwaca, B.; Motsieloa, L.; Global Aids Monitoring Report: Analysis of Current Status and Progress Towards Targets. CESAR. 2021. Available online: https://sanac.org.za/wp-content/uploads/2022/04/South-Africa-Global-AIDS-Monitoring_GAM-Report-2020.pdf (accessed on 6 August 2024).
- Saczynski, J.S.; McManus, D.D.; Goldberg, R.J. Commonly used data-collection approaches in clinical research. Am. J. Med. 2013, 126, 946–950. [Google Scholar] [CrossRef]
- Wagner, A.K.; Soumerai, S.B.; Zhang, F.; Ross-Degnan, D. Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 2002, 27, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- South African Government. Coronavirus COVID-19 Alert Level 2. 2021. Available online: https://www.gov.za/coronavirus/alert-level-2 (accessed on 15 November 2023).
- Moonasar, D.; Pillay, A.; Leonard, E.; Naidoo, R.; Mngemane, S.; Ramkrishna, W.; Jamaloodien, K.; Lebese, L.; Chetty, K.; Bamford, L.; et al. COVID-19: Lessons and experiences from South Africa’s first surge. BMJ Glob. Health 2021, 6, e004393. [Google Scholar] [CrossRef] [PubMed]
- Iversen, J.; Sabin, K.; Chang, J.; Thomas, R.M.; Prestage, G.; Strathdee, S.A.; Maher, L. COVID-19, HIV and key populations: Cross-cutting issues and the need for population-specific responses. J. Int. AIDS Soc. 2020, 23, e25632. [Google Scholar] [CrossRef] [PubMed]
- Cascalheira, C.J.; Morrison, C.; D’Angelo, A.B.; Garcia Villanueva, O.; Grov, C. The impact of the COVID-19 pandemic on HIV-positive men who have sex with men:(Dis) connection to social, sexual, and health networks. Psychol. Sex. 2023, 14, 306–320. [Google Scholar] [CrossRef]
- Mulaudzi, M.; Kiguwa, P.; Zharima, C.; Otwombe, K.; Hlongwane, K.; Dietrich, J.J. Sexual risk behaviors among youth in Soweto, South Africa during the COVID-19 national lockdown. Sex. Med. 2022, 10, 100487. [Google Scholar] [CrossRef]
- Yao, D.; Hill, N.; Brown, B.; Gule, D.; Chabane, M.; Mcingana, M.; Willis, K.; Shiba, V.; Olawore, O.; Nel, D.; et al. The impact of COVID-19 restrictions on HIV prevention and treatment services for key populations in South Africa: An interrupted time series analysis. BMC Public Health 2024, 24, 2386. [Google Scholar] [CrossRef]
- Miller, R.L.; McLaughlin, A.; Montoya, V.; Toy, J.; Stone, S.; Harding, J.; Liang, R.H.; Wong, J.; Barrios, R.; Montaner, J.S.; et al. Impact of SARS-CoV-2 lockdown on expansion of HIV transmission clusters among key populations: A retrospective phylogenetic analysis. Lancet Reg. Health Am. 2022, 16, 100369. [Google Scholar] [CrossRef]
- Jo, Y.; Rosen, S.; Sy, K.T.L.; Phiri, B.; Huber, A.N.; Mwansa, M.; Shakwelele, H.; Haimbe, P.; Mwenechanya, M.M.; Mulenga, P.L.; et al. Changes in HIV Treatment Differentiated Care Utilization During the COVID-19 Pandemic in Zambia. medRxiv 2021. [Google Scholar] [CrossRef]
- Ahmad, S.; Fuller, S.; Sohn, A.H. The impact of COVID-19 on HIV treatment and care delivery in South and Southeast Asia: A qualitative study. HIV Res. Clin. Pract. 2024, 25, 2355763. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Updated Recommendations on Service Delivery for the Treatment and Care of People Living with HIV; World Health Organization: Geneva, Switzerland, 2021; Available online: https://www.who.int/publications/i/item/9789240023581 (accessed on 23 February 2025).
- Collins, L.F.; Colasanti, J.A.; Nguyen, M.L.; Moran, C.A.; Lahiri, C.D.; Marconi, V.C.; Armstrong, W.S.; Shah, N.S. The COVID-19 pandemic as a catalyst for differentiated care models to end the HIV epidemic in the United States: Applying lessons from high-burden settings. AIDS 2021, 35, 337–341. [Google Scholar] [CrossRef]
- Siedner, M.J.; Kraemer, J.D.; Meyer, M.J.; Harling, G.; Mngomezulu, T.; Gabela, P.; Dlamini, S.; Gareta, D.; Majozi, N.; Ngwenya, N.; et al. Access to primary healthcare during lockdown measures for COVID-19 in rural South Africa: An interrupted time series analysis. BMJ Open 2020, 10, e043763. [Google Scholar] [CrossRef] [PubMed]
| Measure | Operational Definition |
|---|---|
| Consent for HIV testing | The process of pre-counselling, informing the MSM about HIV tests, including the potential risks, benefits, and outcomes and letting them decide if they would like to continue taking the test or not. |
| Positive HIV tests | HIV tests that were reactive and underwent confirmatory tests. |
| Linkage to HIV care | The process of connecting MSM with confirmed reactive HIV tests for healthcare to begin taking treatment for their HIV infection. |
| ART initiation | The process of starting to receive HIV treatment after being diagnosed with HIV. |
| ART collection | The ability of HIV-diagnosed MSM to collect their HIV medication from the healthcare provider/facility/relevant party as required. |
| Viral load test | Whether MSM living with HIV were able to undertake a viral load test, regardless of their viral load/viral suppression state. |
| Age | Age at last birthday taken on the day of data collection. |
| Location | The geographical district where the MSM included in the study resided. |
| Before COVID-19 Lockdown (January 2018–February 2020) | During COVID-19 Lockdown (March 2020–December 2022) | January 2018–December 2022 | |
|---|---|---|---|
| n (%) | n (%) | Total | |
| Median age (years) | 31 (25–38) | 28 (23–34) | 30 (23–38) |
| District GP City of Tshwane Metropolitan Municipality GP Ekurhuleni Metropolitan Municipality KZ eThekwini Metropolitan Municipality KZ uMgungundlovu District Municipality MP Ehlanzeni District Municipality | 217 (70.5) 40 (13.0) 24 (7.8) 11 (3.6) 16 (5.2) | 11,930 (12.3) 32,729 (33.9) 36,676 (37.9) 6159 (6.4) 9187 (9.5) | 12,147 (12.5) 32,769 (33.8) 36,700 (37.8) 6170 (6.4) 9203 (9.5) 96,989 (100) |
| Consent for HIV testing No Yes | 3 (1.0) 305 (99.0) | 28,030 (29.0) 68,652 (71.0) | 28,033 (28.9) 68,957 (71.1) 96,990 (100) |
| HIV test result Negative Positive | 125 (42.8) 167 (57.2) | 40,394 (89.8) 4572 (10.2) | 40,519 (89.5) 4739 (10.5) 45,258 (100) |
| Linkage to HIV care Yes No | 165 (98.8) 2 (0.6) | 4273 (98.4) 70 (1.6) | 4438 (98.4) 72 (1.6) 4510 (100) |
| ART initiation No Yes | 221 (75.7) 71 (24.3) | 55,704 (93.3) 4014 (6.7) | 55,925 (93.2) 4085 (6.8) 60,010 (100) |
| ART collection No Yes | 167 (57.2) 125 (42.8) | 55,822 (93.7) 3748 (6.3) | 55,989 (93.5) 3873 (6.5) 59,862 (100) |
| Viral load test done 12 months 6 months Other | 127 (97.0) 2 (1.5) 2 (1.5) | 919 (67.7) 293 (21.6) 145 (10.7) | 1046 (70.3) 295 (19.8) 147 (9.9) 1488 (100) |
| Before COVID-19 Lockdown | During COVID-19 Lockdown | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome Variable | Q1 | Median | Q3 | IQR | Q1 | Median | Q3 | IQR |
| Positive HIV test results | 3.25 | 6.00 | 7.00 | 3.75 | 89.25 | 154.00 | 181.00 | 91.75 |
| Linkage to HIV care | 2.50 | 6.00 | 7.00 | 4.50 | 81.25 | 132.00 | 176.50 | 95.25 |
| ART initiation | 0.25 | 2.00 | 2.00 | 1.75 | 76.75 | 125.50 | 163.00 | 86.25 |
| ART collection | 2.00 | 4.00 | 6.00 | 4.00 | 65.75 | 114.00 | 154.75 | 89.00 |
| Viral load test | 3.00 | 5.00 | 6.00 | 3.00 | 22.00 | 41.50 | 53.00 | 31.00 |
| Outcome Variable | W | p-Value |
|---|---|---|
| Positive HIV test results | 5.50 | <0.001 |
| Linkage to HIV care | 5.50 | <0.001 |
| ART initiation | 16.5 | <0.001 |
| ART collection | 8.50 | <0.001 |
| Viral load test | 19.5 | <0.001 |
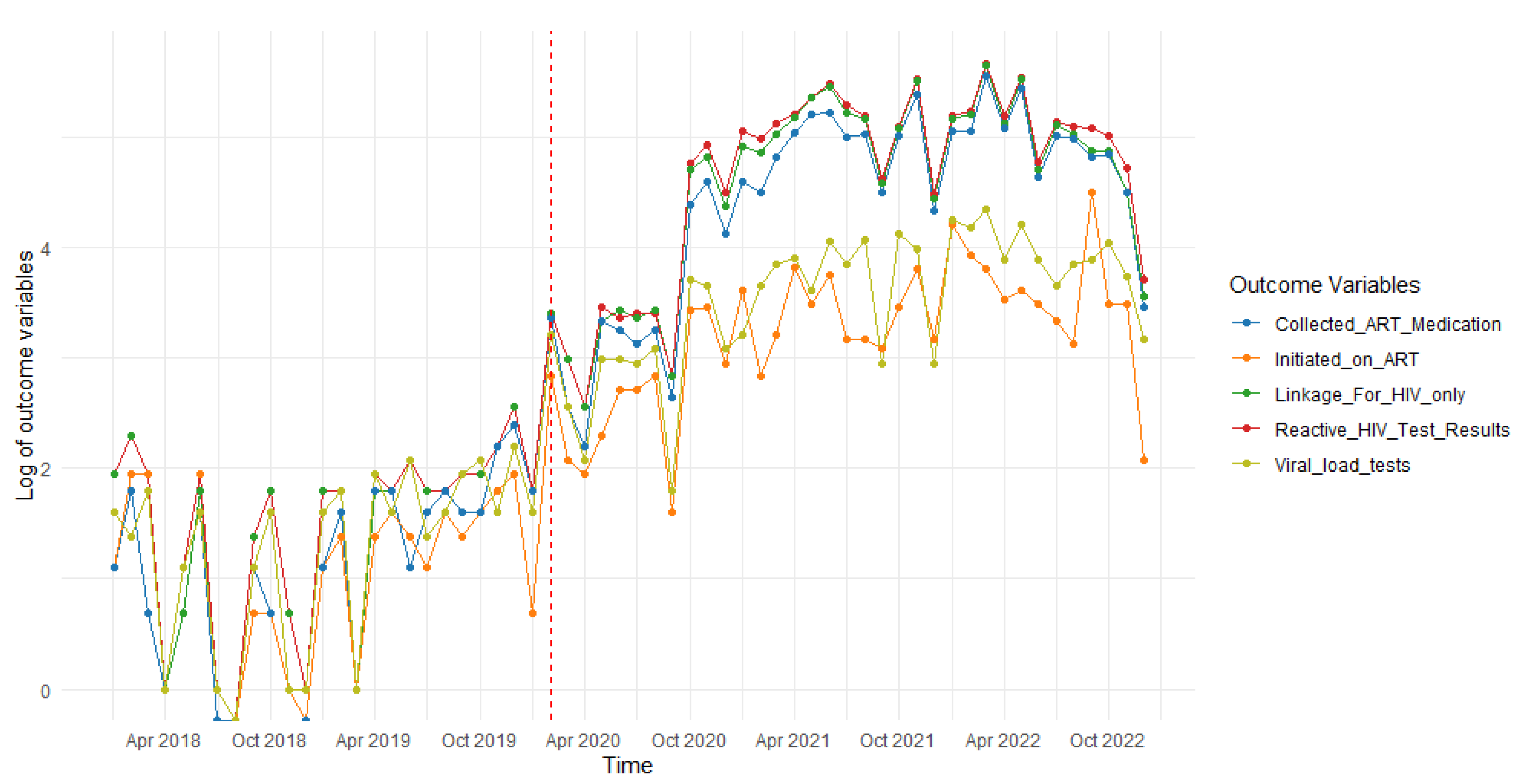
| HIV Treatment Outcome | Time Before the COVID-19 Lockdown | COVID-19 Lockdown | Time After the COVID-19 Lockdown |
|---|---|---|---|
| Positive HIV tests | |||
| Estimate | 0.00009885 (0.0000833, 0.000114) | −26.79 (−42.17, −11.40) | 0.001572 (0.00075, 0.00239) |
| Standard error | 0.000007777 | 7.69 | 0.0004082 |
| t-value | 12.71 | −3.49 | 3.85 |
| p-value | <0.001 | 0.010 | <0.001 |
| Adjusted R-squared | 0.9648 | ||
| Linkage to HIV care | |||
| Estimate | 0.00009767 (0.0000820, 0.000113) | −25.21 (−40.70, −9.71) | 0.001486 (0.00066, 0.00231) |
| Standard error | 0.000007842 | 7.737 | 0.000411 |
| t-value | 12.47 | −3.26 | 3.62 |
| p-value | <0.001 | 0.002 | <0.001 |
| Adjusted R-squared | 0.9634 | ||
| ART initiation | |||
| Estimate | 0.00007869 (0.0000657, 0.0000917) | −17.10 (−29.92, −4.29) | 0.001003 (0.00032, 0.00168) |
| Standard error | 0.000006478 | 6.4 | 0.00034 |
| t-value | 12.15 | −2.67 | 2.95 |
| p-value | <0.001 | 0.009 | 0.004 |
| Adjusted R-squared | 0.952 | ||
| ART collection | |||
| Estimate | 0.00008209 (0.000066, 0.0000982) | −30.00 (−45.87, −14.13) | 0.001748 (0.00091, 0.00259) |
| Standard error | 0.000008022 | 7.925 | 0.000421 |
| t-value | 10.23 | −3.79 | 4.15 |
| p-value | <0.001 | <0.001 | <0.001 |
| Adjusted R-squared | 0.9582 | ||
| Viral load tests | |||
| Estimate | 0.00008998 (0.0000774, 0.000103) | −19.00 (−31.39, −6.61) | 0.001109 (0.00045, 0.00177) |
| Standard error | 0.000006262 | 6.186 | 0.0003286 |
| t-value | 14.37 | −3.07 | 3.38 |
| p-value | <0.001 | 0.003 | 0.001 |
| Adjusted R-squared | 0.9626 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sebati, B.; Phalane, E.; Shiferaw, Y.A.; Pienaar, J.; Furamera, S.; Phaswana-Mafuya, R.N. Impact of COVID-19 on the HIV Treatment Outcomes Among Men Who Have Sex with Men in South Africa After the Implementation of a Differentiated Service Delivery Model: An Interrupted Time Series Analysis. Int. J. Environ. Res. Public Health 2025, 22, 452. https://doi.org/10.3390/ijerph22030452
Sebati B, Phalane E, Shiferaw YA, Pienaar J, Furamera S, Phaswana-Mafuya RN. Impact of COVID-19 on the HIV Treatment Outcomes Among Men Who Have Sex with Men in South Africa After the Implementation of a Differentiated Service Delivery Model: An Interrupted Time Series Analysis. International Journal of Environmental Research and Public Health. 2025; 22(3):452. https://doi.org/10.3390/ijerph22030452
Chicago/Turabian StyleSebati, Betty, Edith Phalane, Yegnanew A. Shiferaw, Jacqueline Pienaar, Stanford Furamera, and Refilwe Nancy Phaswana-Mafuya. 2025. "Impact of COVID-19 on the HIV Treatment Outcomes Among Men Who Have Sex with Men in South Africa After the Implementation of a Differentiated Service Delivery Model: An Interrupted Time Series Analysis" International Journal of Environmental Research and Public Health 22, no. 3: 452. https://doi.org/10.3390/ijerph22030452
APA StyleSebati, B., Phalane, E., Shiferaw, Y. A., Pienaar, J., Furamera, S., & Phaswana-Mafuya, R. N. (2025). Impact of COVID-19 on the HIV Treatment Outcomes Among Men Who Have Sex with Men in South Africa After the Implementation of a Differentiated Service Delivery Model: An Interrupted Time Series Analysis. International Journal of Environmental Research and Public Health, 22(3), 452. https://doi.org/10.3390/ijerph22030452






