Comparing Short Cognitive Screening Instruments in an Outreach Memory Clinic in Primary Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Data Collection
2.3. Measures
2.4. Analysis
2.5. Inter-Rater Reliability Testing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Derby, C.A. Trends in the public health significance, definitions of disease, and implications for prevention of Alzheimer’s disease. Curr. Epidemiol. Rep. 2020, 7, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.; Knopman, D.S. Classification and epidemiology of MCI. Clin. Geriatr. Med. 2013, 29, 753–772. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Wells, Y.; Yu, D. Challenges in detecting and managing mild cognitive impairment in primary care: A focus group study in Shanghai, China. BMJ Open 2022, 12, e062240. [Google Scholar] [CrossRef]
- Jerjes, W. The importance of attentive primary care in the early identification of mild cognitive impairment: Case series. AME Case Rep. 2024, 8, 56. [Google Scholar] [CrossRef]
- Liss, J.L.; Assunção, S.S.M.; Cummings, J.; Atri, A.; Geldmacher, D.S.; Candela, S.F.; Devanand, D.P.; Fillit, H.M.; Susman, J.; Mintzer, J.; et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: A review and synthesis. J. Int. Med. 2021, 290, 310–334. [Google Scholar] [CrossRef] [PubMed]
- Bradford, A.; Kunik, M.E.; Schulz, P.; Williams, S.P.; Singh, H. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Dis. Assoc. Disord. 2009, 23, 306–314. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Boada, M.; Borson, S.; Chilukuri, M.; Dubois, B.; Ingram, J.; Iwata, A.; Porsteinsson, A.P.; Possin, K.L.; Rabinovici, G.D.; et al. Early Detection of Mild Cognitive Impairment (MCI) in Primary Care. J. Prev. Alzheimer’s Dis. 2020, 7, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Borson, S.; Small, G.W.; O’Brien, Q.; Morrello, A.; Boustani, M. Understanding barriers to and facilitators of clinician-patient conversations about brain health and cognitive concerns in primary care: A systematic review and practical considerations for the clinician. BMC Prim. Care 2023, 24, 233. [Google Scholar] [CrossRef]
- Rameez, S.; Nasir, A. Barriers to mental health treatment in primary care practice in low- and middle-income countries in a post-covid era: A systematic review. J. Fam. Med. Prim. Care 2023, 12, 1485–1504. [Google Scholar] [CrossRef]
- Wakida, E.K.; Talib, Z.M.; Akena, D.; Okello, E.S.; Kinengyere, A.; Mindra, A.; Obua, C. Barriers and facilitators to the integration of mental health services into primary health care: A systematic review. Syst. Rev. 2018, 7, 211. [Google Scholar] [CrossRef]
- Wainberg, M.L.; Scorza, P.; Shultz, J.M.; Helpman, L.; Mootz, J.J.; Johnson, K.A.; Neria, Y.; Bradford, J.E.; Oquendo, M.A.; Arbuckle, M.R. Challenges and Opportunities in Global Mental Health: A Research-to-Practice Perspective. Curr. Psychiatry Rep. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Huntley, J.; Liu, K.Y.; Costafreda, S.G.; Selbæk, G.; Alladi, S.; Ames, D.; Banerjee, S.; Burns, A.; Brayne, C.; et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing Commission. Lancet 2024, 404, 572–628. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.; Wallace, L.; Kuhn, I.; Mytton, O.; Lafortune, L.; Wills, W.; Mukadam, N.; Brayne, C. Population-level interventions for the primary prevention of dementia: A complex evidence review. Lancet 2023, 402, S13. [Google Scholar] [CrossRef]
- Jones, D.; Drewery, R.; Windle, K.; Humphrey, S.; de Paiva, A.F. Dementia prevention and the GP’s role: A qualitative interview study. Br. J. Gen. Pract. 2024, 74, 242–249. [Google Scholar] [CrossRef]
- Sideman, A.B.; Ma, M.; de Jesus, A.H.; Alagappan, C.; Razon, N.; Dohan, D.; Chodos, A.; Al-Rousan, T.; Alving, L.I.; Segal-Gidan, F.; et al. Primary Care Practitioner Perspectives on the Role of Primary Care in Dementia Diagnosis and Care. JAMA Netw. Open 2023, 6, e2336030. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.C.; Woodward, E. A national survey of memory clinics in Australia. Int. Psychogeriatr. 2009, 21, 696–702. [Google Scholar] [CrossRef]
- Brodaty, H.; Clarke, J.; Ganguli, M.; Grek, A.; Jorm, A.F.; Khachaturian, Z.; Scherr, P. Screening for cognitive impairment in general practice: Toward a consensus. Alzheimer Dis. Assoc. Disord. 1998, 12, 1–13. [Google Scholar] [CrossRef]
- Boustani, M.; Callahan, C.M.; Unverzagt, F.W.; Austrom, M.G.; Perkins, A.J.; Fultz, B.A.; Hui, S.L.; Hendrie, H.C. Implementing a Screening and Diagnosis Program for Dementia in Primary Care. J. Gen. Intern. Med. 2005, 20, 572–577. [Google Scholar] [CrossRef]
- Valcour, V.G.; Masaki, K.H.; Curb, J.D.; Blanchette, P.L. The detection of dementia in the primary care setting. Arch. Int. Med. 2006, 160, 2964–2968. [Google Scholar] [CrossRef]
- Knopman, D.S. Current treatment of mild cognitive impairment and Alzheimer’s disease. Curr. Neurol. Neurosci. Rep. 2006, 6, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Dobbs, B.; McKay, R.; Kirwan, C.; Cooper, T.; Marin, A.; Gupta, N. Diagnosis and management of dementia in primary care: Exploratory study. Can. Fam. Physician 2014, 60, 457–465. [Google Scholar] [PubMed]
- Kaduszkiewicz, H.; Zimmermann, T.; Van den Bussche, H.; Bachmann, C.; Wiese, B.; Bickel, H.; Mösch, E.; Romberg, H.-P.; Jessen, F.; Cvetanovska-Pllashniku, G.; et al. Do general practitioners recognize mild cognitive impairment in their patients? J. Nutr. Health Aging 2010, 14, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jun, H.; Becker, A.; Wallick, C.; Mattke, S. Detection Rates of Mild Cognitive Impairment in Primary Care for the United States Medicare Population. J. Prev. Alzheimer’s Dis. 2024, 11, 7–12. [Google Scholar] [CrossRef]
- Boustani, M.; Peterson, B.; Hanson, L.; Harris, R.; Lohr, K.N.; U.S. Preventive Services Task Force. Screening for dementia in primary care: A summary of the evidence for the U.S. Preventive Services Task Force. Ann. Int. Med. 2003, 138, 927–937. [Google Scholar] [CrossRef]
- Karimi, L.; Mahboub-Ahari, A.; Jahangiry, L.; Sadeghi-Bazargani, H.; Farahbakhsh, M. A systematic review and meta-analysis of studies on screening for mild cognitive impairment in primary healthcare. BMC Psychiatry 2022, 22, 97. [Google Scholar] [CrossRef]
- Mattke, S.; Batie, D.; Chodosh, J.; Felten, K.; Flaherty, E.; Fowler, N.R.; Kobylarz, F.A.; O’Brien, K.; Paulsen, R.; Pohnert, A.; et al. Expanding the use of brief cognitive assessments to detect suspected early-stage cognitive impairment in primary care. Alzheimer’s Dement. 2023, 19, 4252–4259. [Google Scholar] [CrossRef]
- Brodaty, H.; Low, L.F.; Gibson, L.; Burns, K. What is the best dementia screening instrument for general practitioners to use? Am. J. Geriatr. Psychiatry 2006, 14, 391–400. [Google Scholar] [CrossRef]
- Siddiqui, M.; Nyahoda, T.; Traber, C.; Elliott, S.; Wang, V.; Toledo-Franco, L.; Rodin, M.B. Screening for Cognitive Impairment in Primary Care: Rationale and Tools. Mo. Med. 2023, 120, 431–439. [Google Scholar]
- Ismail, Z.; Rajji, T.K.; Shulman, K.I. Brief cognitive screening instruments: An update. Int. J. Geriatr. Psychiatry 2010, 25, 111–120. [Google Scholar] [CrossRef]
- Brodaty, H.; Pond, D.; Kemp, N.M.; Luscombe, G.; Harding, L.; Berman, K.; Huppert, F.A. The GPCOG: A new screening test for dementia designed for general practice. J. Am. Geriatr. Soc. 2002, 50, 530–534. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Carton, C.; Calafiore, M.; Cauet, C.; Messaadi, N.; Bayen, M.; Wyts, D.; Messaadi, W.; Richebe, T.; Bayen, S. MoCA use in general practice for the early detection of cognitive impairment. BJGP Open 2025, BJGPO.2024.0039. [Google Scholar] [CrossRef]
- Xu, F.; Ma, J.J.; Sun, F.; Lee, J.; Coon, D.W.; Xiao, Q.; Huang, Y.; Zhang, L.; Liang, Z.H. The Efficacy of General Practitioner Assessment of Cognition in Chinese Elders Aged 80 and Older. Am. J. Alzheimer’s Dis. Other Dementiasr 2019, 34, 523–529. [Google Scholar] [CrossRef]
- Brodaty, H.; Connors, M.H.; Loy, C.; Teixeira-Pinto, A.; Stocks, N.; Gunn, J.; Mate, K.E.; Pond, C.D. Screening for Dementia in Primary Care: A Comparison of the GPCOG and the MMSE. Dement. Geriatr. Cogn. Disord. 2016, 42, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Iatraki, E.; Simos, P.G.; Bertsias, A.; Duijker, G.; Zaganas, I.; Tziraki, C.; Vgontzas, A.N.; Lionis, C.; THALIS Primary Health Care Research Team/Network. Cognitive screening tools for primary care settings: Examining the ‘Test Your Memory’ and ‘General Practitioner assessment of Cognition’ tools in a rural aging population in Greece. Eur. J. Gen. Pract. 2017, 23, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.S.; O’Connor, E.; Rossom, R.C.; Perdue, L.A.; Eckstrom, E. Screening for cognitive impairment in older adults: A systematic review for the U.S. Preventive Services Task Force. Ann. Int. Med. 2013, 15, 601–612. [Google Scholar] [CrossRef]
- Patnode, C.D.; Perdue, L.A.; Rossom, R.C.; Rushkin, M.C.; Redmond, N.; Thomas, R.G.; Lin, J.S. Screening for Cognitive Impairment in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2020, 323, 764–785. [Google Scholar] [CrossRef]
- Dhikav, V.; Jadeja, B.; Gupta, P. Community Screening of Probable Dementia at Primary Care Center in Western India: A Pilot Project. J. Neurosci. Rural. Pract. 2022, 13, 490–494. [Google Scholar] [CrossRef]
- Stone, C.; Copeland, B.; Collier, C.; Cheung, G. Memory clinic survey in New Zealand: A second look. Australas. Psychiatry 2019, 27, 486–490. [Google Scholar] [CrossRef]
- McWhirter, L.; Ritchie, C.; Stone, J.; Carson, A. Functional cognitive disorders: A systematic review. Lancet Psychiatry 2020, 7, 191–207. [Google Scholar] [CrossRef]
- Morgan, D.G.; Crossley, M.; Kirk, A.; D’Arcy, C.; Stewart, N.; Biem, J.; Forbes, D.; Harder, S.; Basran, J.; Dal Bello-Haas, V.; et al. Improving access to dementia care: Development and evaluation of a rural and remote memory clinic. Aging Ment. Health 2009, 13, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Disler, R.; Pascoe, A.; Anderson, H.; Piejko, E.; Asaid, A.; Disler, P. A new model for general practice-led, regional, community-based, memory clinics. BMC Prim. Care 2022, 23, 242. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R. The Quick Mild Cognitive Impairment (Qmci) Screen: Developing a New Screening Test for Mild Cognitive Impairment and Dementia. Ph.D. Thesis, University College Cork, Cork, Ireland, 2015. Available online: https://hdl.handle.net/10468/2170 (accessed on 18 February 2025).
- O’Caoimh, R.; Gao, Y.; McGlade, C.; Healy, L.; Gallagher, P.; Timmons, S.; Molloy, D.W. Comparison of the quick mild cognitive impairment (Qmci) screen and the SMMSE in screening for mild cognitive impairment. Age Ageing 2012, 41, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Caviness, J.N.; Driver-Dunckley, E.; Connor, D.J.; Sabbagh, M.N.; Hentz, J.G.; Noble, B.; Evidente, V.G.H.; Shill, H.A.; Adler, C.H. Defining mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2007, 22, 1272–1277. [Google Scholar] [CrossRef]
- Jorm, A.F. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): Development and cross-validation. Psychol. Med. 1994, 24, 145–153. [Google Scholar] [CrossRef]
- Galvin, J.E.; Roe, C.M.; Powlishta, K.K.; Coats, M.A.; Muich, S.J.; Grant, E.; Miller, J.P.; Storandt, M.; Morris, J.C. The AD8: A brief informant interview to detect dementia. Neurology 2005, 65, 559–564. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef]
- Paradise, M.B.; Glozier, N.S.; Naismith, S.L.; Davenport, T.A.; Hickie, I.B. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: A cross-sectional study. BMC Psychiatry 2011, 11, 108. [Google Scholar] [CrossRef]
- Yesavage, J.A. Geriatric Depression Scale. Psychopharmacol. Bull. 1988, 24, 709–711. [Google Scholar] [PubMed]
- Cullen, B.; O’Neill, B.; Evans, J.J.; Coen, R.F.; Lawlor, B.A. A review of screening tests for cognitive impairment. J. Neurol. Neurosurg. Psychiatry 2007, 78, 790–799. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Gallagher, P.F.; Eustace, J.; McGlade, C.; Molloy, D.W. Which part of the Quick mild cognitive impairment screen (Qmci) discriminates between normal cognition, mild cognitive impairment and dementia? Age Ageing 2013, 42, 324–330. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Svendrovski, A.; Johnston, B.C.; Gao, Y.; McGlade, C.; Eustace, J.; Timmons, S.; Guyatt, G.; Molloy, D.W. The Quick Mild Cognitive Impairment screen correlated with the Standardized Alzheimer’s Disease Assessment Scale-cognitive section in clinical trials. J. Clin. Epidemiol. 2014, 67, 87–92. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Gao, Y.; Svendovski, A.; Gallagher, P.; Eustace, J.; Molloy, D.W. Comparing Approaches to Optimize Cut-off Scores for Short Cognitive Screening Instruments in Mild Cognitive Impairment and Dementia. J. Alzheimer’s Dis. 2017, 57, 123–133. [Google Scholar] [CrossRef]
- Machado Dos Santos, P.; O’Caoimh, R.; Svendrovski, A.; Casanovas, C.; Orfila Pernas, F.; Illario, M.; Molloy, W.; Paul, C. The RAPid COmmunity COGnitive screening Programme (RAPCOG): Developing the Portuguese version of the Quick Mild Cognitive Impairment (Qmci-P) screen as part of the EIP on AHA Twinning Scheme. Trans. Med.@ UniSa 2019, 19, 82–89. [Google Scholar]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1998, 44, 837–845. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Glynn, K.; Coen, R.; Lawlor, B.A. Is the Quick Mild Cognitive Impairment Screen (QMCI) more accurate at detecting mild cognitive impairment than existing short cognitive screening tests? A systematic review of the current literature. Int. J. Geriatr. Psychiatry 2019, 34, 1739–1746. [Google Scholar] [CrossRef]
- O’Caoimh, R.; Molloy, D.W.; Clarnette, R. The quick mild cognitive impairment screen and applications to dementia. In Diagnosis and Management in Dementia; Academic Press: Cambridge, MA, USA, 2020; pp. 429–440. [Google Scholar] [CrossRef]
- Tan, J.-P.; Li, N.; Gao, J.; Wang, L.-N.; Zhao, Y.-M.; Yu, B.-C.; Du, W.; Zhang, W.-J.; Cui, L.-Q.; Wang, Q.-S.; et al. Optimal cutoff scores for dementia and mild cognitive impairment of the Montreal Cognitive Assessment among elderly and oldest-old Chinese population. J. Alzheimers Dis. 2015, 43, 1403–1412. [Google Scholar] [CrossRef]
- Patil, M.; Borkar, M.; Rao, S.; Nandedkar, K.U.; Wakure, A.R. Comparative study of 3 tests of cognitive impairment MMSE, AD-8, GPCOG in 200 geriatric cases suspected to have cognitive impairment out of 3750 screened. Int. J. Adv. Med. 2020, 7, 1150. [Google Scholar] [CrossRef]
- Abner, E.L.; Kryscio, R.J.; Caban-Holt, A.M.; Schmitt, F.A. Baseline subjective memory complaints associate with increased risk of incident dementia: The PREADVISE trial. J. Prev. Alzheimer’s Dis. 2015, 2, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Clionsky, M.; Clionsky, E. Dementia Screening: Saying No to the USPSTF and Yes to Brief Cognitive Evaluation. J. Alzheimer’s Dis. Park. 2024, 4, e132. [Google Scholar] [CrossRef]
| Diagnosis | Total (n = 63) | SMC (n = 32) | MCI (n = 16) | Dementia (n = 16) | p = x |
|---|---|---|---|---|---|
| Age in years (Median and IQR) | 73 (81–64 = ±17) | 66 (74–59 = ±15) | 74.5 (81–69 = ±12) | 79.5 (83–75 = ±8) | X2 = 16 (df = 2) <0.001 |
| Education (Median and IQR) | 11 (14–10 = ±4) | 12 (15–11 = ±4) | 11 (14–10 = ±4) | 12 (13–11 = ±2) | X2 = 1.3 (df = 2) 0.522 |
| Sex (% Female) | 67% | 61% | 69% | 75% | X2 = 0.93 (df = 2) 0.693 |
| IQCODE (Median and IQR) | 3.9 (4.3–3.5 = ±0.8) | 3.16 (3.7–3 = ±0.7) | 3.7 (4–3.6 = ±0.4) | 4.3 (4.7–4 = ±0.7) | X2 = 12 (df = 2) 0.002 |
| AD8 (Median and IQR) | 5 (7–4 = ±3) | 2 (5–0 = ±5) | 4 (5–3 = ±2) | 7 (8–6 = ±2) | X2 = 17 (df = 2) <0.001 |
| SMMSE (Median and IQR) | 27 (29–25 = ±4) | 29 (30–28 = ±2) | 26.5 (28–25 = ±3) | 20.5 (24–18 = ±6) | X2 = 36 (df = 2) <0.001 |
| Qmci screen (Median and IQR) | 60 (71–47 = ±24) | 71 (74–59 = ±15) | 54.5 (59–43 = ±16) | 40.5 (48–32 = ±16) | X2 = 40 (df = 2) <0.001 |
| Qmci admin time (Median and IQR) | 4.4 (5–4 = ±1) | 4.0 (4–4 = 0) | 4.4 (5–4 = ±1) | 4.84 (5–4 = ±1) | X2 = 4 (df = 2) 0.144 |
| MoCA (Median and IQR) | 22 (25–17 = ±8) | 25 (27–22 = ±5) | 18 (22–17.5 = ±4.5) | 14.5 (17–11 = ±6) | X2 = 35 (df = 2) <0.001 |
| MoCA admin time (Median and IQR) | 8.2 (10–7.5 = ±2.5) | 7.7 (8–6.5 = ±1.5) | 8.5 (9–8 = ±1) | 10 (10–10 = ±0) | X2 = 6 (df = 2) 0.046 |
| GPCOG (Part 1: Examination) (Median and IQR) | 8 (9–4 = ±5) | 8 (9–8 = ±1) | 7 (9–4 = ±5) | 2.5 (4–1 = ±3) | X2 = 22 (df = 2) <0.001 |
| GPCOG (Part 2: Informant) (Median and IQR) | 4 (6–2 = ±4) | 6 (6–4 = ±2) | 4 (4–3 = ±1) | 2 (3–0 = ±3) | X2 = 32 (df = 2) <0.001 |
| GPCOG admin time (Median and IQR) | 2.5 (3.4–2.4 = ±1) | 2.4 (2.8–2.2 = ±0.6) | 2.5 (3–2.5 = ±0.5) | 3 (3–2.5 = ±0.5) | X2 = 1 (df = 2) 0.468 |
| GPCOG (% Cognitive impairment) | 34% | 3.6% | 36% | 93% | X2 = 33 (df = 2) <0.001 |
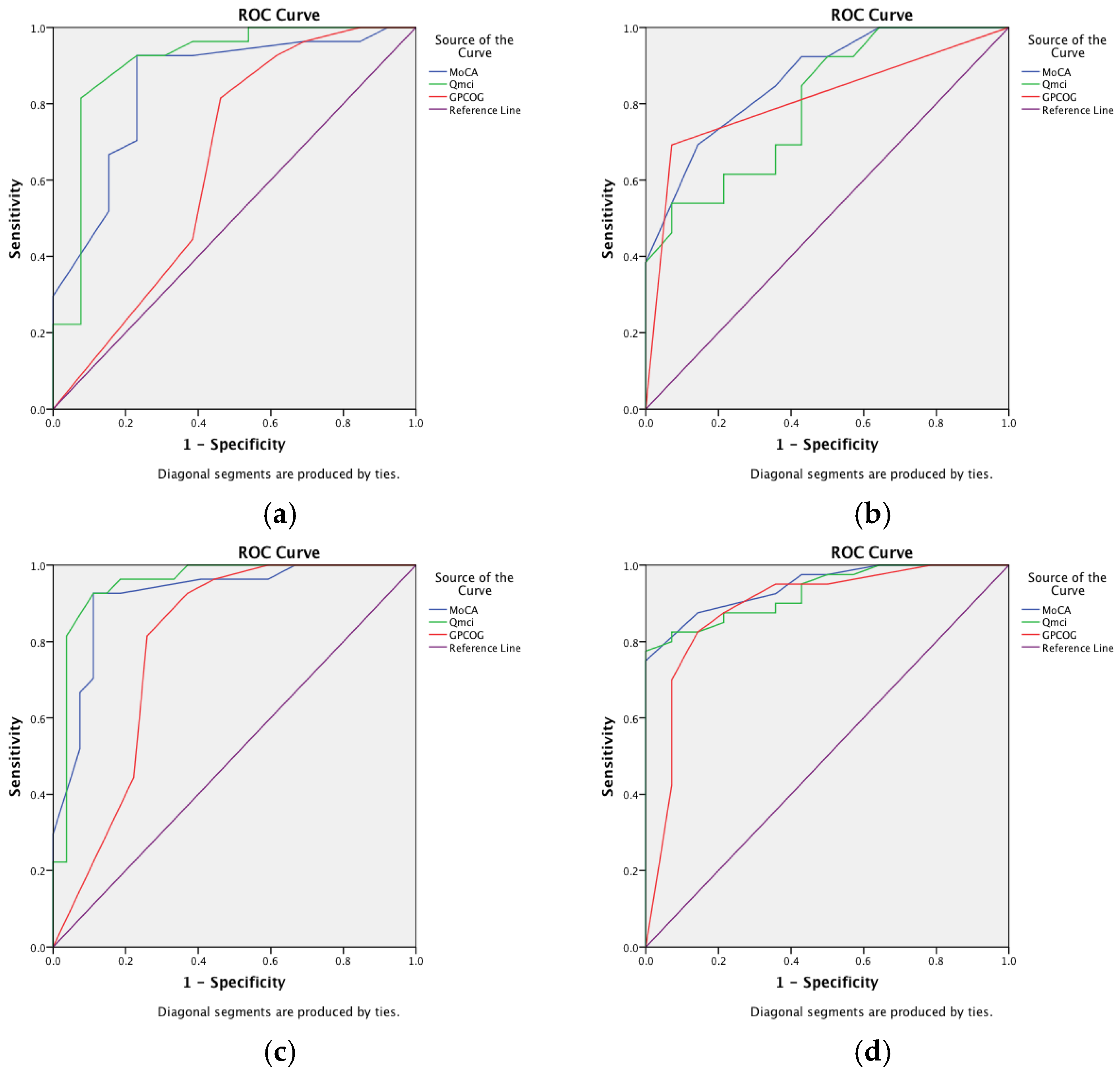
| Corresponding Figure | Comparison * | MoCA | Qmci Screen | GPCOG |
|---|---|---|---|---|
| Area Under the Curve (95% Confidence Intervals) | ||||
| a | MCI versus SMC | 0.84 (0.72–0.98) | 0.91 (0.79–1.0) | 0.67 (0.44–0.85) |
| MoCA vs. Qmci; p = 0.19 MoCA vs. GPCOG; p = 0.02 * Qmci vs. GPCOG; p = 0.01 * | ||||
| b | MCI versus Dementia | 0.87 (0.73–1.0) | 0.80 (0.63–0.97) | 0.81 (0.64–0.99) |
| MoCA vs. Qmci; p = 0.364 MoCA vs. GPCOG; p = 0.397 Qmci vs. GPCOG; p = 0.491 | ||||
| c | Cognitive Impairment (MCI and dementia) versus SMC | 0.91 (0.84–1.0) | 0.95 (0.89–1.0) | 0.82 (0.69–0.94) |
| MoCA vs. Qmci; p = 0.117 MoCA vs. GPCOG; p = 0.02 * Qmci vs. GPCOG; p = 0.008 * | ||||
| d | Dementia versus MCI and SMC | 0.94 (0.89–1.0) | 0.93 (0.86–1.0) | 0. 91 (0.82–1.0) |
| MoCA vs. Qmci; p = 0.910 MoCA vs. GPCOG; p = 0.271 Qmci vs. GPCOG; p = 0.614 | ||||
| Instrument | MoCA | Qmci Screen | GPCOG | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Diagnostic Category | Youden’s Index (Optimal Cut-Off) | Sensitivity | Specificity | Youden’s Index (Optimal Cut-Off) | Sensitivity | Specificity | Youden’s Index (Optimal Cut-Off) | Sensitivity | Specificity |
| MCI versus SMC | <21/30 | 0.73 | 0.87 | <61/100 | 0.94 | 0.81 | <7/9 | 0.57 | 0.82 |
| MCI versus Dementia | <17/30 | 0.81 | 0.73 | <53/100 | 0.86 | 0.56 | <3/9 | 0.64 | 0.86 |
| MCI and Dementia versus SMC | <20/30 | 0.81 | 0.93 | <60/100 | 0.91 | 0.90 | <5/9 | 0.64 | 0.93 |
| Dementia versus SMC and MCI | <18/30 | 0.94 | 0.78 | <55/100 | 0.94 | 0.79 | <5/9 | 0.86 | 0.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Caoimh, R.; Cadoo, S.; Daly, B.; Molloy, D.W. Comparing Short Cognitive Screening Instruments in an Outreach Memory Clinic in Primary Care. Int. J. Environ. Res. Public Health 2025, 22, 410. https://doi.org/10.3390/ijerph22030410
O’Caoimh R, Cadoo S, Daly B, Molloy DW. Comparing Short Cognitive Screening Instruments in an Outreach Memory Clinic in Primary Care. International Journal of Environmental Research and Public Health. 2025; 22(3):410. https://doi.org/10.3390/ijerph22030410
Chicago/Turabian StyleO’Caoimh, Rónán, Sheena Cadoo, Brian Daly, and D. William Molloy. 2025. "Comparing Short Cognitive Screening Instruments in an Outreach Memory Clinic in Primary Care" International Journal of Environmental Research and Public Health 22, no. 3: 410. https://doi.org/10.3390/ijerph22030410
APA StyleO’Caoimh, R., Cadoo, S., Daly, B., & Molloy, D. W. (2025). Comparing Short Cognitive Screening Instruments in an Outreach Memory Clinic in Primary Care. International Journal of Environmental Research and Public Health, 22(3), 410. https://doi.org/10.3390/ijerph22030410






