Association Between Renal Dysfunction and Lipid Ratios in Rural Black South Africans
Abstract
1. Introduction
2. Materials and Methods
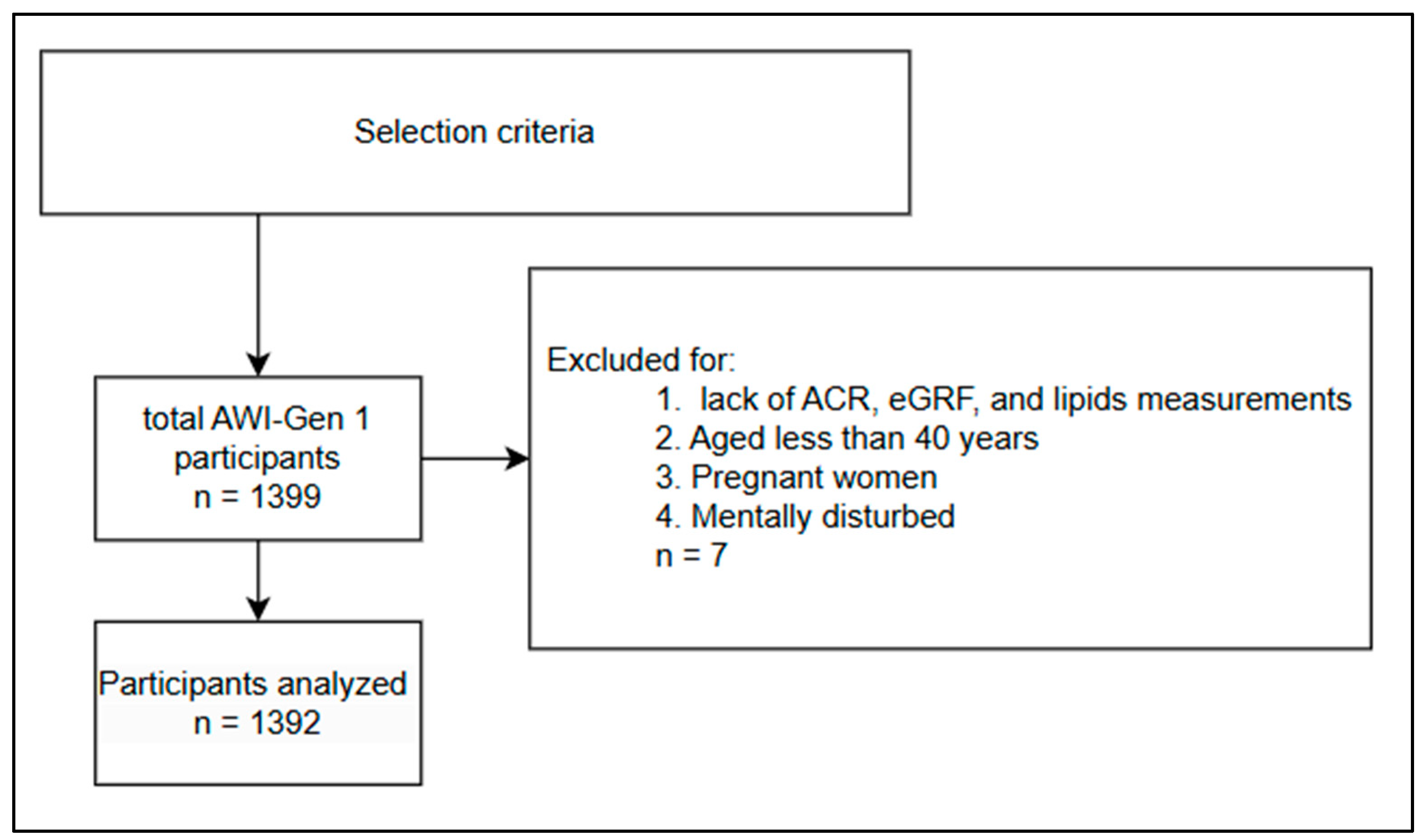
2.1. Study Design
2.2. Selection Criteria
2.3. Measurements
2.4. Serum Lipid Profiles and Lipid-Related Ratios
2.5. Determination of CKD
2.6. Determination of Obesity
2.7. Determination of Hypertension
2.8. Determination of Diabetes
2.9. Data Analysis
2.9.1. Exposure
2.9.2. Outcome
2.9.3. Covariates
2.9.4. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Navise, N.H.; Mokwatsi, G.G.; Gafane-Matemane, L.F.; Fabian, J.; Lammertyn, L. Kidney dysfunction: Prevalence and associated risk factors in a community-based study from the North West Province of South Africa. BMC Nephrol. 2023, 24, 23. [Google Scholar] [CrossRef]
- Liang, Z.; Wang, W.; Yang, C.; Wang, Y.; Shen, J.; Li, P.; Ma, L.; Wei, F.; Chen, R.; Liang, C.; et al. Residential greenness and prevalence of chronic kidney disease: Findings from the China National Survey of Chronic Kidney Disease. Sci. Total Environ. 2022, 806, 150628. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; Mcgaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.-W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–2040 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef] [PubMed]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- George, J.A.; Brandenburg, J.-T.; Fabian, J.; Crowther, N.J.; Agongo, G.; Alberts, M.; Ali, S.; Asiki, G.; Boua, P.R.; Gómez-Olivé, F.X.; et al. Kidney damage and associated risk factors in rural and urban sub-Saharan Africa (AWI-Gen): A cross-sectional population study. Lancet Glob. Health 2019, 7, e1632–e1643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yuan, Z.; Chen, W.; Chen, S.; Liu, X.; Liang, Y.; Shao, X.; Zou, H. Serum Lipid Profiles, Lipid Ratios and Chronic Kidney Disease in a Chinese Population. Int. J. Environ. Res. Public Health 2014, 11, 7622–7635. [Google Scholar] [CrossRef]
- Yang, C.-W.; Harris, D.C.H.; Luyckx, V.A.; Nangaku, M.; Hou, F.F.; Garcia Garcia, G.; Abu-Aisha, H.; Niang, A.; Sola, L.; Bunnag, S.; et al. Global case studies for chronic kidney disease/end-stage kidney disease care. Kidney Int. Suppl. 2020, 10, e24–e48. [Google Scholar] [CrossRef]
- Suriyong, P.; Ruengorn, C.; Shayakul, C.; Anantachoti, P.; Kanjanarat, P. Prevalence of chronic kidney disease stages 3–5 in low- and middle-income countries in Asia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0264393. [Google Scholar] [CrossRef] [PubMed]
- Matsha, T.E.; Erasmus, R.T. Chronic kidney disease in sub-Saharan Africa. Lancet Glob. Health 2019, 7, e1587–e1588. [Google Scholar] [CrossRef]
- Moosa, M.R.; Van der Walt, I.; Naicker, S.; Meyers, A.M. Important causes of chronic kidney disease in South Africa. S. Afr. Med. J. 2015, 105, 320–327. [Google Scholar] [CrossRef]
- Reiger, S.; Jardim, T.V.; Abrahams-Gessel, S.; Crowther, N.J.; Wade, A.; Gomez-Olive, F.X.; Salomon, J.; Tollman, S.; Gaziano, T.A. Awareness, treatment, and control of dyslipidemia in rural South Africa: The HAALSI (Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study. PLoS ONE 2017, 12, e0187347. [Google Scholar] [CrossRef]
- Maimela, E.; Alberts, M.; Modjadji, S.E.P.; Choma, S.S.R.; Dikotope, S.A.; Ntuli, T.S.; Van Geertruyden, J.-P. The Prevalence and Determinants of Chronic Non-Communicable Disease Risk Factors amongst Adults in the Dikgale Health Demographic and Surveillance System (HDSS) Site, Limpopo Province of South Africa. PLoS ONE 2016, 11, e0147926. [Google Scholar] [CrossRef]
- Masilela, C.; Adeniyi, O.V.; Benjeddou, M. Prevalence, patterns and determinants of dyslipidaemia among South African adults with comorbidities. Sci. Rep. 2022, 12, 337. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, J.W.; Jing, B.; Tolan, S.; Helmke, N.; Mukerjee, R.; Naicker, S.; Patel, U. The epidemiology of chronic kidney disease in sub-Saharan Africa: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e174–e181. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Tonelli, M.; Bonventre, J.; Coresh, J.; Donner, J.-A.; Fogo, A.B.; Fox, C.S.; Gansevoort, R.T.; Heerspink, H.J.; Jardine, M. Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 2017, 390, 1888–1917. [Google Scholar] [CrossRef]
- Weldegiorgis, M.; Woodward, M. Elevated triglycerides and reduced high-density lipoprotein cholesterol are independently associated with the onset of advanced chronic kidney disease: A cohort study of 911,360 individuals from the United Kingdom. BMC Nephrol. 2022, 23, 312. [Google Scholar]
- Kim, Y.; Jeong, S.M.; Yoo, B.; Oh, B.; Kang, H.-C. Associations of smoking with overall obesity, and central obesity: A cross-sectional study from the Korea National Health and Nutrition Examination Survey (2010–2013). Epidemiol. Health 2016, 38, e2016020. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Chen, Y.; Huang, Y.; Lu, Y.; Liu, X.; Zhou, H.; Yuan, H. Association of the TG/HDL-C and Non-HDL-C/HDL-C Ratios with Chronic Kidney Disease in an Adult Chinese Population. Kidney Blood Press. Res. 2017, 42, 1141–1154. [Google Scholar] [CrossRef]
- Mihaylova, B.; Emberson, J.; Blackwell, L.; Keech, A.; Simes, J.; Barnes, E.H.; Voysey, M.; Gray, A.; Collins, R.; Baigent, C. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: Meta-analysis of individual data from 27 randomised trials. Lancet Lond. Engl. 2012, 380, 581–590. [Google Scholar]
- Rahman, M.; Yang, W.; Akkina, S.; Alper, A.; Anderson, A.H.; Appel, L.J.; He, J.; Raj, D.S.; Schelling, J.; Strauss, L. Relation of serum lipids and lipoproteins with progression of CKD: The CRIC study. Clin. J. Am. Soc. Nephrol. 2014, 9, 1190–1198. [Google Scholar] [CrossRef] [PubMed]
- Chawla, V.; Greene, T.; Beck, G.J.; Kusek, J.W.; Collins, A.J.; Sarnak, M.J.; Menon, V. Hyperlipidemia and long-term outcomes in nondiabetic chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1582–1587. [Google Scholar] [CrossRef] [PubMed]
- Sparling, P.B.; Noakes, T.D.; Steyn, K.; Jordaan, E.; Jooste, P.L.; Bourne, L.T.; Badenhorst, C. Level of physical activity and CHD risk factors in black South African men. Med. Sci. Sports Exerc. 1994, 26, 896–902. [Google Scholar] [CrossRef]
- Parsa, A.; Kao, W.H.L.; Xie, D.; Astor, B.C.; Li, M.; Hsu, C.; Feldman, H.I.; Parekh, R.S.; Kusek, J.W.; Greene, T.H.; et al. APOL1 Risk Variants, Race, and Progression of Chronic Kidney Disease. N. Engl. J. Med. 2013, 369, 2183–2196. [Google Scholar] [CrossRef]
- Lipworth, L.; Mumma, M.T.; Cavanaugh, K.L.; Edwards, T.L.; Ikizler, T.A.; E.Tarone, R.; McLaughlin, J.K.; Blot, W.J. Incidence and Predictors of End Stage Renal Disease among Low-Income Blacks and Whites. PLoS ONE 2012, 7, e48407. [Google Scholar] [CrossRef]
- Grams, M.E.; Chow, E.K.H.; Segev, D.L.; Coresh, J. Lifetime Incidence of CKD Stages 3-5 in the United States. Am. J. Kidney Dis. 2013, 62, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Peralta, C.A.; Katz, R.; DeBoer, I.; Ix, J.; Sarnak, M.; Kramer, H.; Siscovick, D.; Shea, S.; Szklo, M.; Shlipak, M. Racial and Ethnic Differences in Kidney Function Decline among Persons without Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 1327. [Google Scholar] [CrossRef] [PubMed]
- Emerson, R.W. Convenience Sampling, Random Sampling, and Snowball Sampling: How Does Sampling Affect the Validity of Research? J. Vis. Impair. Blind. 2015, 109, 164–168. [Google Scholar] [CrossRef]
- Ali, S.A.; Soo, C.; Agongo, G.; Alberts, M.; Amenga-Etego, L.; Boua, R.P.; Choudhury, A.; Crowther, N.J.; Depuur, C.; Gómez-Olivé, F.X.; et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: Methods used for Phase 1 of the AWI-Gen population cross-sectional study. Glob. Health Action 2018, 11, 1507133. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Giugliano, R.P.; Murphy, S.A.; Wasserman, S.M.; Stein, E.A.; Češka, R.; López-Miranda, J.; Georgiev, B.; Lorenzatti, A.J.; Tikkanen, M.J. Comparison of low-density lipoprotein cholesterol assessment by Martin/Hopkins estimation, Friedewald estimation, and preparative ultracentrifugation: Insights from the FOURIER trial. JAMA Cardiol. 2018, 3, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Klug, E.; Raal, F.J.; Marais, A.D.; Smuts, C.M.; Schamroth, C.; Jankelow, D.; Blom, D.J.; Webb, D.A. South African dyslipidaemia guideline consensus statement: 2018 update A joint statement from the South African Heart Association (SA Heart) and the Lipid and Atherosclerosis Society of Southern Africa (LASSA). S. Afr. Med. J. 2018, 108, 973–1000. [Google Scholar] [CrossRef] [PubMed]
- Magwai, T.; Modjadji, P.; Choma, S. Association of microalbuminuria with serum lipids and inflammatory markers in an adult population in the Dikgale Health and Demographic Surveillance System site, South Africa. Cardiovasc. J. Afr. 2022, 33, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Delanaye, P.; Schaeffner, E.; Cozzolino, M.; Langlois, M.; Plebani, M.; Ozben, T.; Cavalier, E. The new, race-free, Chronic Kidney Disease Epidemiology Consortium (CKD-EPI) equation to estimate glomerular filtration rate: Is it applicable in Europe? A position statement by the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM). Clin. Chem. Lab. Med. CCLM 2023, 61, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Xie, Q.; Wang, H.; Wu, F.; He, D.; Huang, Y.; He, Y.; Dai, S.; Chen, J.; Zhang, Y. Biological variation of estimated glomerular filtrations rate in apparently healthy individuals within 24 h calculated using new CKD-EPI equations. Ir. J. Med. Sci. 2024, 193, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Salehipour Bavarsad, S.; Jalali, M.T.; Shahbazian, H.; Saadati, S.M.; Hesam, S.; Mohammadtaghvaie, N. Performance of Creatinine and Cystatin C-Based Equations to Estimate Kidney Function in Renal Transplant Recipients. Nephro-Urol. Mon. 2023, 15, e129099. [Google Scholar] [CrossRef]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic kidney disease diagnosis and management: A review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Mashinya, F.; Alberts, M.; Cook, I.; Ntuli, S. Determinants of body mass index by gender in the Dikgale Health and Demographic Surveillance System site, South Africa. Glob. Health Action 2018, 11, 1537613. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, J. Overweight and Obesity: A Brief Challenge on Prevalence, Complications and Physical Activity among Men and Women. Womens Health 2018, 7, 00161. [Google Scholar] [CrossRef][Green Version]
- Ahmad, N.; Adam, S.M.; Nawi, A.; Hassan, M.; Ghazi, H. Abdominal obesity indicators: Waist circumference or waist-to-hip ratio in Malaysian adults population. Int. J. Prev. Med. 2016, 7, 82. [Google Scholar] [CrossRef]
- Ntimana, C.B.; Choma, S.S.R. Modifiable determinants of central obesity among the rural black population in the DIMAMO HDSS, Limpopo, South Africa. Front. Public Health 2023, 11, 1165662. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.P.; Awasthi, A.; Kondal, D.; Kapoor, M.; Roy, A.; Prabhakaran, D. Impact of repeated blood pressure measurement on blood pressure categorization in a population-based study from India. J. Hum. Hypertens. 2019, 33, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Oh, A.R.; Lee, S.; Lee, J.; Min, J.J.; Kwon, J.; Kim, J.; Yang, K.; Choi, J.; Lee, S.; et al. Associations Between Preoperative Glucose and Hemoglobin A1c Level and Myocardial Injury After Noncardiac Surgery. J. Am. Heart Assoc. 2021, 10, 019216. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Matsha, T.E.; Yako, Y.Y.; Rensburg, M.A.; Hassan, M.S.; Kengne, A.P.; Erasmus, R.T. Chronic kidney diseases in mixed ancestry south African populations: Prevalence, determinants and concordance between kidney function estimators. BMC Nephrol. 2013, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, C.M. Kidney disease and HIV infection. Top. Antivir. Med. 2017, 25, 13. [Google Scholar]
- Naicker, S.; Rahmania, S.; Kopp, J.B. HIV and chronic kidney disease. Clin. Nephrol. 2015, 83, S32. [Google Scholar] [CrossRef]
- ben Khadda, Z.; Bungau, S.G.; El Balkhi, S.; Ezrari, S.; Radu, A.-F.; Houssaini, T.S.; Achour, S. Urinary biomonitoring of exposure to glyphosate and its metabolite amino-methyl phosphonic acid among farmers and non-farmers in Morocco. Environ. Toxicol. Pharmacol. 2025, 113, 104620. [Google Scholar] [CrossRef] [PubMed]
- Bouya, S.; Balouchi, A.; Rafiemanesh, H.; Hesaraki, M. Prevalence of chronic kidney disease in Iranian general population: A meta-analysis and systematic review. Ther. Apher. Dial. 2018, 22, 594–599. [Google Scholar] [CrossRef]
- Conte, C.; Antonelli, G.; Melica, M.E.; Tarocchi, M.; Romagnani, P.; Peired, A.J. Role of sex hormones in prevalent kidney diseases. Int. J. Mol. Sci. 2023, 24, 8244. [Google Scholar] [CrossRef]
- Cobo, G.; Hecking, M.; Port, F.K.; Exner, I.; Lindholm, B.; Stenvinkel, P.; Carrero, J.J. Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin. Sci. Lond. Engl. 1979 2016, 130, 1147–1163. [Google Scholar] [CrossRef] [PubMed]
- DeFronzo, R.A.; Reeves, W.B.; Awad, A.S. Pathophysiology of diabetic kidney disease: Impact of SGLT2 inhibitors. Nat. Rev. Nephrol. 2021, 17, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.M.; Chou, J.; Ahmadi, S.F.; Park, J.; Chen, J.L.; Amin, A.N. The obesity paradox in kidney disease: How to reconcile it with obesity management. Kidney Int. Rep. 2017, 2, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Cai, A.; Mo, Y.; Zhang, Y.; Li, J.; Chen, J.; Zhou, Y.; Chen, R.; Wei, R.; Huang, Y.; Tang, S.; et al. Relationship of pulse pressure index and carotid intima-media thickness in hypertensive adults. Clin. Exp. Hypertens. 2015, 37, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Alberts, M.; Dikotope, S.A.; Choma, S.R.; Masemola, M.L.; Modjadji, S.E.; Mashinya, F.; Burger, S.; Cook, I.; Brits, S.J.; Byass, P. Health & Demographic Surveillance System Profile: The Dikgale Health and Demographic Surveillance System. Int. J. Epidemiol. 2015, 44, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.R.; Mabhida, S.E.; Myers, B.; Apalata, T.; Nicol, E.; Benjeddou, M.; Muller, C.; Johnson, R. Prevalence of hypertension and its associated risk factors in a rural black population of Mthatha town, South Africa. Int. J. Environ. Res. Public Health 2021, 18, 1215. [Google Scholar] [CrossRef] [PubMed]
- Ringane, M.C.; Choma, S.S.R. The optimal WC cut-off points for the prediction of subclinical CVD as measured by carotid intima-media thickness among African adults: A cross-sectional study. BMC Cardiovasc. Disord. 2021, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mhundwa, W.; Joubert, G.; Mofokeng, T.R. The prevalence of chronic kidney disease among type 2 diabetes mellitus patients in central South Africa. S. Afr. Fam. Pract. 2023, 65, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M.; Ng, S.W. The nutrition transition to a stage of high obesity and noncommunicable disease prevalence dominated by ultra-processed foods is not inevitable. Obes. Rev. 2022, 23, 13366. [Google Scholar] [CrossRef] [PubMed]
- Kolovou, G.D.; Anagnostopoulou, K.K.; Damaskos, D.S.; Bilianou, H.I.; Mihas, C.; Milionis, H.J.; Kostakou, P.M.; Cokkinos, D.V. Gender differences in the lipid profile of dyslipidemic subjects. Eur. J. Intern. Med. 2009, 20, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, M.; Chrysohoou, C.; Georgousopoulou, E.; Damigou, E.; Skoumas, I.; Pitsavos, C.; Panagiotakos, D. Long-term prognostic value of LDL-C, HDL-C, lp(a) and TG levels on cardiovascular disease incidence, by body weight status, dietary habits and lipid-lowering treatment: The ATTICA epidemiological cohort study (2002–2012). Lipids Health Dis. 2022, 21, 141. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Du, Z.; Wei, H.; Dong, J. Total cholesterol to high-density lipoprotein cholesterol ratio is independently associated with CKD progression. Int. Urol. Nephrol. 2022, 54, 2057–2063. [Google Scholar] [CrossRef] [PubMed]
- Casari, S.; Di Paola, M.; Banci, E.; Diallo, S.; Scarallo, L.; Renzo, S.; Gori, A.; Renzi, S.; Paci, M.; De Mast, Q.; et al. Changing Dietary Habits: The Impact of Urbanization and Rising Socio-Economic Status in Families from Burkina Faso in Sub-Saharan Africa. Nutrients 2022, 14, 1782. [Google Scholar] [CrossRef] [PubMed]
- Adeniyi, A.B.; Laurence, C.E.; Volmink, J.A.; Davids, M.R. Prevalence of chronic kidney disease and association with cardiovascular risk factors among teachers in Cape Town, South Africa. Clin. Kidney J. 2017, 10, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Kosmas, C.E.; Rodriguez Polanco, S.; Bousvarou, M.D.; Papakonstantinou, E.J.; Peña Genao, E.; Guzman, E.; Kostara, C.E. The triglyceride/high-density lipoprotein cholesterol (TG/HDL-C) ratio as a risk marker for metabolic syndrome and cardiovascular disease. Diagnostics 2023, 13, 929. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Tani, S.; Matsuo, R.; Matsumoto, N. Increased triglyceride/high-density lipoprotein cholesterol ratio may be associated with reduction in the low-density lipoprotein particle size: Assessment of atherosclerotic cardiovascular disease risk. Heart Vessels 2019, 34, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-T.; Gao, Y.; Zheng, Y.-Y.; Ma, Y.-T.; Xie, X. Atherogenic index of plasma (AIP): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 1–7. [Google Scholar] [CrossRef]
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Variables | Non-Kidney Dysfunction (N = 391) | Kidney Dysfunction (N = 36) | p-Value | Non-Kidney Dysfunction (N = 838) | Kidney Dysfunction (N = 127) | p-Value |
| Age (mean ± SD) | 51.50 ± 8.03 | 56.75 ± 9.68 | 0.003 | 51.78 ± 8.13 | 54.4 ± 8.64 | 0.001 |
| Age (≤45 yrs) n (%) | 104 (26.6) | 6 (16.7) | 0.008 | 208 (24.8) | 19 (15.0) | 0.020 |
| Age (46–55 yrs) n (%) | 169 (43.2) | 10 (27.8) | 352 (42.0) | 53 (41.7) | ||
| Age (≥56 yrs) n (%) | 118 (30.2) | 20 (55.6) | 278 (33.2) | 55 (43.3) | ||
| BMI (kg/m2) | 21.57 ± 3.92 | 23.31 ± 5.02 | 0.051 | 30.55 ± 7.98 | 30.3 ± 7.26 | 0.740 |
| Obesity n (%) | 11 (2.8) | 3 (8.3) | 0.105 | 420 (50.1) | 58 (45.7) | 0.392 |
| WC (cm) | 80.10 ± 11.29 | 86.4 ± 13.55 | 0.010 | 93.58 ± 15.91 | 95.62 ± 17.06 | 0.205 |
| Central obesity n (%) | 50 (12.8) | 10 (27.8) | 0.022 | 653 (78.0) | 102 (80.3) | 0.644 |
| SBP (mmHg) | 124.76 ± 20.36 | 140.24 ± 27.74 | 0.002 | 125.3 ± 20.63 | 132.2 ± 26.03 | 0.005 |
| DBP (mmHg) | 78.09 ± 12.55 | 84.90 ± 13.86 | 0.007 | 81.14 ± 12.43 | 85.14 ± 15.21 | 0.004 |
| Hypertension n (%) | 84 (21.5) | 18 (50.0) | <0.001 | 246 (29.4) | 51 (40.2) | 0.017 |
| Current smoker n (%) | 247 (63.2) | 10 (27.8) | <0.001 | 27 (3.2) | 4 (3.1) | 1.000 |
| Current alcohol consumption n (%) | 237 (60.8) | 19 (52.8) | 0.377 | 114 (13.6) | 16 (12.6) | 0.889 |
| Glucose (mmol/L) | 4.91 ± 1.56 | 6.51 ± 4.78 | 0.053 | 5.22 ± 2.22 | 6.07 ± 3.57 | 0.014 |
| Diabetes mellitus n (%) | 18 (4.7) | 6 (16.7) | 0.011 | 18 (15.1) | 52 (6.3) | 0.002 |
| TC (mmol/L) | 3.95 ± 1.03 | 3.95 ± 1.04 | 0.994 | 4.19 ± 1.09 | 4.49 ± 1.68 | 0.050 |
| TG (mmol/L) | 0.95 (1.34–0.682) | 0.99 (1.29–0.73) | 0.785 | 0.95 (1.32–0.71) | 1.15 (1.51–0.78) | 0.003 |
| LDL-C (mmol/L) | 2.36 ± 1.03 | 2.27 ± 0.95 | 0.614 | 2.64 ± 1.02 | 2.59 ± 1.01 | 0.549 |
| HDL-C (mmol/L) | 1.26 ± 0.47 | 1.20 ± 0.57 | 0.524 | 1.19 ± 0.36 | 1.13 ± 0.34 | 0.117 |
| TG/HDL-C | 0.83 (1.27–0.53) | 0.73 (1.38–0.58) | 0.725 | 0.84 (1.28–0.56) | 1.03 (1.45–0.78) | <0.001 |
| TC/HDL-C | 3.51 ± 1.68 | 3.72 ± 1.52 | 0.430 | 3.74 ± 1.16 | 4.14 ± 1.41 | 0.003 |
| LDL-C/HDL-C | 2.22 ± 2.7 | 2.16 ± 1.06 | 0.808 | 2.39 ± 1.03 | 2.47 ± 1.11 | 0.494 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| eGFR | ACR | eGFR | ACR | |||||
| Variables | Correlation | p-Value | Correlation | p-Value | Correlation | p-Value | Correlation | p-Value |
| HDL-C (mmol/L) | −0.054 | 0.266 | −0.085 | 0.265 | 0.006 | 0.849 | −0.044 | 0.399 |
| LDL-C (mmol/L) | −0.044 | 0.370 | 0.006 | 0.940 | −0.022 | 0.515 | −0.045 | 0.398 |
| TC (mmol/L) | 0.063 | 0.196 | −0.020 | 0.798 | 0.142 | <0.001 | 0.054 | 0.296 |
| TG (mmol/L) | 0.054 | 0.267 | 0.024 | 0.754 | 0.108 | 0.001 | 0.032 | 0.534 |
| LDL/HDL-C | −0.008 | 0.868 | 0.085 | 0.272 | −0.031 | 0.353 | 0.010 | 0.846 |
| TC/HDL-C | 0.089 | 0.065 | 0.051 | 0.503 | 0.075 | 0.020 | 0.092 | 0.074 |
| TG/HDL-C | 0.081 | 0.097 | 0.053 | 0.488 | 0.069 | 0.032 | 0.071 | 0.169 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| eGFR | ACR | eGFR | ACR | |||||
| Variables | Correlation | p-Value | Correlation | p-Value | Correlation | p-Value | Correlation | p-Value |
| HDL-C (mmol/L) | −0.013 | 0.801 | −0.085 | 0.265 | −0.028 | 0.394 | −0.080 | 0.140 |
| LDL-C (mmol/L) | −0.049 | 0.325 | 0.006 | 0.940 | −0.049 | 0.139 | −0.043 | 0.429 |
| TC (mmol/L) | 0.049 | 0.322 | −0.020 | 0.798 | 0.067 | 0.046 | −0.026 | 0.633 |
| TG (mmol/L) | 0.048 | 0.333 | 0.024 | 0.754 | 0.052 | 0.122 | 0.042 | 0.436 |
| LDL/HDL-C | −0.010 | 0.840 | 0.085 | 0.272 | −0.045 | 0.180 | 0.024 | 0.656 |
| TC/HDL-C | 0.069 | 0.165 | 0.051 | 0.503 | 0.048 | 0.148 | 0.080 | 0.139 |
| TG/HDL-C | 0.105 | 0.035 | 0.053 | 0.488 | 0.033 | 0.319 | 0.087 | 0.168 |
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| eGFR | ACR | eGFR | ACR | |||||
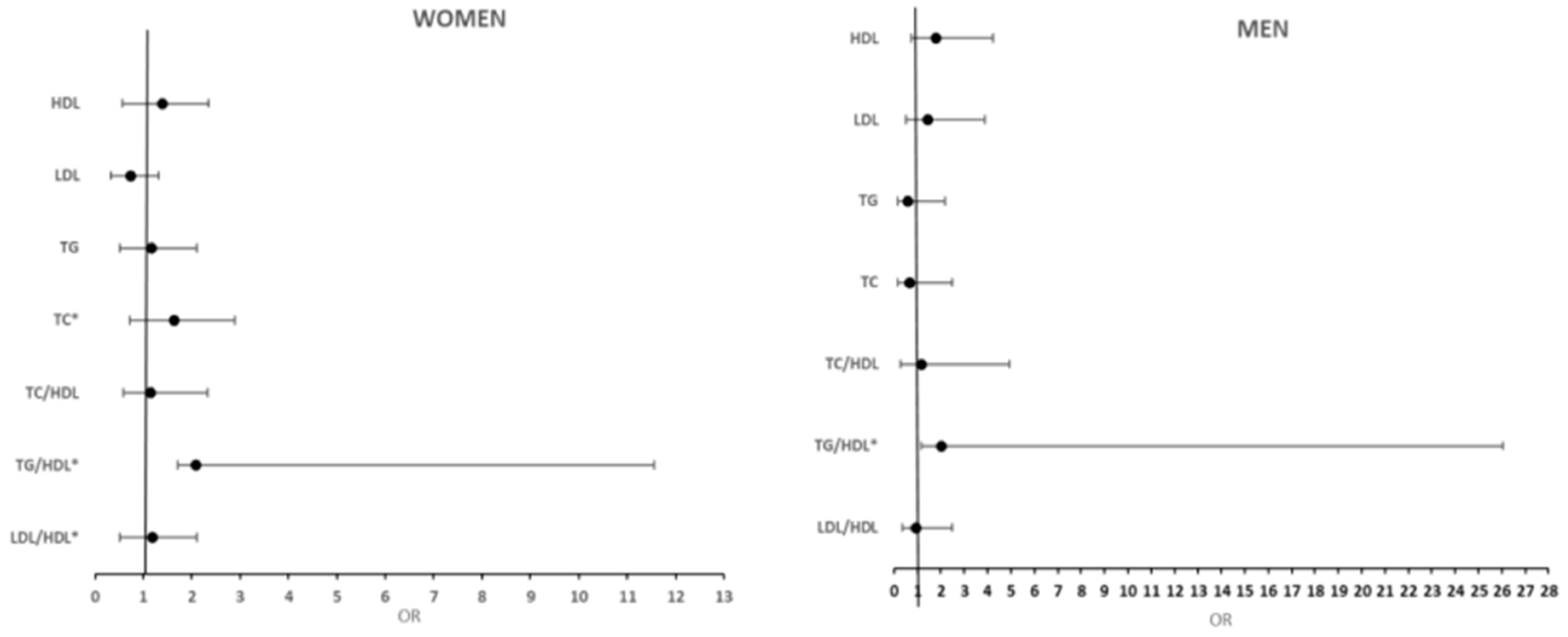
| Variables | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value | OR (95%CI) | p-Value |
| TC | 1.47 (0.83;2.69) | 0.190 | 0.95 (0.66;1.38) | 0.79 | 1.55 (1.23;1.94) | <0.001 | 1.10 (0.92;1.32) | 0.30 |
| HDL | 2.41 (0.58;10.0) | 0.23 | 2.79 (1.19;6.53) | 0.018 | 1.03 (0.50;2.11) | 0.94 | 1.31 (0.70;2.45) | 0.39 |
| LDL | 0.44 (0.52;3.67) | 0.45 | 2.05 (0.83;5.06) | 0.12 | 0.66 (0.33;1.29) | 0.23 | 0.98 (0.59;1.65) | 0.95 |
| TG | 1.00 (0.12;8.67) | 0.99 | 0.60 (0.15;2.44) | 0.48 | 2.75 (1.32;3.75) | 0.007 | 0.98 (0.49;1.99) | 0.98 |
| TC/HDL-C | 1.20 (0.97;1.49) | 0.86 | 1.06 (0.89;1.26) | 0.50 | 1.26 (1.03;1.53) | 0.021 | 1.17 (0.98;1.40) | 0.08 |
| TG/HDL-C | 1.22 (0.94;1.59) | 0.141 | 1.10 (0.83;1.46) | 0.49 | 1.41 (1.03;1.94) | 0.035 | 1.24 (0.91;1.69) | 0.17 |
| LDL/HDL-C | 0.96 (0.57;1.61) | 0.87 | 1.21 (0.86;1.72) | 0.27 | 0.86 (0.64;1.18) | 0.35 | 1.02 (0.82;1.27) | 0.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntimana, C.B.; Mashaba, R.G.; Seakamela, K.P.; Mphekgwana, P.M.; Nemuramba, R.; Mothapo, K.; Tlouyamma, J.; Choma, S.S.R.; Maimela, E. Association Between Renal Dysfunction and Lipid Ratios in Rural Black South Africans. Int. J. Environ. Res. Public Health 2025, 22, 324. https://doi.org/10.3390/ijerph22030324
Ntimana CB, Mashaba RG, Seakamela KP, Mphekgwana PM, Nemuramba R, Mothapo K, Tlouyamma J, Choma SSR, Maimela E. Association Between Renal Dysfunction and Lipid Ratios in Rural Black South Africans. International Journal of Environmental Research and Public Health. 2025; 22(3):324. https://doi.org/10.3390/ijerph22030324
Chicago/Turabian StyleNtimana, Cairo B., Reneilwe G. Mashaba, Kagiso P. Seakamela, Peter M. Mphekgwana, Rathani Nemuramba, Katlego Mothapo, Joseph Tlouyamma, Solomon S. R. Choma, and Eric Maimela. 2025. "Association Between Renal Dysfunction and Lipid Ratios in Rural Black South Africans" International Journal of Environmental Research and Public Health 22, no. 3: 324. https://doi.org/10.3390/ijerph22030324
APA StyleNtimana, C. B., Mashaba, R. G., Seakamela, K. P., Mphekgwana, P. M., Nemuramba, R., Mothapo, K., Tlouyamma, J., Choma, S. S. R., & Maimela, E. (2025). Association Between Renal Dysfunction and Lipid Ratios in Rural Black South Africans. International Journal of Environmental Research and Public Health, 22(3), 324. https://doi.org/10.3390/ijerph22030324






