IgE and Eosinophilia in Newly Arrived Refugees in Denmark: A Cross-Sectional Study of Prevalence and Clinical Management in Primary Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
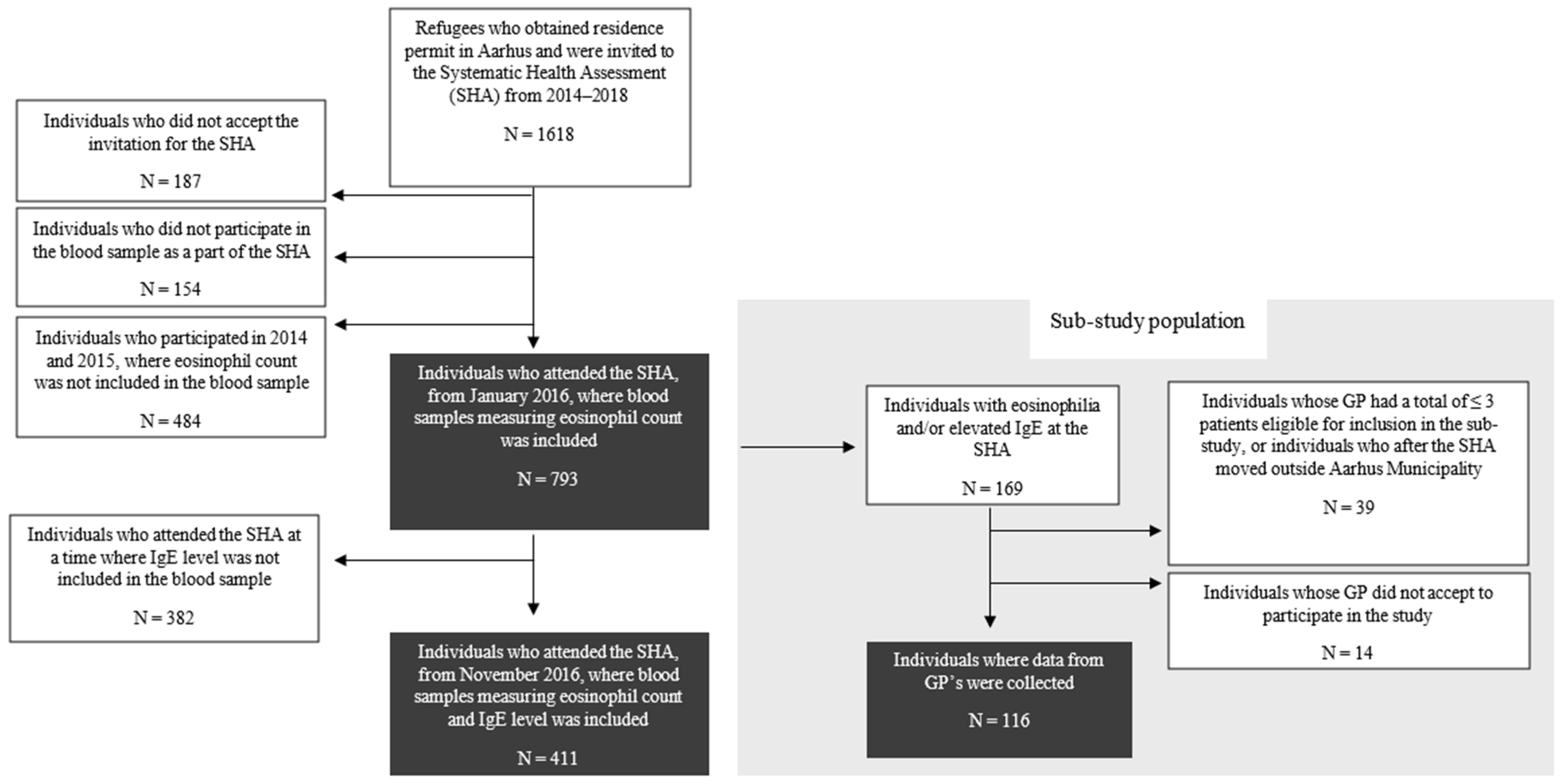
2.2. Study Population
2.3. Data Collection
2.4. Processing of Laboratory Samples
2.5. Analyses and Statistics
2.6. Ethics
3. Results
3.1. Eosinophilia and Elevated IgE
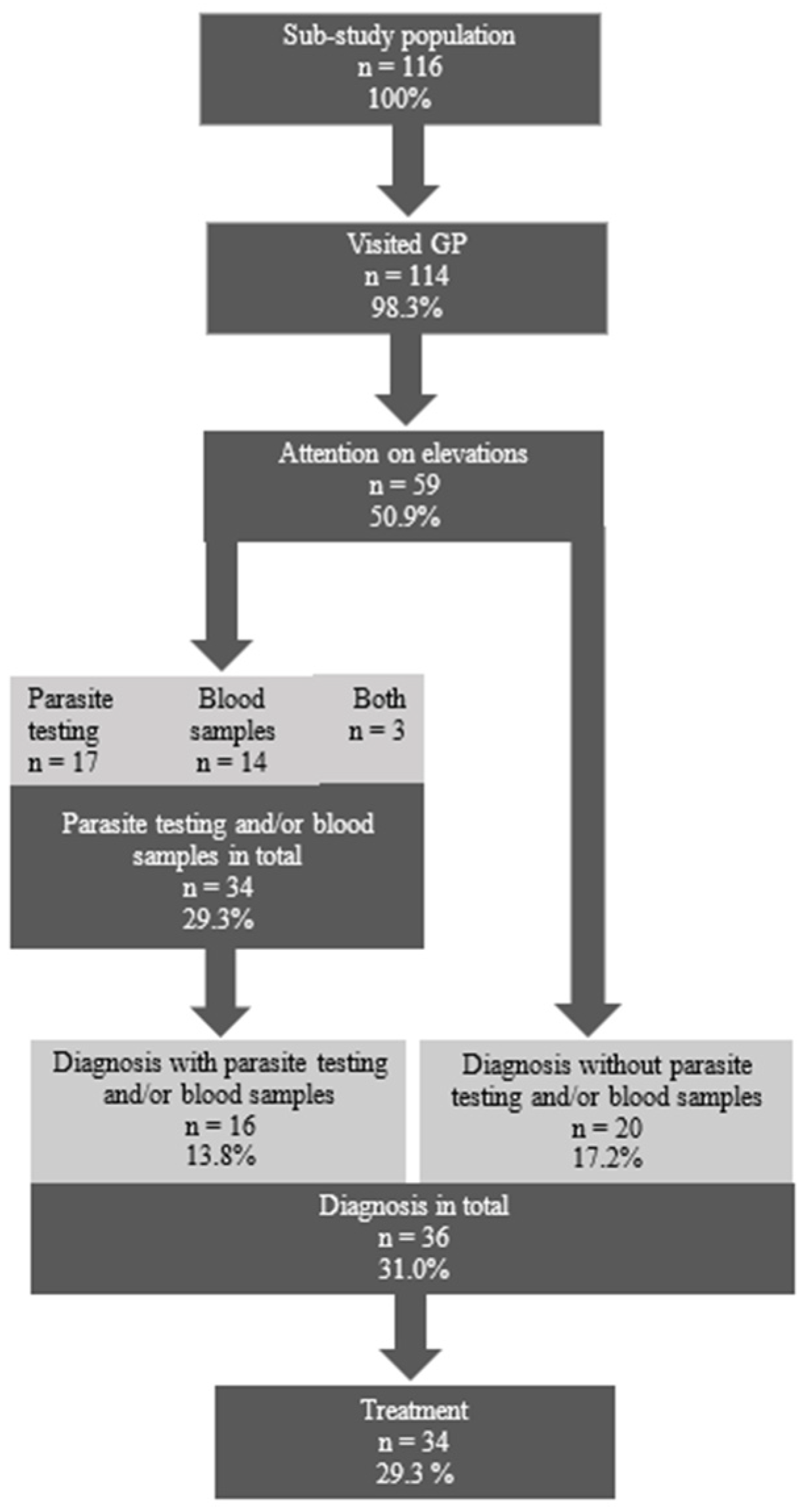
3.2. Clinical Management in Primary Care Settings
4. Discussion
4.1. Prevalence of Eosinophilia and Elevated IgE
4.2. Clinical Management of Eosinophilia and Elevated IgE
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medecins Sans Frontières: Obstacle Course to Europe (MSF Report 2016). Available online: https://www.msf.org/sites/default/files/msf_obstacle_course_to_europe_0.pdf (accessed on 23 January 2025).
- Pavli, A.; Maltezou, H. Health problems of newly arrived migrants and refugees in Europe. J. Travel Med. 2017, 24, tax016. [Google Scholar] [CrossRef]
- Barnett, E.D.; Weld, L.H.; McCarthy, A.E.; So, H.; Walker, P.F.; Stauffer, W.; Cetron, M. Spectrum of illness in international migrants seen at GeoSentinel clinics in 1997–2009, part 1: US-bound migrants evaluated by comprehensive protocol-based health assessment. Clin. Infect. Dis. 2013, 56, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulou, I.D.; Tanaka, M.; Dikalioti, S.; Samoli, E.; Nisianakis, P.; Boleti, O.D.; Tsoumakas, K. Clinical and laboratory evaluation of new immigrant and refugee children arriving in Greece. BMC Pediatr. 2017, 17, 132. [Google Scholar] [CrossRef]
- Belhassen-García, M.; Pardo-Lledías, J.; Pérez Del Villar, L.; Muro, A.; Velasco-Tirado, V.; Blázquez de Castro, A.; Vicente, B.; García García, M.I.; Luis Muñoz Bellido, J.; Cordero-Sánchez, M. Relevance of eosinophilia and hyper-IgE in immigrant children. Medicine 2014, 93, e43. [Google Scholar] [CrossRef] [PubMed]
- Pardo, J.; Carranza, C.; Muro, A.; Angel-Moreno, A.; Martín, A.M.; Martín, T.; Hernández-Cabrera, M.; Pérez-Arellano, J.L. Helminth-related Eosinophilia in African immigrants, Gran Canaria. Emerg. Infect. Dis. 2006, 12, 1587–1589. [Google Scholar] [CrossRef] [PubMed]
- Salzer, H.J.F.; Rolling, T.; Vinnemeier, C.D.; Tannich, E.; Schmiedel, S.; Addo, M.M.; Cramer, J.P. Helminthic infections in returning travelers and migrants with eosinophilia: Diagnostic value of medical history, eosinophil count and IgE. Travel Med. Infect. Dis. 2017, 20, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Whetham, J.; Day, J.N.; Armstrong, M.; Chiodini, P.L.; Whitty, C.J. Investigation of tropical eosinophilia; assessing a strategy based on geographical area. J. Infect. 2003, 46, 180–185. [Google Scholar] [CrossRef]
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Requena-Méndez, A.; Chiodini, P.; Bisoffi, Z.; Buonfrate, D.; Gotuzzo, E.; Muñoz, J. The laboratory diagnosis and follow up of strongyloidiasis: A systematic review. PLoS Negl. Trop. Dis. 2013, 7, e2002. [Google Scholar] [CrossRef] [PubMed]
- Greaves, D.; Coggle, S.; Pollard, C.; Aliyu, S.H.; Moore, E.M. Strongyloides stercoralis infection. BMJ 2013, 347, f4610. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Alvarado, M.; Basáñez, M.G.; Bolliger, I.; Bourne, R.; Boussinesq, M.; Brooker, S.J.; Brown, A.S.; Buckle, G.; Budke, C.M.; et al. The global burden of disease study 2010: Interpretation and implications for the neglected tropical diseases. PLoS Negl. Trop. Dis. 2014, 8, e2865. [Google Scholar] [CrossRef] [PubMed]
- Deniaud, F.; Rouessé, C.; Collignon, A.; Domingo, A.; Rigal, L. Failure to offer parasitology screening to vulnerable migrants in France: Epidemiology and consequences. Sante 2010, 20, 201–208. [Google Scholar]
- Buonfrate, D.; Requena-Mendez, A.; Angheben, A.; Muñoz, J.; Gobbi, F.; Van Den Ende, J.; Bisoffi, Z. Severe strongyloidiasis: A systematic review of case reports. BMC Infect. Dis. 2013, 13, 78. [Google Scholar] [CrossRef]
- Diaz, E.; Calderón-Larrañaga, A.; Prado-Torres, A.; Poblador-Plou, B.; Gimeno-Feliu, L.A. How do immigrants use primary health care services? A register-based study in Norway. Eur. J. Public Health 2015, 25, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Szczepura, A. Access to health care for ethnic minority populations. Postgrad. Med. J. 2005, 81, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Hvass, A.M.F.; Nyboe, L.; Lanng, K.; Nielsen, C.V.; Wejse, C. A Mental Health Profile of 900 Newly Arrived Refugees in Denmark Using ICD-10 Diagnoses. Sustainability 2022, 14, 418. [Google Scholar] [CrossRef]
- Brandenberger, J.; Tylleskär, T.; Sontag, K.; Peterhans, B.; Ritz, N. A systematic literature review of reported challenges in health care delivery to migrants and refugees in high-income countries—The 3C model. BMC Public Health 2019, 19, 755. [Google Scholar] [CrossRef] [PubMed]
- Storgaard, S.F.; Wejse, C.; Agergaard, J.; Eiset, A.H. Is being a refugee associated with increased 30-day mortality after visiting the emergency department? A register-based cohort study using Danish data. Scand. J. Public Health 2023, 52, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Hvass, A.M.F.; Norredam, M.; Sodemann, M.; Wejse, C. Is there a need of health assessments for resettling refugees? A cross-sectional study of 1431 refugees who arrived in Denmark between 2014 and 2018. J. Migr. Health 2021, 3, 100044. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, O.W.; Fassi, D.E.; Madsen, G.; Stentoft, J.; Vestergaard, H.; Rønnov-Jessen, D.; Pedersen, P.T.; Pulczynski, S.; Overgaard, U.M.; Andersen, C.L. Eosinophilia. Ugeskr. Laeger 2018, 180, V01180032. [Google Scholar]
- United Nations Statistics Division. Standard Country or Area Codes for Statistical Use (M49). Available online: https://unstats.un.org/unsd/methodology/m49/ (accessed on 23 January 2025).
- Eurostat: Asylum Statistics 2019. Available online: https://ec.europa.eu/eurostat/cache/infographs/asylum/asylum_2019/ (accessed on 23 January 2025).
- Danish Ministry of Immigration and Integration: International Migration—Denmark. Report to OECD (2019). Available online: https://uim.dk/publikationer/2019/international-migration-denmark-2019/ (accessed on 23 January 2025).
- Barrett, J.; Warrell, C.E.; Macpherson, L.; Watson, J.; Lowe, P.; Armstrong, M.; Whitty, C.J.M. The changing aetiology of eosinophilia in migrants and returning travellers in the Hospital for Tropical Diseases, London 2002–2015: An observational study. J. Infect. 2017, 75, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Mejia, R.; Nutman, T.B. Evaluation and differential diagnosis of marked, persistent eosinophilia. Semin. Hematol. 2012, 49, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, D.H.; Zagaria, N. Lymphatic filariasis elimination: Progress in global programme development. Ann. Trop. Med. Parasitol. 2002, 96 (Suppl. S2), S15–S40. [Google Scholar] [CrossRef]
- Turner, H.C.; Bettis, A.A.; Chu, B.K.; McFarland, D.A.; Hooper, P.J.; Ottesen, E.A.; Bradley, M.H. The health and economic benefits of the global programme to eliminate lymphatic filariasis (2000–2014). Infect. Dis. Poverty 2016, 5, 54. [Google Scholar] [CrossRef]
- Fenwick, A.; Webster, J.P. Schistosomiasis: Challenges for control, treatment and drug resistance. Curr. Opin. Infect. Dis. 2006, 19, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Weatherhead, J.E.; Hotez, P.J.; Mejia, R. The Global State of Helminth Control and Elimination in Children. Pediatr. Clin. N. Am. 2017, 64, 867–877. [Google Scholar] [CrossRef]
- Pion, S.D.S.; Chesnais, C.B.; Awaca-Uvon, N.P.; Vlaminck, J.; Abdou, A.; Kunyu-Shako, B.; Kuyangisa Simuna, G.; Tambwe, J.P.; Weil, G.J.; Boussinesq, M. The impact of four years of semiannual treatments with albendazole alone on lymphatic filariasis and soil-transmitted helminth infections: A community-based study in the Democratic Republic of the Congo. PLoS Negl. Trop. Dis. 2020, 14, e0008322. [Google Scholar] [CrossRef]
- Mduluza, T.; Jones, C.; Osakunor, D.N.M.; Lim, R.; Kuebel, J.K.; Phiri, I.; Manangazira, P.; Tagwireyi, P.; Mutapi, F. Six rounds of annual praziquantel treatment during a national helminth control program significantly reduced schistosome infection and morbidity levels in a cohort of schoolchildren in Zimbabwe. PLoS Negl. Trop. Dis. 2020, 14, e0008388. [Google Scholar] [CrossRef] [PubMed]
- Janda, A.; Eder, K.; Fressle, R.; Geweniger, A.; Diffloth, N.; Heeg, M.; Binder, N.; Sitaru, A.G.; Rohr, J.; Henneke, P.; et al. Comprehensive infectious disease screening in a cohort of unaccompanied refugee minors in Germany from 2016 to 2017: A cross-sectional study. PLoS Med. 2020, 17, e1003076. [Google Scholar] [CrossRef] [PubMed]
- Libman, M.D.; MacLean, J.D.; Gyorkos, T.W. Screening for schistosomiasis, filariasis, and strongyloidiasis among expatriates returning from the tropics. Clin. Infect. Dis. 1993, 17, 353–359. [Google Scholar] [CrossRef]
- Hvass, A.M.F.; Wejse, C. Systematic health screening of refugees after resettlement in recipient countries: A scoping review. Ann. Hum. Biol. 2017, 44, 475–483. [Google Scholar] [CrossRef]
- Lægehåndbogen (Danish Physician’s Handbook), Imported Parasitic Diseases. Available online: https://www.sundhed.dk/sundhedsfaglig/laegehaandbogen/infektioner/symptomer-og-tegn/importerede-parasitaere-sygdomme/ (accessed on 23 January 2025).
- Seybolt, L.M.; Christiansen, D.; Barnett, E.D. Diagnostic evaluation of newly arrived asymptomatic refugees with eosinophilia. Clin. Infect. Dis. 2006, 42, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Lam, M.C.; Leung, T.F.; Wong, K.Y.; Chow, C.M.; Fok, T.F.; Ng, P.C. Are age-specific high serum IgE levels associated with worse symptomatology in children with atopic dermatitis? Int. J. Dermatol. 2007, 46, 1258–1262. [Google Scholar] [CrossRef] [PubMed]
- Lo, N.C.; Addiss, D.G.; Hotez, P.J.; King, C.H.; Stothard, J.R.; Evans, D.S.; Colley, D.G.; Lin, W.; Coulibaly, J.T.; Bustinduy, A.L.; et al. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: The time is now. Lancet Infect. Dis. 2017, 17, e64–e69. [Google Scholar] [CrossRef]
- Kortas, A.Z.; Polenz, J.; von Hayek, J.; Rüdiger, S.; Rottbauer, W.; Storr, U.; Wibmer, T. Screening for infectious diseases among asylum seekers newly arrived in Germany in 2015: A systematic single-centre analysis. Public Health 2017, 153, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Aagaard-Hansen, J.; Nombela, N.; Alvar, J. Population movement: A key factor in the epidemiology of neglected tropical diseases. Trop. Med. Int. Health 2010, 15, 1281–1288. [Google Scholar] [CrossRef]
- Deen, L.; Cowan, S.; Wejse, C.; Petersen, J.H.; Norredam, M. Refugees and family-reunified immigrants have a high incidence of HIV diagnosis and late presentation compared with Danish born: A nationwide register-based cohort study. Infection 2018, 46, 659–667. [Google Scholar] [CrossRef]
- Frederiksen, N.W.; Christoffersen, N.M.; Haugaard, A.K.; Ahmadi, A.; Poulsen, A.; Norredam, M.; Kruse, A. Health screening among children newly granted asylum in Denmark. Acta Paediatr. 2021, 110, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.H.; Kruse, A.; Frederiksen, H.W.; Ahmadi, A.; Norredam, M. Health status of refugees newly resettled in Denmark. Dan. Med. J. 2020, 67, A08200567. [Google Scholar] [PubMed]
- Bustamante, J.; García López-Hortelano, M.; Barcia, C.; Díaz Almirón, M.; Subirats, M.; Montero Vega, D.; Mellado, M.J.; Sainz, T. Eosinophilia in Migrant Children: How Should We Proceed? Pediatr. Infect. Dis. J. 2022, 41, 102–107. [Google Scholar] [CrossRef]
- Bustamante, J.; Sainz, T.; Ara-Montojo, M.F.; Almirón, M.D.; Subirats, M.; Vega, D.M.; Mellado, M.J.; López-Hortelano, M.G. Screening for parasites in migrant children. Travel. Med. Infect. Dis. 2022, 47, 102287. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control (ECDC): Public Health Guidance on Screening and Vaccination for Infectious Diseases in Newly Arrived Migrants Within the EU/EEA. 2018. Available online: www.ecdc.europa.eu/sites/default/files/documents/Public%20health%20guidance%20on%20screening%20and%20vaccination%20of%20migrants%20in%20the%20EU%20EEA.pdf (accessed on 23 January 2025).

| Origin | Partici- pants | Age Median [Range] | Female | Eosinophilia | Elevated IgE | Eosinophilia and Elevated IgE |
|---|---|---|---|---|---|---|
| Region Country | N | Years | % |
n/N (%) [95% CI] p-Value |
n/N (%) [95% CI] p-Value |
n/N (%) [95% CI] p-Value |
| Eastern Africa | 104 | 18 [1.1–69.7] | 54.8 | 14/104 (13.5) [7.6; 21.6] p = 0.004 * | 32/69 (46.4) [34.3; 58.8] p = 0.005 * | 10/69 (14.5) [7.2; 25.0] p ≤ 0.001 |
| Eritrea | 57 | 18.9 [1.1–65.6] | 56.1 | 12/57 (21.1) [11.4; 33.9] p ≤ 0.001 | 18/38 (47.4) [31.0; 64.2] p = 0.044 | 9/38 (23.7) [11.4; 40.3] p ≤ 0.001 |
| Ethiopia | 9 | 27.2 [6.9–36.9] | 66.7 | 1/9 (11.1) [0.3; 4.8] p = 0.472 | 3/4 (75.0) [19.4; 99.4] p = 0.100 | 0/4 (0) [0.0; 60.2] p = 1.000 |
| Somalia | 37 | 16.0 [4.1–69.7] | 51.4 | 1/37 (2.7) [0.1; 14.2] p = 0.505 | 11/27 (40.7) [22.4; 61.2] p = 0.394 | 1/27 (3.7) [0.1; 19.1] p = 1.000 |
| Other ** | 1 | 19.4 [19.4–19.4] | 0.0 | 0/1 (0) [0; 97.5] p = 1.000 | 0/0 | 0/0 |
| Middle Africa | 13 | 16.5 [3.0–64.2] | 46.2 | 3/13 (23.1) [5.0; 53.8] p = 0.052 | 0/0 | 0/0 |
| Congo | 9 | 19.1 [5.8–64.2] | 44.4 | 2/9 (22.2) [2.8; 60.0] p = 0.120 | 0/0 | 0/0 |
| Other ** | 4 | 12.2 [3.0–42.0] | 50.0 | 1/4 (25.0) [0.6; 80.6] p = 0.246 | 0/0 | 0/0 |
| Western Asia | 531 | 23.6 [0.4–67.8] | 49.5 | 27/531 (5.1) [3.4; 7.3] p = 0.006 * | 55/206 (26.7) [20.8; 33.3] p = 0.018 * | 4/206 (1.94) [0.5; 4.9] p = 0.027 |
| Syria | 503 | 23.2 [0.4–67.6] | 49.1 | 27/503 (5.4) [3.6; 7.7] p = 0.040 | 46/187 (24.6) [18.6; 31.4] p = 0.003 | 4/187 (2.1) [0.6; 5.4] p = 0.082 |
| Lebanon | 8 | 24.8 [17.7–52.0] | 62.5 | 0/8 (0) [0.0; 36.9] p = 1.000 | 2/5 (40.0) [5.3; 85.3] p = 0.658 | 0/5 (0) [0.0; 5.2] p = 1.000 |
| Iraq | 8 | 26.1 [5.5–31.9] | 50.0 | 0/8 (0) [0.0; 36.9] p = 1.000 | 3/6 (50.0) [11.8; 88.2] p = 0.391 | 0/6 (0) [0.0; 45.9] p = 1.000 |
| Unknown *** | 8 | 49.9 [26.2–67.8] | 62.5 | 0/8 (0) [0.0; 36.9] p = 1.000 | 4/7 (57.1) [18.4; 90.1] p = 0.218 | 0/7 (0) [0.0; 41.0] p = 1.000 |
| Other ** | 4 | 49.1 [42.1–56.0] | 50.0 | 0/4 (0) [0.0; 60.2] p = 1.000 | 0/1 (0) [0.0; 97.5] p = 1.000 | 0/1 (0) [0.0; 97.5] p = 1.000 |
| Southern Asia | 138 | 22.9 [0.4–63.7] | 39.9 | 9/138 (6.5) [3.0; 12.0] p = 0.883 * | 45/131 (34.4) [26.3; 43.1] p = 0.507 * | 3/131 (2.3) [0.5; 6.5] p = 0.289 |
| Afghanistan | 46 | 17.7 [3.1–57.5] | 30.4 | 2/46 (4.4) [0.5; 14.8] p = 0.762 | 17/45 (37.8) [23.8; 53.5] p = 0.401 | 0/45 (0) [0.0; 7.9] p = 0.237 |
| Iran | 90 | 27.0 [0.4–63.7] | 44.4 | 7/90 (7.8) [3.2; 15.4] p = 0.658 | 27/84 (32.1) [22.4; 43.2] p = 1.000 | 3/84 (3.6) [0.7; 10.1] p = 1.000 |
| Other ** | 2 | 29.3 [16.2–42.4] | 50.0 | 0/2 (0) [0.0; 84.2] p = 1.000 | 1/2 (50.0) [1.3; 98.7] p = 0.540 | 0/2 (0) [0.0; 84.2] p = 1.000 |
| Other **** | 7 | 20.8 [2.4–38.1] | 57.1 | 1/7 (14.3) [0.4; 5.8] p = 0.391 | 0/5 (0) [0.0; 52.2] p = 0.181 | 0/5 (0) [0.0; 52.2] p = 1.000 |
| Total | 793 | 22.9 [0.4–69.7] | 48.6 | 54/793 (6.8) [5.2; 8.8] | 132/411 (32.1) [27.7; 36.9] | 17/411 (4.1) [2.4; 6.5] |
| All | Attention to Elevations | No Attention to Elevations | p-Value | |||
|---|---|---|---|---|---|---|
| Participants | n | 116 | 59 | 57 | ||
| Elevations | Isolated eosinophilia | n (%) | 24 (20.7) | 10 (17.0) | 14 (24.6) | 0.312 |
| Isolated IgE | 79 (68.1) | 40 (67.8) | 39 (68.4) | 0.942 | ||
| Both elevated | 13 (11.21) | 9 (15.3) | 4 (7.0) | 0.160 | ||
| Eosinophil count, 100 cells/µL * | Median [range] | 0.61 [0.5–1.76] | 0.66 [0.52–1.76] | 0.60 [0.5–1.4] | 0.236 ** | |
| IgE level, IU/L * | 237.5 [105–1627] | 273 [120–1627] | 182 [105–928] | 0.040 ** | ||
| Symptoms | Gastrointestinal | n (%) | 46 (39.7) | 30 (50.9) | 16 (28.1) | 0.012 |
| Dermatological | 26 (22.4) | 13 (22.0) | 13 (22.8) | 0.920 | ||
| Respiratory | 21 (18.1) | 18 (30.5) | 3 (5.3) | 0.000 | ||
| Urological | 18 (15.5) | 9 (15.3) | 9 (15.8) | 0.937 | ||
| Known disease related to eosinophilia or elevated IgE *** | 26 (22.4) | 21 (35.6) | 5 (8.8) | 0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanng, K.; Margolinsky, R.V.; Wejse, C.; Kallestrup, P.; Hvass, A.M.F. IgE and Eosinophilia in Newly Arrived Refugees in Denmark: A Cross-Sectional Study of Prevalence and Clinical Management in Primary Care. Int. J. Environ. Res. Public Health 2025, 22, 180. https://doi.org/10.3390/ijerph22020180
Lanng K, Margolinsky RV, Wejse C, Kallestrup P, Hvass AMF. IgE and Eosinophilia in Newly Arrived Refugees in Denmark: A Cross-Sectional Study of Prevalence and Clinical Management in Primary Care. International Journal of Environmental Research and Public Health. 2025; 22(2):180. https://doi.org/10.3390/ijerph22020180
Chicago/Turabian StyleLanng, Kamilla, Rebecca Vigh Margolinsky, Christian Wejse, Per Kallestrup, and Anne Mette Fløe Hvass. 2025. "IgE and Eosinophilia in Newly Arrived Refugees in Denmark: A Cross-Sectional Study of Prevalence and Clinical Management in Primary Care" International Journal of Environmental Research and Public Health 22, no. 2: 180. https://doi.org/10.3390/ijerph22020180
APA StyleLanng, K., Margolinsky, R. V., Wejse, C., Kallestrup, P., & Hvass, A. M. F. (2025). IgE and Eosinophilia in Newly Arrived Refugees in Denmark: A Cross-Sectional Study of Prevalence and Clinical Management in Primary Care. International Journal of Environmental Research and Public Health, 22(2), 180. https://doi.org/10.3390/ijerph22020180








