COVID-19 Infections Still Occur: How Do Pregnant and Non-Pregnant Individuals Compare? A Study from the Canadian Mother–Child Initiative on Drug Safety in Pregnancy (CAMCCO)
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Comparisons Between Pregnant and Non-Pregnant Individuals with COVID-19
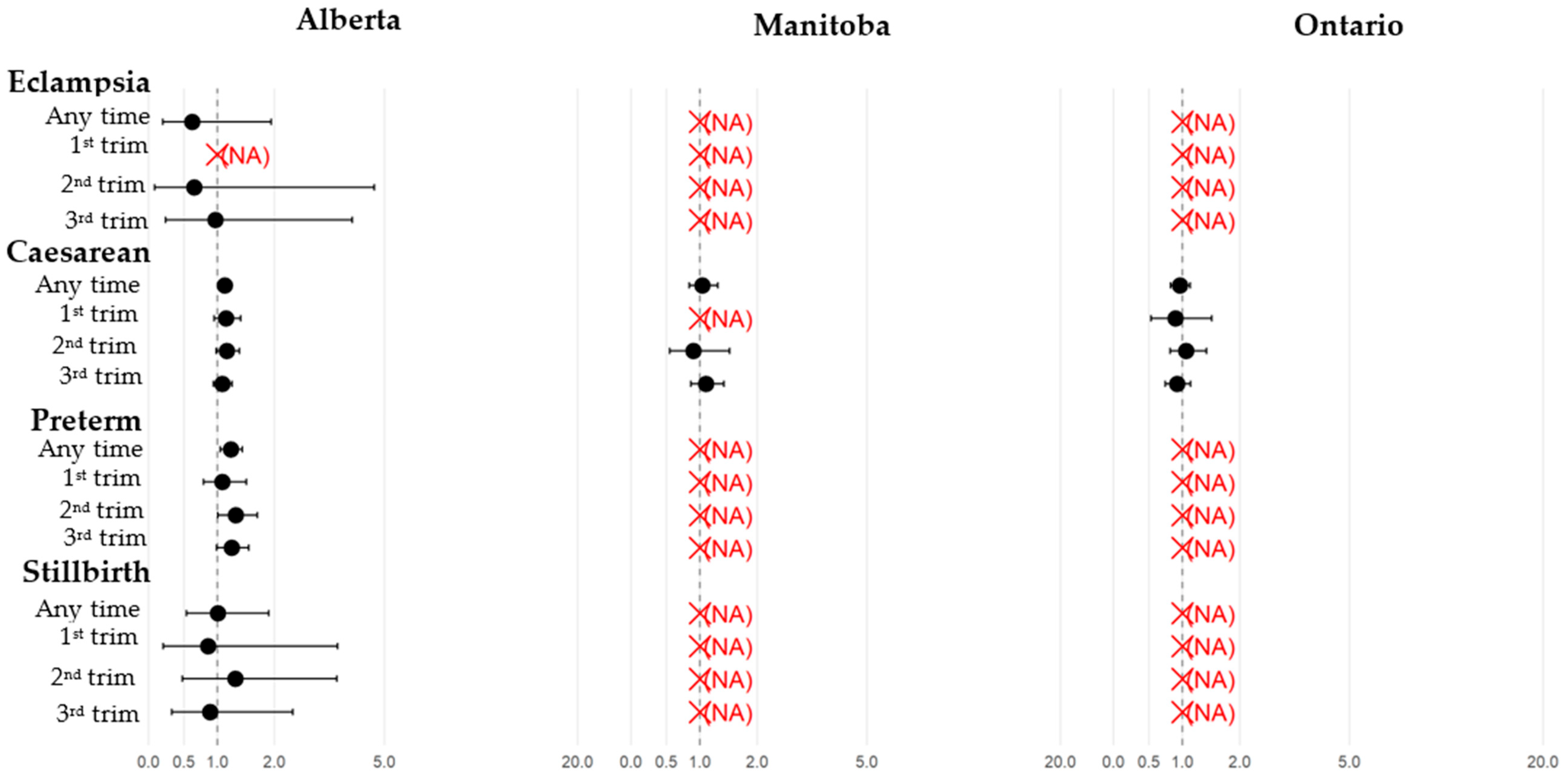
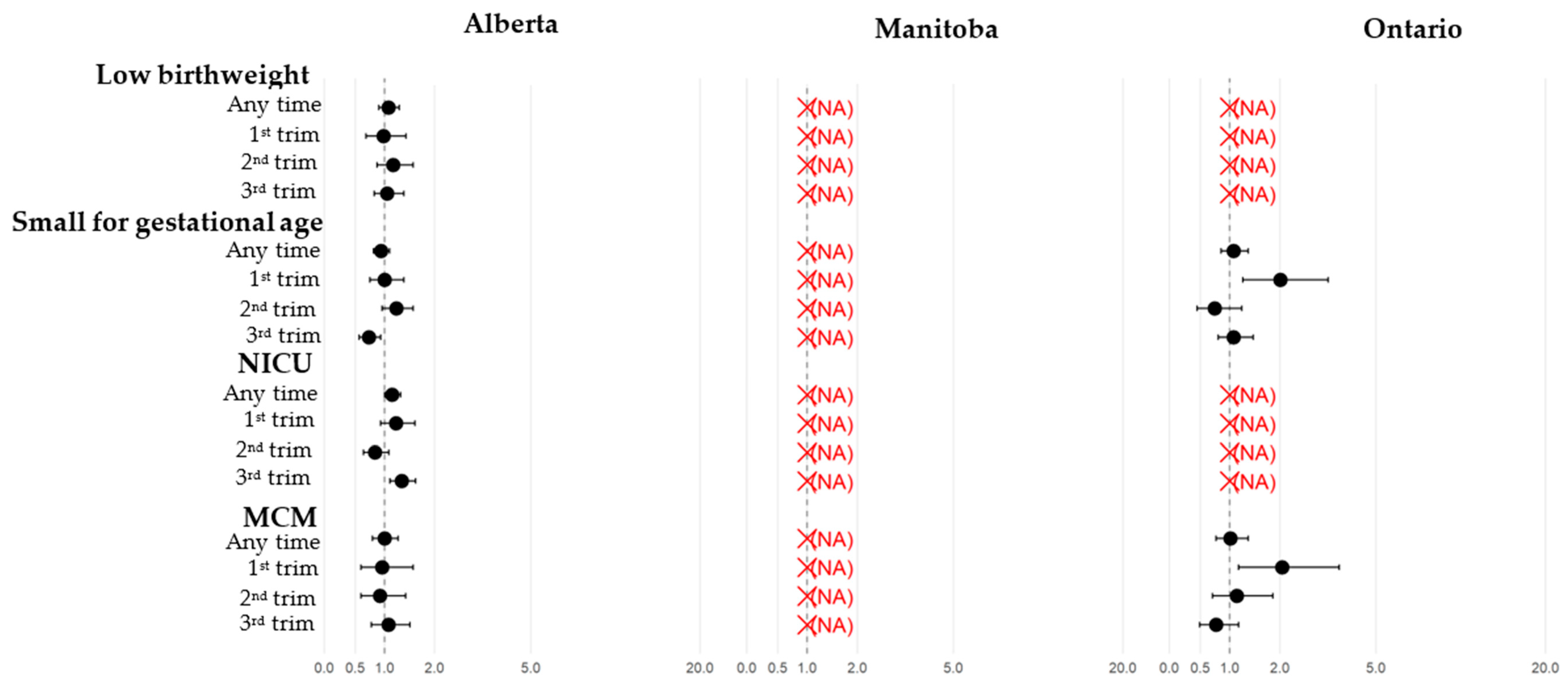
3.2. Pregnant Individuals: Comparisons Between Those with and Without COVID-19
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATC | Anatomical Therapeutic Chemical |
| CAMCCO | Canadian Mother–Child Cohort |
| CANCOVID-preg | Canadian Surveillance program of COVID-19 in pregnancy |
| CONSIGN | COVID-19 infectiOn aNd medicineS In pregnaNcy |
| COVID-19 | Coronavirus Disease 2019 |
| DG | Day of Gestation |
| ICD | International Classification of Disease |
| LBW | Low birth weight |
| MCM | Major Congenital Malformation |
| NICU | Neonatal Intensive Care Unit |
| RR | Relative Risk |
| SGA | Small-for-gestational age |
| TOPFA | Termination of Pregnancy due to Fetal Anomaly |
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Liu, Y.Y.; Wu, C.H.; Wang, C.Y.; Wang, C.H.; Long, C.Y. Impact of COVID-19 on Pregnancy. Int. J. Med. Sci. 2021, 18, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Update to living systematic review on COVID-19 in pregnancy. BMJ 2021, 372, n615. [CrossRef] [PubMed]
- McClymont, E.; Albert, A.Y.; Alton, G.D.; Boucoiran, I.; Castillo, E.; Fell, D.B.; Kuret, V.; Poliquin, V.; Reeve, T.; Scott, H.; et al. Association of SARS-CoV-2 Infection During Pregnancy with Maternal and Perinatal Outcomes. JAMA 2022, 327, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- National Institutes of Health. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines; National Institutes of Health: Bethesda, MD, USA, 2024.
- Nadig, N.; Bhimraj, A.; Cawcutt, K.; Chiotos, K.; Dzierba, A.L.; Kim, A.Y.; Martin, G.S.; Pearson, J.C.; Shumaker, A.H.; Baden, L.R.; et al. 2025 Clinical Practice Guideline Update by the Infectious Diseases Society of America on the Treatment and Management of COVID-19: Infliximab. Clin Infect Dis. 2025, ciaf355. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.A.; Jamieson, D.J. COVID-19 and Pregnancy. Infect. Dis. Clin. N. Am. 2022, 36, 423–433. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.; Ashraf, R.; Rowe, H.; Zipursky, J.; Clarfield, L.; Maxwell, C.; Arzola, C.; Lapinsky, S.; Paquette, K.; Murthy, S.; et al. Pregnancy and COVID-19: Pharmacologic considerations. Ultrasound Obstet. Gynecol. 2021, 57, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Poliquin, V.; Castillo, E.; Boucoiran, I.; Wong, J.M.H.; Watson, H.; Yudin, M.; Money, D.; Van Schalkwyk, J.; Elwood, C. SOGC Statement on COVID-19 Vaccination in Pregnancy; Infectious Disease Committee of the Society of Obstetricians and Gynaecologists of Canada: Ottawa, ON, Canada, 2022. [Google Scholar]
- Mota-Pérez, M.; Huerta-Álvarez, C.; Llorente, A.; Cea-Soriano, L. COVID-19 Distribution in Pregnancy, Drug Use Patterns and COVID-19 Medication during the Pandemic in Spain: Data from Real-World Electronic Health Records. Pharmaceuticals 2024, 17, 207. [Google Scholar] [CrossRef] [PubMed]
- Berard, A.; Kaul, P.; Eltonsy, S.; Winquist, B.; Chateau, D.; Hawken, S.; Sprague, A.; Walker, M.; Bernatsky, S.; Abrahamowicz, M.; et al. The Canadian Mother-Child Cohort Active Surveillance Initiative (CAMCCO): Comparisons between Quebec, Manitoba, Saskatchewan, and Alberta. PLoS ONE 2022, 17, e0274355. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Shinde, M.; Cosgrove, A.; Lyons, J.G.; Kempner, M.E.; Mosley, J.; Cole, D.; Hoffman, E.; Messenger-Jones, E.; Hernández-Muñoz, J.J.; Stojanovic, D.; et al. Characteristics and Medication Use Patterns of Pregnancies with COVID-19 Ending in Live-Birth in the Sentinel System. Pharmacoepidemiol. Drug Saf. 2025, 34, e70121. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Drouin, J.; Le Vu, S.; Botton, J.; Semenzato, L.; Bertrand, M.; Jabagi, M.J.; Miranda, S.; Dray-Spira, R.; Weill, A.; et al. COVID-19 vaccination rates among pregnant women in France: A nationwide cohort study. Vaccine 2025, 53, 127070. [Google Scholar] [CrossRef] [PubMed]
- Unruh, L.; Allin, S.; Marchildon, G.; Burke, S.; Barry, S.; Siersbaek, R.; Thomas, S.; Rajan, S.; Koval, A.; Alexander, M.; et al. A comparison of 2020 health policy responses to the COVID-19 pandemic in Canada, Ireland, the United Kingdom and the United States of America. Health Policy 2022, 126, 427–437. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allotey, J.; Stallings, E.; Bonet, M.; Yap, M.; Chatterjee, S.; Kew, T.; Debenham, L.; Llavall, A.C.; Dixit, A.; Zhou, D.; et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: Living systematic review and meta-analysis. BMJ 2020, 370, m3320. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.P.; Sheehy, O.; Gorgui, J.; Bérard, A. Can We Rely on Pharmacy Claims Databases to Ascertain Maternal Use of Medications during Pregnancy? Birth Defects Res. 2017, 109, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nauman, A.; Paul, P.; Ganesan, S.; Chen, K.H.; Jalil, S.M.S.; Jaouni, S.H.; Kawas, H.; Khan, W.A.; Vattoth, A.L.; et al. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: A systematic review. Hum. Vaccines Immunother. 2022, 18, 2027160. [Google Scholar] [CrossRef] [PubMed]
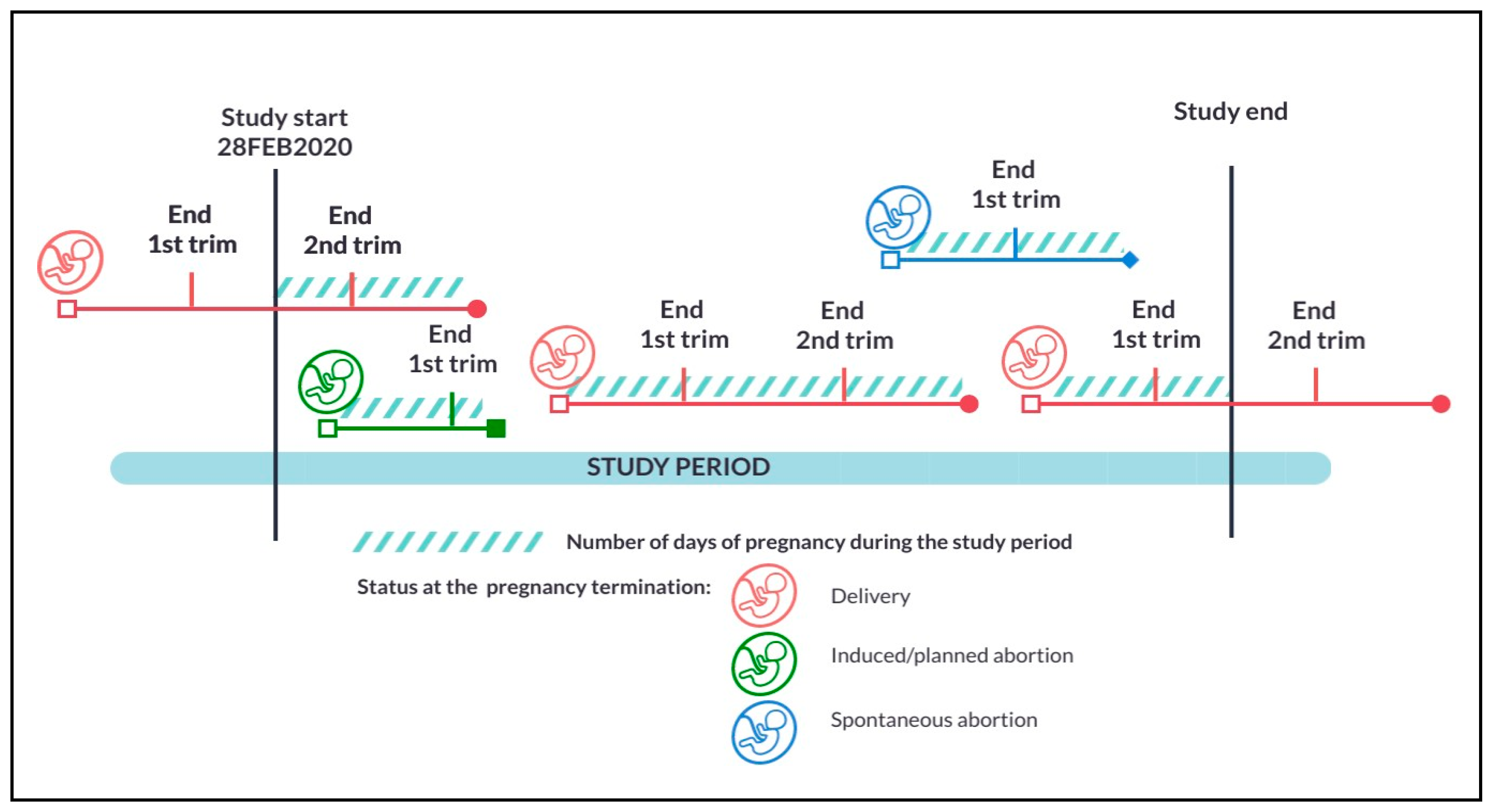
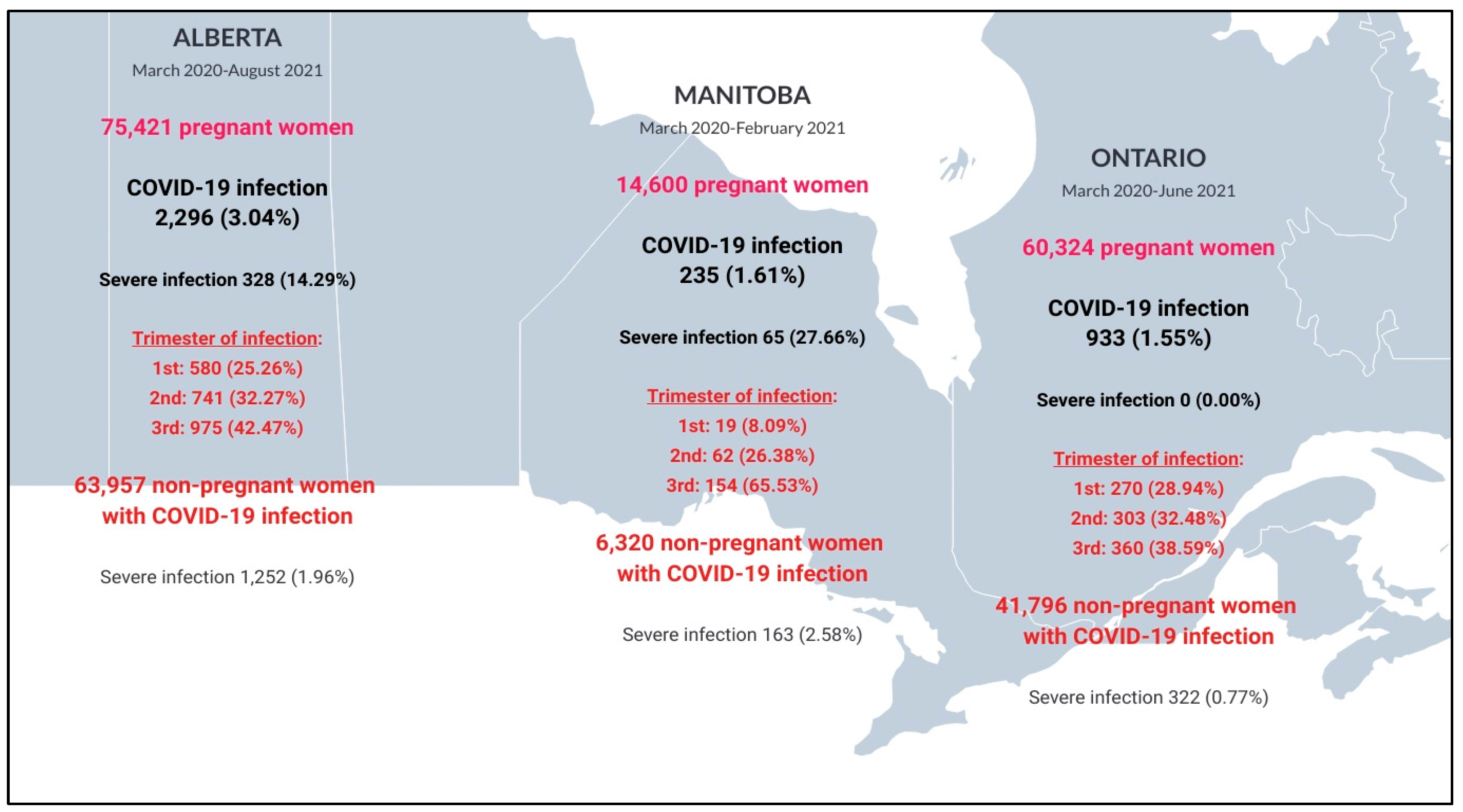

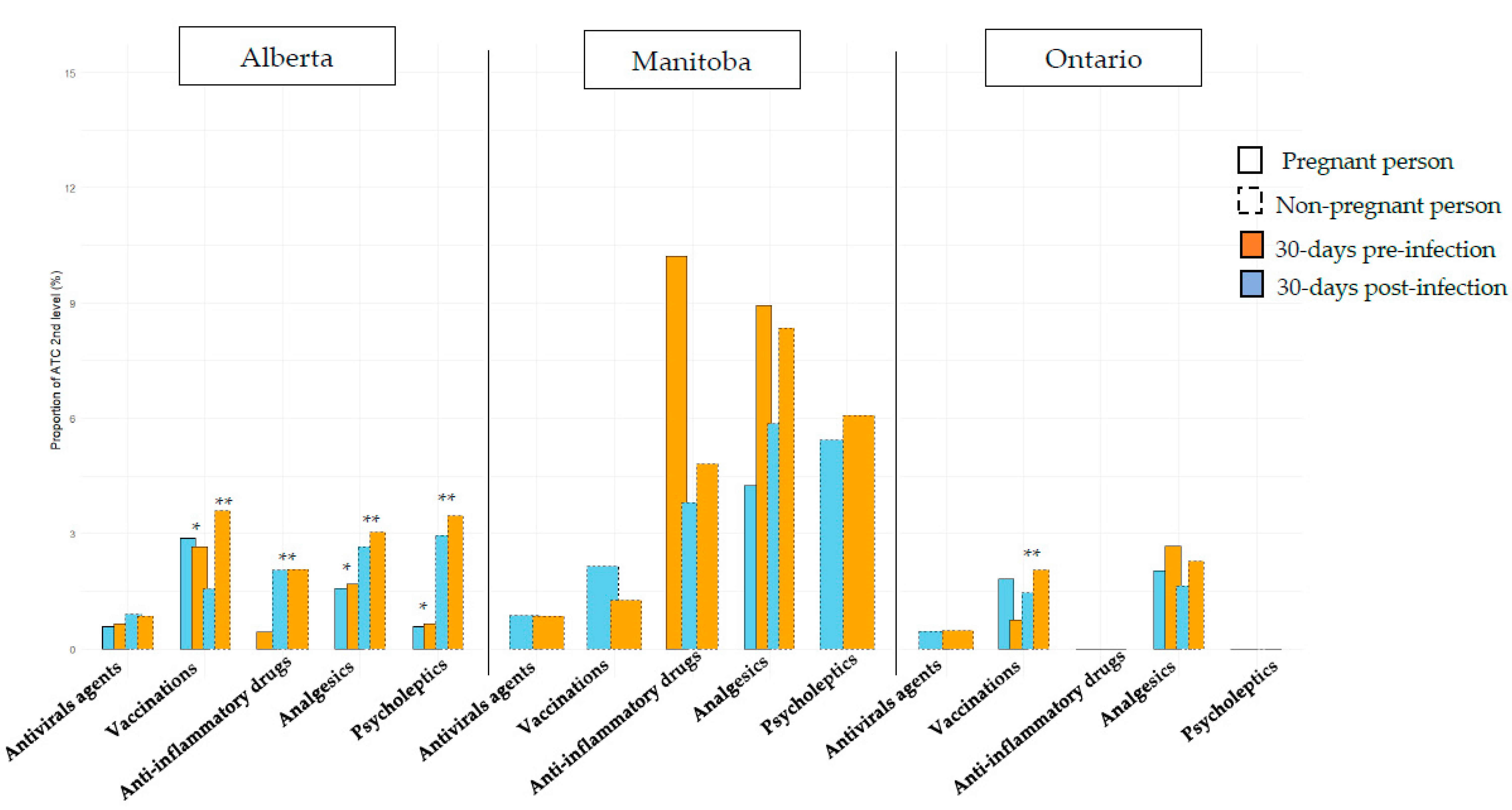
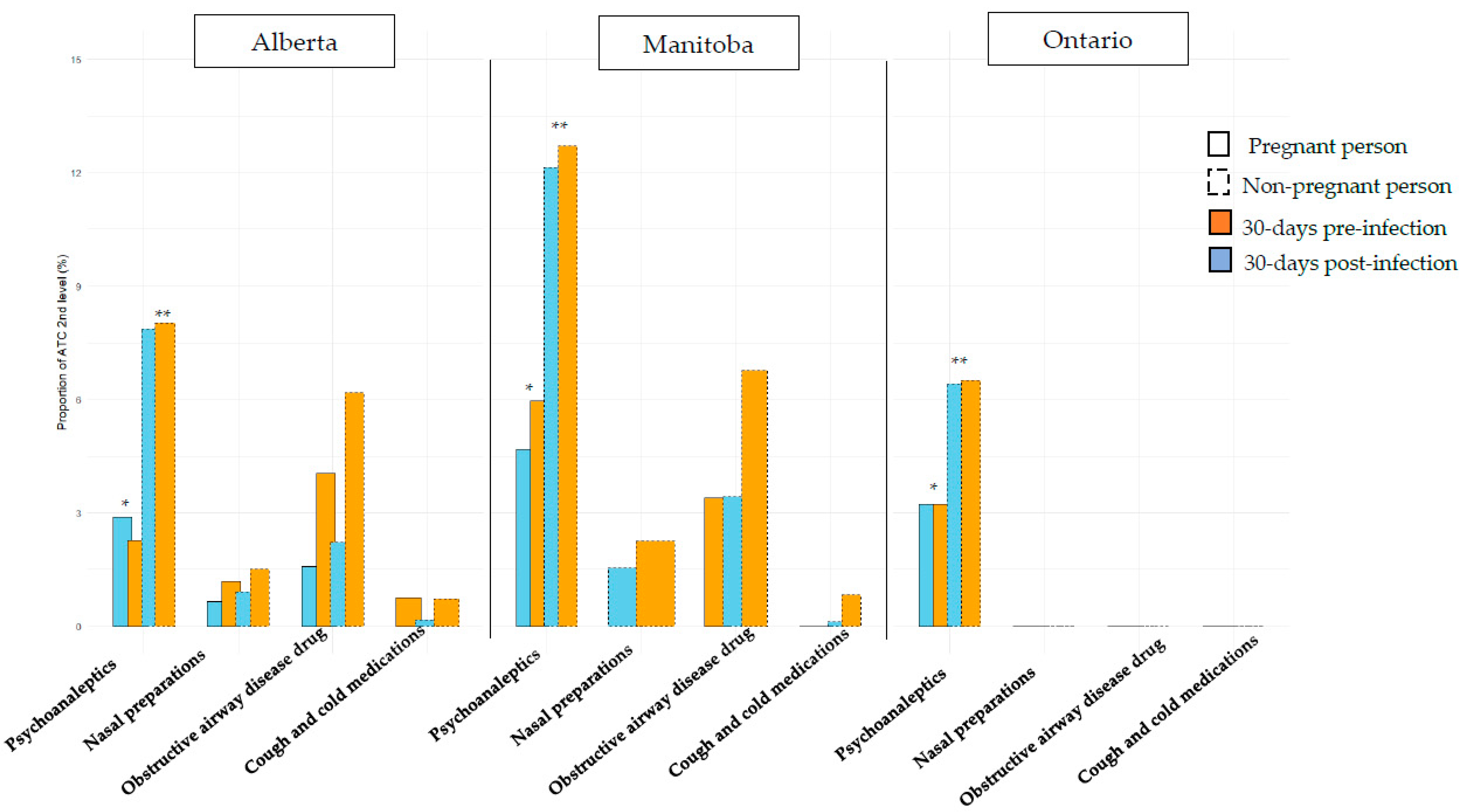

| Alberta | Manitoba | Ontario | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | Pregnant People with COVID-19 Infection n = 2296 | Non-Pregnant People with COVID-19 Infection n = 63,957 | p-Value | Pregnant People with COVID-19 Infection n = 235 | Non-Pregnant People with COVID-19 Infection n = 6320 | p-Value | Pregnant People with COVID-19 Infection n = 933 (1.55%) | Non-Pregnant People with COVID-19 Infection n = 41,796 | p-Value |
| Follow-up in months after index date —mean ± SD | 8.5 ± 1.1 | 6.2 ± 3.2 | <0.001 | 7.7 ± 1.5 | 3.7 ± 1.6 | <0.001 | 7.5 ± 2.1 | 6.2 ± 3.2 | <0.001 |
| Maternal age at the 1st day of gestation (1DG) mean ± SD | 30.0 ± 5.2 | 30.4 ± 8.9 | <0.001 | 28.5 ± 6.1 | 30.0 ± 8.8 | 0.045 | 28.1 ± 6.2 | 28.7 ± 9.0 | 0.045 |
| Severe COVID-19 infection | 328 (14.3) | 1252 (2.0) | <0.001 | 65 (27.7) | 163 (2.6) | <0.001 | 0 (0.0) | 322 (0.7) | N.A. |
| Site of the COVID-19 diagnosis—n (%) | <0.001 | <0.001 | <0.001 | ||||||
| Acute inpatient hospitalization | 30 (1.3) | 398 (0.6) | 65 (27.7) | 163 (2.6) | 70 (7.5) | 361 (0.9) | |||
| Physician’s office/Test site | 2266 (98.7) | 63,559 (99.4) | 170 (72.3) | 6157 (97.4) | 863 (92.5) | 41,435 (99.1) | |||
| Maternal morbidity and vaccination during pregnancy or during study period for the non- pregnant women: | |||||||||
| Influenza vaccination | 68 (28.9) | 1465 (23.2) | 0.0407 | 115 (12.3) | 805 (1.9) | <0.001 | |||
| Pneumococcal vaccination | 0 (0.0) | 18 (0.3) | N.A. | 0 (0.0) | 0 (0.0) | N.A. | |||
| Pertussis vaccination | 93 (39.6) | 305 (4.8) | <0.0001 | 0 (0.0) | 0 (0.0) | N.A. | |||
| Proxy for maternal lifestyle in the year prior to the index date: | |||||||||
| Tobacco dependence | 27 (1.2) | 873 (1.4) | 0.442 | 8 (3.4) | 168 (2.7) | 0.4872 | <6 | 67 (0.2) | N.A. |
| Alcohol dependence | 35 (1.5) | 850 (1.6) | 0.868 | 10 (4.3) | 275 (4.4) | 0.9435 | <6 | 135 (0.3) | N.A. |
| Co-morbid conditions in the year prior to the index date1: | |||||||||
| Cardiovascular disease | 58 (2.5) | 1530 (2.4) | 0.68 | <6 | 95 (1.5) | 0.5856 | 0 (0.0) | 0.316 | |
| Hypertension | 92 (4.0) | 4467 (7.0) | <0.001 | 16 (6.8) | 570 (9.0) | 0.2435 | 14 (1.5) | 0.193 | |
| Sickle cell disease | 0 (0.0) | 22 (0.03) | 0.37 | 0 (0.0) | <6 | N.A. | <6 | N.A. | |
| Respiratory disease | 175 (7.6) | 5769 (9.0) | 0.02 | 17 (7.2) | 503 (8.0) | 0.6864 | 24 (2.6) | 0.523 | |
| Diabetes type 1 or 2 | 78 (3.4) | 2456 (3.8) | 0.28 | 11 (4.7) | 472 (7.5) | 0.1083 | 34 (3.6) | 0.728 | |
| Obesity | 41 (1.8) | 1335 (2.1) | 0.32 | 8 (3.4) | 109 (1.7) | 0.0718 | 11 (1.2) | 0.009 | |
| Chronic kidney disease | 29 (1.3) | 1084 (1.7) | 0.11 | <6 | 81 (1.3) | N.A. | <6 | N.A. | |
| HIV | <10 | 113 (0.2) | 0.13 | 9 (3.8) | 288 (4.6) | 0.5987 | <6 | N.A. | |
| Use of immunosuppressants | 107 (4.7) | 3087 (4.8) | 0.71 | 10 (4.3) | 239 (3.8) | 0.7092 | 15 (1.6) | 0.102 | |
| Cancer | 241 (10.5) | 5541 (8.7) | <0.001 | <6 | 94 (1.5) | 0.7803 | 45 (4.8) | 0.241 | |
| Any mental disorders | 512 (22.3) | 19,485 (30.5) | <0.001 | 51 (21.7) | 1659 (26.3) | 0.119 | 150 (16.1) | <0.001 | |
| Mood and anxiety disorders | 447 (19.5) | 17,197 (26.9) | <0.001 | 49 (20.9) | 1542 (24.4) | 0.2129 | 131 (14.0) | <0.001 | |
| Other mental disorders | 193 (8.4) | 8156 (12.8) | <0.001 | 12 (5.1) | 458 (7.3) | 0.2117 | 32 (3.4) | 0.793 | |
| Common rheumatic diseases | 70 (3.1) | 3110 (4.9) | <0.001 | <6 | 233 (3.7) | 0.0082 | 36 (3.9) | 0.262 | |
| Any previous co-morbid conditions | 962 (41.9) | 30,567 (47.8) | <0.001 | 91 (38.7) | 2721 (43.1) | 0.1878 | 270 (28.9) | 0.115 | |
| Alberta | Manitoba | Ontario | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnant Women ( n = 75,421) | Pregnant Women (n = 14,600) | Pregnant Women ( n = 60,324) | |||||||
| Characteristics | COVID-19 Infection n = 2296 (3.04%) | No COVID-19 Infection n = 73,125 | p-Value | COVID-19 Infection n = 235 (1.61%) | No COVID-19 Infection n = 14,365 | p-Value | COVID-19 Infection n = 933 (1.55%) | No COVID-19 Infection n = 59,391 | p-Value |
| Maternal age at the 1st day of gestation (1DG)—mean ± SD | 30.0 ± 5.2 | 30.3 ± 5.2 | 0.59 | 28.5 ± 6.1 | 29.1 ± 5.8 | 0.12 | 28.08 ± 6.21 | 28.19 ± 6.39 | 0.29 |
| Status at the end of the pregnancy—n (%) | 0.61 | 0.0003 | <0.001 | ||||||
| Delivery | 2175 (93.5) | 66,921 (90.3) | N.A. | 10,594 (73.7) | 604 (64.7) | 32,249 (54.3) | |||
| Planned/induced abortion | 26 (1.1) | 1512 (2.0) | 14 (6.0) | 1448 (10.1) | 243 (26.0) | 20,431 (34.4) | |||
| Spontaneous abortion | 125 (5.4) | 5710 (7.7) | 20 (8.5) | 2236 (15.6) | 86 (9.2) | 6711(11.3) | |||
| Gestational age at the end of the pregnancy—mean ± SD | 37.3 ± 4.6 | 36.9 ± 5.5 | <0.001 | 35.6 ± 6.6 | 34.1 ± 8.0 | 0.0004 | 31.8 ± 9.1 | 29.9 ± 9.5 | <0.001 |
| Sex of the baby(ies)—n (%) | 0.27 | 0.48 | 0.53 | ||||||
| Female | 1044 (48.2) | 32,375 (48.6) | 92 (46.0) | 5216 (48.5) | 308 (50.2) | 16,036 (48.9) | |||
| Male | 1121 (51.8) | 34,240 (51.4) | 108 (54.0) | 5535 (51.5) | 306 (49.8) | 16,776 (51.1) | |||
| Type of delivery—n (%) | 0.03 | 0.68 | 0.37 | ||||||
| Vaginal | 1419 (66.2) | 45,076 (68.4) | 145 (72.1) | 7822 (73.2) | 482 (79.8) | 25,442 (78.9) | |||
| cesarean section | 726 (33.8) | 20,827 (31.6) | 56 (27.9) | 2859 (26.8) | 122 (20.2) | 6802 (21.1) | |||
| Proxy for maternal lifestyle in the year prior to the 1DG: | |||||||||
| Tobacco dependence | 27 (1.2) | 1086 (1.5) | 0.26 | 8 (3.4) | 356 (2.5) | 0.37 | ≤5 | 181 (0.3) | N.A. |
| Alcohol dependence | 35 (1.5) | 828 (1.1) | 0.10 | 10 (4.3) | 450 (3.1) | 0.33 | ≤5 | 249 (0.4) | N.A. |
| Co-morbid conditions in the year prior to the 1DG: | |||||||||
| Cardiovascular disease | 58 (2.5) | 1964 (2.7) | 0.69 | <6 | 195 (1.4) | 0.77 | 0 (0.0) | 18 (0.0) | N.A. |
| Hypertension | 92 (4.0) | 3186 (4.4) | 0.45 | 16 (6.8) | 809 (5.6) | 0.44 | 14 (1.5) | 599 (1.0) | 0.14 |
| Sickle cell disease | 0 (0.0) | 19 (0.03) | N.A. | 0 (0.0) | <6 | N.A. | <6 | 73 (0.1) | N.A. |
| Respiratory disease | 175 (7.6) | 6611 (9.0) | 0.02 | 17 (7.2) | 1322 (9.2) | 0.30 | 24 (2.6) | 1628 (2.7) | 0.75 |
| Diabetes type 1 or 2 | 78 (3.4) | 2512 (3.4) | 0.97 | 11 (4.7) | 578 (4.0) | 0.61 | 34 (3.6) | 1182 (2.0) | <0.001 |
| Obesity | 41 (1.8) | 990 (1.4) | 0.10 | 8 (3.4) | 291 (2.0) | 0.16 | 11 (1.2) | 979 (1.6) | 0.26 |
| Chronic kidney disease | 29 (1.3) | 965 (1.3) | 0.89 | <6 | 81 (0.6) | 0.15 | <6 | 83 (0.1) | N.A. |
| HIV | <10 | 73 (0.1) | 0.61 | 9 (3.8) | 589 (4.1) | 0.84 | <6 | 86 (0.1) | N.A. |
| Use of immunosuppressants | 107 (4.7) | 3109 (4.3) | 0.37 | 10 (4.3) | 706 (2.0) | 0.64 | 15 (1.6) | 1393 (2.3) | 0.14 |
| Cancer | 241 (10.5) | 8147 (11.1) | 0.35 | <6 | 532 (3.7) | 0.11 | 45 (4.8) | 2959 (5.0) | 0.83 |
| Any mental disorders | 512 (22.3) | 17,325 (23.7) | 0.13 | 51 (21.7) | 3629 (25.3) | 0.21 | 150 (16.1) | 13,414 (22.6) | <0.01 |
| Mood and anxiety disorders | 447 (19.5) | 15,010 (20.5) | 0.23 | 49 (20.9) | 3392 (23.6) | 0.32 | 131 (14.0) | 11,912 (20.1) | <0.01 |
| Other mental disorders | 193 (8.4) | 6500 (8.9) | 0.44 | 12 (5.1) | 910 (6.3) | 0.44 | 32 (3.4) | 2864 (4.8) | 0.05 |
| Common rheumatic diseases | 70 (3.1) | 2192 (3.0) | 0.94 | <6 | 322 (2.2) | 0.06 | 36 (3.9) | 1729 (2.9) | 0.09 |
| Any previous co-morbid conditions | 962 (41.9) | 31,464 (43.0) | 0.29 | 91 (38.7) | 6119 (42.6) | 0.23 | 270 (28.9) | 20,073 (33.8) | 0.002 |
| Adverse pregnancy outcome in the 2-years prior to the 1DG: | |||||||||
| Prior history of gestational diabetes | 75 (3.3) | 2275 (3.1) | 0.72 | 26 (11.1) | 824 (5.7) | 0.001 | 19 (2.0) | 948 (1.6) | 0.29 |
| Prior history of hypertensive disorders | 37 (1.6) | 835 (1.1) | 0.05 | 7 (3.0) | 259 (1.8) | 0.21 | ≤5 | 310 (0.5) | N.A. |
| Any of the above adverse reproductive history | 103 (4.5) | 2959 (4.1) | 0.33 | 46 (19.6) | 2115 (14.7) | 0.04 | 23 (2.5) | 1216 (2.0) | 0.37 |
| Previous adverse reproductive history in the 2 years prior to the 1DG: | |||||||||
| Prior Stillbirth | <10 | 247 (0.3) | 0.43 | <6 | 74 (0.5) | N.A. | 0 (0.0) | 9 (0.0) | 0.71 |
| Prior small for gestational age | 68 (3.0) | 1950 (2.7) | 0.43 | <6 | 44 (0.3) | N.A. | 21 (2.3) | 1372 (2.3) | 0.91 |
| Prior congenital malformation | 66 (2.7) | 1992 (2.7) | 0.71 | 7 (3.0) | 274 (1.9) | 0.23 | 7 (0.8) | 832 (1.4) | 0.09 |
| Any of the above adverse reproductive history | 124 (5.4) | 3810 (5.2) | 0.72 | 10 (4.3) | 385 (2.7) | 0.14 | 26 (2.8) | 2103 (3.5) | 0.22 |
| Maternal morbidity and vaccination during pregnancy: | 0.03 | ||||||||
| Influenza vaccination | N.A. | N.A. | 44 (18.7) | 3140 (21.9) | 0.009 | 115 (12.3) | 8818 (14.8) | ||
| Pneumococcal vaccination | N.A. | N.A. | 0 (0.0) | <6 | N.A. | 0 (0.0) | 0 (0.0) | ||
| Pertussis vaccination | N.A. | N.A. | 93 (39.6) | 5844 (40.7) | 0.73 | 0 (0.0) | 0 (0.0) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bérard, A.; Sheehy, O.; Kaul, P.; Eltonsy, S.; Walker, M.; Hawken, S.; Bernatsky, S.; Pugliese, M.; Barrett, O.; Savu, A.; et al. COVID-19 Infections Still Occur: How Do Pregnant and Non-Pregnant Individuals Compare? A Study from the Canadian Mother–Child Initiative on Drug Safety in Pregnancy (CAMCCO). Int. J. Environ. Res. Public Health 2025, 22, 1756. https://doi.org/10.3390/ijerph22111756
Bérard A, Sheehy O, Kaul P, Eltonsy S, Walker M, Hawken S, Bernatsky S, Pugliese M, Barrett O, Savu A, et al. COVID-19 Infections Still Occur: How Do Pregnant and Non-Pregnant Individuals Compare? A Study from the Canadian Mother–Child Initiative on Drug Safety in Pregnancy (CAMCCO). International Journal of Environmental Research and Public Health. 2025; 22(11):1756. https://doi.org/10.3390/ijerph22111756
Chicago/Turabian StyleBérard, Anick, Odile Sheehy, Padma Kaul, Sherif Eltonsy, Mark Walker, Steven Hawken, Sasha Bernatsky, Michael Pugliese, Olesya Barrett, Anamaria Savu, and et al. 2025. "COVID-19 Infections Still Occur: How Do Pregnant and Non-Pregnant Individuals Compare? A Study from the Canadian Mother–Child Initiative on Drug Safety in Pregnancy (CAMCCO)" International Journal of Environmental Research and Public Health 22, no. 11: 1756. https://doi.org/10.3390/ijerph22111756
APA StyleBérard, A., Sheehy, O., Kaul, P., Eltonsy, S., Walker, M., Hawken, S., Bernatsky, S., Pugliese, M., Barrett, O., Savu, A., & Dragan, R. (2025). COVID-19 Infections Still Occur: How Do Pregnant and Non-Pregnant Individuals Compare? A Study from the Canadian Mother–Child Initiative on Drug Safety in Pregnancy (CAMCCO). International Journal of Environmental Research and Public Health, 22(11), 1756. https://doi.org/10.3390/ijerph22111756






