Relationship Between Physical Activity and Autonomic Responses in Adults with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.2.1. International Physical Activity Questionnaire (IPAQ) Questionnaire
2.2.2. Anthropometric Assessment
2.2.3. HRV Assessment
2.3. Statistical Analysis
3. Results
3.1. Anthropometric and Metabolic Parameters
3.2. Resting Blood Pressure and Heart Rate
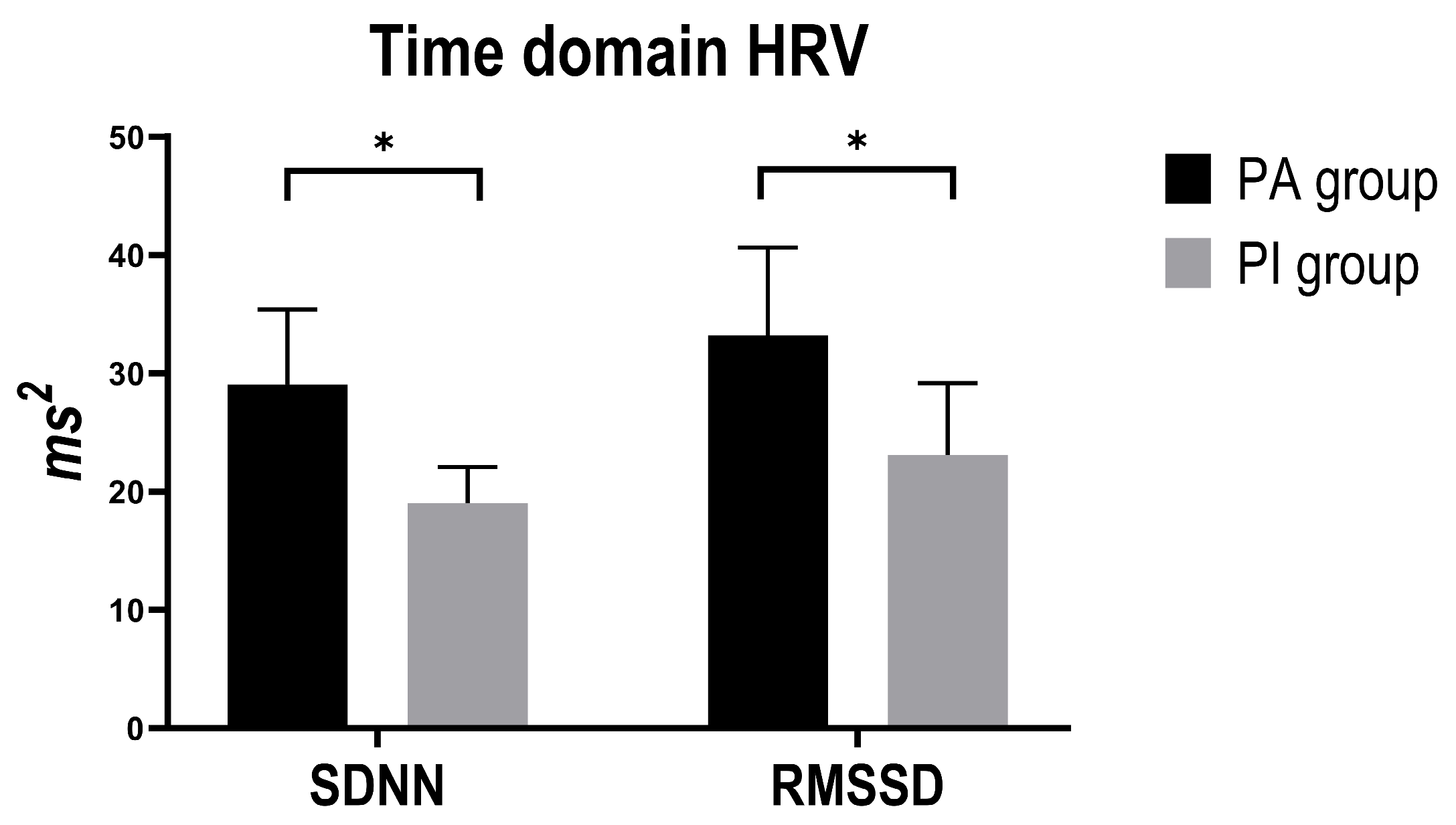
3.3. Time-Domain HRV Indices
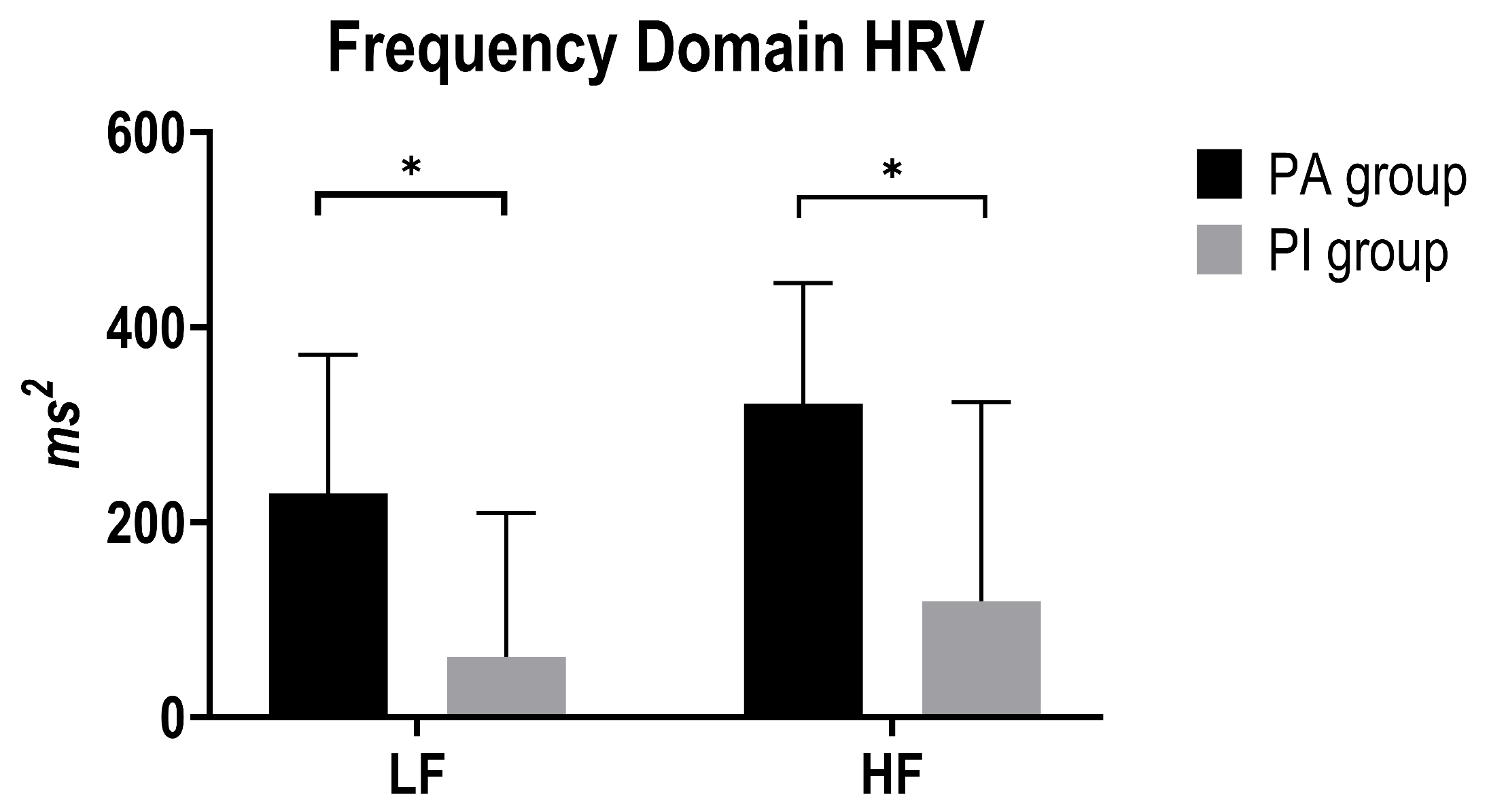
3.4. Frequency-Domain HRV Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| CI | Confidence Interval |
| fiDIA | Diastolic Blood Pressure |
| FGP | Fasting Glucose Panel |
| HbA1c | Glycated Hemoglobin |
| HDL | High-Density Lipoprotein |
| HF | High-Frequency Power (HRV) |
| HR | Heart Rate |
| HRV | Heart Rate Variability |
| IQR | Interquartile Range |
| IPAQ | International Physical Activity Questionnaire |
| IPAQ-S | International Physical Activity Questionnaire, Short Form |
| LDL | Low-Density Lipoprotein |
| LF | Low-Frequency Power (HRV) |
| LF/HF ratio | Low-Frequency/High-Frequency Ratio |
| MAP/fiMAP | Mean Arterial Pressure |
| Mean RR | Mean of Normal-to-Normal RR Intervals |
| MET | Metabolic Equivalent of Task |
| PA | Physically Active |
| PI | Physically Inactive |
| RMSSD | Root Mean Square of Successive Differences (HRV index) |
| fiSYS | Systolic Blood Pressure |
| SD | Standard Deviation |
| SDNN | Standard Deviation of Normal-to-Normal RR Intervals (HRV index) |
| SE | Standard Error |
| T2DM | Type 2 Diabetes Mellitus |
| WHO | World Health Organization |
References
- WHO. The Guidance on Global Monitoring for Diabetes Prevention and Control. Available online: https://www.who.int/publications/i/item/9789240102248 (accessed on 20 July 2025).
- 11th Edition|2025 Diabetes Atlas. Available online: https://diabetesatlas.org/resources/idf-diabetes-atlas-2025/ (accessed on 20 July 2025).
- Zhou, B.; Rayner, A.W.; Gregg, E.W.; Sheffer, K.E.; Carrillo-Larco, R.M.; Bennett, J.E.; Shaw, J.E.; Paciorek, C.J.; Singleton, R.K.; Pires, A.B.; et al. Worldwide trends in diabetes prevalence and treatment from 1990 to 2022: A pooled analysis of 1108 population-representative studies with 141 million participants. Lancet 2024, 404, 2077–2093. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Shadangi, S.; Gupta, P.K.; Rana, S. Type 2 Diabetes Mellitus: A Comprehensive Review of Pathophysiology, Comorbidities, and Emerging Therapies. Compr. Physiol. 2025, 15, e70003. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Wefers, J.; Meier, J.J. Treatment of type 2 diabetes: Challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 2021, 9, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Cannata, F.; Vadalà, G.; Russo, F.; Papalia, R.; Napoli, N.; Pozzilli, P. Beneficial Effects of Physical Activity in Diabetic Patients. J. Funct. Morphol. Kinesiol. 2020, 5, 70. [Google Scholar] [CrossRef]
- Chowdhury, M.; Nevitt, S.; Eleftheriadou, A.; Kanagala, P.; Esa, H.; Cuthbertson, D.J.; Tahrani, A.; Alam, U. Cardiac autonomic neuropathy and risk of cardiovascular disease and mortality in type 1 and type 2 diabetes: A meta-analysis. BMJ Open Diabetes Res. Care 2021, 9, e002480. [Google Scholar] [CrossRef]
- Eleftheriadou, A.; Williams, S.; Nevitt, S.; Brown, E.; Roylance, R.; Wilding, J.P.H.; Cuthbertson, D.J.; Alam, U. The prevalence of cardiac autonomic neuropathy in prediabetes: A systematic review. Diabetologia 2021, 64, 288–303. [Google Scholar] [CrossRef]
- Coopmans, C.; Zhou, T.L.; Henry, R.M.; Heijman, J.; Schaper, N.C.; Koster, A.; Schram, M.T.; van der Kallen, C.J.; Wesselius, A.; Engelsman, R.J.D.; et al. Both prediabetes and type 2 diabetes are associated with lower heart rate variability: The Maastricht Study. Diabetes Care 2020, 43, 1126–1133. [Google Scholar] [CrossRef]
- Benichou, T.; Pereira, B.; Mermillod, M.; Tauveron, I.; Pfabigan, D.; Maqdasy, S.; Dutheil, F. Heart rate variability in type 2 diabetes mellitus: A systematic review and meta–analysis. PLoS ONE 2018, 13, e0195166. [Google Scholar] [CrossRef]
- Zaki, S.; Alam, F.; Faizan, M.; Sharma, S.; Naqvi, I.H. Association between heart rate variability and cardiorespiratory fitness in individuals with type 2 diabetes mellitus: A cross-sectional study. J. Hum. Sport Exerc. 2024, 19, 779–791. [Google Scholar] [CrossRef]
- Kase, M.; Iijima, T.; Niitani, T.; Sagara, M.; Sakurai, S.; Tomaru, T.; Jojima, T.; Usui, I.; Aso, Y. Relationship between reduced heart rate variability and increased arterial stiffness evaluated by the cardio-ankle vascular index in people with type 2 diabetes. Diabetol. Int. 2023, 14, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Hu, L.; Xu, Y.; Wu, S.; Chen, Y.; Zou, W.; Zhang, M.; Wang, Y.; Gu, Y. Impact of blood glucose control on sympathetic and vagus nerve functional status in patients with type 2 diabetes mellitus. Acta Diabetol. 2020, 57, 141–150. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines on Physical Activity and Sedentary Behaviour; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240015128 (accessed on 20 July 2025).
- American Diabetes Association Professional Practice Committee. Improving care and promoting health in populations: Standards of Care in Diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S14–S26. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Marcotte-Chénard, A.; Little, J. Towards optimizing exercise prescription for type 2 diabetes: Modulating exercise parameters to strategically improve glucose control. Transl. Exerc. Biomed. 2024, 1, 71–88. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee; ElSayed, N.A.; Aleppo, G.; Bannuru, R.R.; Beverly, E.A.; Bruemmer, D.; Collins, B.S.; Darville, A.; Ekhlaspour, L.; Hassanein, M.; et al. 5. Facilitating Positive Health Behaviors and Well-being to Improve Health Outcomes: Standards of Care in Diabetes—2024. Diabetes Care 2024, 47 (Suppl. S1), S77–S110. [Google Scholar]
- Lu, J.; Cao, X.; Chang, X.; Zheng, G.; Zhu, H.; Gao, S.; Yang, Y. Associations between physical activity and all-cause and cardiovascular mortality in adults with type 2 diabetes mellitus: A prospective cohort study from NHANES 2007–2018. Prim. Care Diabetes 2024, 18, 44–51. [Google Scholar] [CrossRef]
- Ahmad, I.; Aung, M.N.; Ueno, S.; Khin, E.T.; Latt, T.S.; Moolphate, S.; Yuasa, M. Physical Activity of Type 2 Diabetes Mellitus Patients and Non-Diabetes Participants in Yangon, Myanmar: A Case-Control Study Applying the International Physical Activity Questionnaires (IPAQ-S). Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1729–1739. [Google Scholar] [CrossRef]
- Dempsey, P.C.; Friedenreich, C.M.; Leitzmann, M.F.; Buman, M.P.; Lambert, E.; Willumsen, J.; Bull, F. Global Public Health Guidelines on Physical Activity and Sedentary Behavior for People Living With Chronic Conditions: A Call to Action. J. Phys. Act. Health 2021, 18, 76–85. [Google Scholar] [CrossRef]
- Engin, B.; Willis, S.A.; Malaikah, S.; Sargeant, J.A.; Yates, T.; Gray, L.J.; Aithal, G.P.; Stensel, D.J.; King, J.A. The effect of exercise training on adipose tissue insulin sensitivity: A systematic review and meta-analysis. Obes. Rev. 2022, 23, e13445. [Google Scholar] [CrossRef]
- Nolan, R.C.; Raynor, A.J.; Berry, N.M.; May, E.J. Self-Reported Physical Activity Using the International Physical Activity Questionnaire (IPAQ) in Australian Adults with Type 2 Diabetes, with and Without Peripheral Neuropathy. Can. J. Diabetes 2016, 40, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Cortesi, M.; Di Michele, R.; Trofè, A.; Raffi, M. Lifetime Exposure to Recreational Swimming Training and its Effects on Autonomic Responses. Int. J. Sports Med. 2021, 42, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Plaza-Florido, A.; Sacha, J.; Alcantara, J.M.A. Associations between different heart rate variability ratios and cardiometabolic risk factors in young adults. Am. J. Hum. Biol. 2022, 34, e23797. [Google Scholar] [CrossRef]
- Papaioannou, V.; Pneumatikos, I.; Maglaveras, N. Association of heart rate variability and inflammatory response in patients with cardiovascular diseases: Current strengths and limitations. Front. Physiol. 2013, 4, 174. [Google Scholar] [CrossRef]
- Pagani, M. Heart rate variability and autonomic diabetic neuropathy. Diabetes Nutr. Metab. 2000, 13, 341–346. [Google Scholar]
- Picard, M.; Tauveron, I.; Magdasy, S.; Benichou, T.; Bagheri, R.; Ugbolue, U.C.; Navel, V.; Dutheil, F. Effect of exercise training on heart rate variability in type 2 diabetes mellitus patients: A systematic review and meta-analysis. Abdelbasset WK, curatore. PLoS ONE 2021, 16, e0251863. [Google Scholar] [CrossRef]
- Bloom, M.J.; Brown, L.M.; Jost, S.R.; Lang, A.S.I.D.; Mankin, N.V.; Mast, Z.W.; McMahan, E.R.; Merheb, J.A.; Nelson, P.P.; Nnaji, J.C.; et al. The Impact of Body Mass Index on Physical Activity and Cardiac Workload. bioRxiv 2021. [Google Scholar] [CrossRef]
- Carter, J.B.; Banister, E.W.; Blaber, A.P. Effect of Endurance Exercise on Autonomic Control of Heart Rate. Sports Med. 2003, 33, 33–46. [Google Scholar] [CrossRef]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart Rate Variability and Cardiac Vagal Tone in Psychophysiological Research—Recommendations for Experiment Planning, Data Analysis, and Data Reporting. Front. Psychol. 2017, 8, 213. [Google Scholar] [CrossRef]
- Soares-Miranda, L.; Sattelmair, J.; Chaves, P.; Duncan, G.E.; Siscovick, D.S.; Stein, P.K.; Mozaffarian, D. Physical Activity and Heart Rate Variability in Older Adults: The Cardiovascular Health Study. Circulation 2014, 129, 2100–2110. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef] [PubMed]
- Vinik, A.I.; Casellini, C.; Parson, H.K.; Colberg, S.R.; Nevoret, M.-L. Cardiac Autonomic Neuropathy in Diabetes: A Predictor of Cardiometabolic Events. Front. Neurosci. 2018, 12, 591. [Google Scholar] [CrossRef] [PubMed]
| PA Group (n. 22) | PI Group (n. 19) | |
|---|---|---|
| n. (%) | n. (%) | |
| Gender | M 5 (22.72) | M 8 (42.10) |
| Anti-diabetic drugs | 14 (63.3) | 9 (47.36) |
| Insulin use | 5 (22.72) | 2 (10.52) |
| Mean ± SD | Mean ± SD | |
| Age (years) | 62.36 ± 11.32 | 61.26 ± 9.86 |
| Weight (Kg) | 81.11 ± 12.93 | 89.73 ± 20.51 |
| Height (m) | 1.64 ± 0.08 | 1.66 ± 0.11 |
| BMI (Kg/m2) | 30.32 ± 5.69 | 32.24 ± 5.88 |
| Waistline (cm) | 103.41 ± 17.26 | 111.79 ± 15.01 |
| HbA1c (mmol/mol) | 54.50 ± 9.96 | 54.38 ± 16.34 |
| FGP (mg/dl) | 130.94 ± 43.39 | 141.5 ± 54.20 |
| Total Cholesterol (mg/dl) | 166.72 ± 34.35 | 171.94 ± 38.32 |
| HDL (mg/dl) | 53.39 ± 13.69 | 56.33 ± 15.25 |
| LDL (mg/dl) | 90.89 ± 27.60 | 90.57 ± 32.51 |
| Triglycerides (mg/dl) | 122.06 ± 62.45 | 138.06 ± 60.19 |
| Variables | Comparisons | Coefficient | Std. Error | t | P > |t| | 95% CI Lower | 95% CI Upper |
|---|---|---|---|---|---|---|---|
| Mean RR | PA vs. PI | −10.03 | 40.87 | −0.25 | 0.80 | −92.78 | 72.72 |
| BMI | −8.09 | 3.14 | −2.57 | 0.014 ** | −14.46 | −1.73 | |
| Mean HR | PA vs. PI | 0.87 | 3.98 | 0.22 | 0.83 | −7.19 | 8.92 |
| BMI | 0.75 | 0.39 | 2.22 | 0.033 * | 0.06 | 1.43 | |
| fiSYS | PA vs. PI | 8.27 | 6.35 | 1.30 | 0.202 | −4.65 | 21.20 |
| HbA1c | 0.55 | 0.27 | 2.02 | 0.052 | −0.01 | 1.11 | |
| SDNN | PA vs. PI | 11.34 | 4.69 | 2.42 | 0.021 * | 1.79 | 20.88 |
| HbA1c | 0.14 | 0.21 | 0.67 | 0.508 | −028 | 0.56 | |
| RMSSD | PA vs. PI | 11.12 | 5.41 | 2.06 | 0.048 * | 0.13 | 22.12 |
| HbA1c | 0.29 | 0.20 | 1.45 | 0.157 | −0.12 | 0.71 | |
| LF | PA vs. PI | 190.04 | 74.51 | 2.55 | 0.015 ** | 39.33 | 340.75 |
| HF | PA vs. PI | 167.63 | 94.57 | 1.77 | 0.086 | −24.76 | 360.03 |
| HbA1c | 5.16 | 4.80 | 1.08 | 0.29 | −4.60 | 14.92 | |
| LF/HF ratio | PA vs. PI | 0.13 | 0.19 | 0.65 | 0.518 | −0.27 | 0.53 |
| Triglycerides | −0.00 | 0.00 | −0.63 | 0.530 | −0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Persiani, M.; Laffi, A.; Piras, A.; Meoni, A.; Brodosi, L.; Nicastri, A.; Petroni, M.L.; Raffi, M. Relationship Between Physical Activity and Autonomic Responses in Adults with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2025, 22, 1702. https://doi.org/10.3390/ijerph22111702
Persiani M, Laffi A, Piras A, Meoni A, Brodosi L, Nicastri A, Petroni ML, Raffi M. Relationship Between Physical Activity and Autonomic Responses in Adults with Type 2 Diabetes. International Journal of Environmental Research and Public Health. 2025; 22(11):1702. https://doi.org/10.3390/ijerph22111702
Chicago/Turabian StylePersiani, Michela, Alessandra Laffi, Alessandro Piras, Andrea Meoni, Lucia Brodosi, Alba Nicastri, Maria Letizia Petroni, and Milena Raffi. 2025. "Relationship Between Physical Activity and Autonomic Responses in Adults with Type 2 Diabetes" International Journal of Environmental Research and Public Health 22, no. 11: 1702. https://doi.org/10.3390/ijerph22111702
APA StylePersiani, M., Laffi, A., Piras, A., Meoni, A., Brodosi, L., Nicastri, A., Petroni, M. L., & Raffi, M. (2025). Relationship Between Physical Activity and Autonomic Responses in Adults with Type 2 Diabetes. International Journal of Environmental Research and Public Health, 22(11), 1702. https://doi.org/10.3390/ijerph22111702









