Quantitative Assessment of Surge Capacity in Rwandan Trauma Hospitals: A Survey Using the 4S Framework
Abstract
1. Introduction
2. Materials and Methods
2.1. Definitions
2.2. Study Location and the Rwandan Health System
2.3. Surgical Capacity in Rwanda
2.4. Survey Design
2.5. Study Participants
2.6. Survey Distribution
2.7. Data Analysis
2.8. Ethics
3. Results
3.1. Hospital and Responder Characteristics
3.2. Descriptive Analysis of the 4S Domains
3.2.1. Staff
3.2.2. Stuff
3.2.3. Systems
3.2.4. Space
3.3. Intra-Class Comparison
3.4. Regression Analysis
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MCIs | Mass casualty incidents |
| LMICs | Low- and middle-income countries |
| NSOAP | National Surgical, Obstetric and Anaesthesia Plan |
| ED | Emergency Department |
| OR | Operating Room |
| HDU | High-Dependency Unit |
| ICU | Intensive Care Unit |
| DMAHS | Director of Medical and Allied Health Services |
| REDCAP | Research Electronic Data Capture |
| CI | Confidence Interval |
| ICC | Intraclass Correlation |
| ANOVA | Analysis of one-way variance |
| CT | Computer tomography |
| CHUB | Butare University Teaching Hospital |
| CHUK | Kigali University Teaching Hospital |
| EMS | Emergency Medical Services |
Appendix A
Appendix A.1
| Question # | Question | Answer Format | 4S Domain |
|---|---|---|---|
| 1a | In which hospital do you work? | (categorical: List of all available hospitals) | N/A (demography) |
| 1b | In which department do you work? | (categorical: emergency department, acute care surgery, surgery) | N/A (demography) |
| 1c | What is your work position in the department? | (categorical: matron/nurse, general practitioner, specialist physician) | N/A (demography) |
| 2 | Does your hospital/clinic have an MCI/surge capacity plan? | (categorical, Yes/No) | Systems |
| 3 | How many patients from an MCI do you think your department can receive at once and still maintain the regular standard of care? | (numeric) | N/A (perceptions) |
| 4 | Is there a standardised system to call in extra staff in case of MCIs? | (categorical, Yes/No/Don’t know) | Systems |
| 5a | If 1b = emergency department, do you have a triage system? | (categorical, Yes/No/Don’t know) | Systems |
| 5b | If 5a = yes, do you have a specific MCI triage system, other than the one normally used? | (categorical, Yes/No/Don’t know) | Systems |
| 6a | If 1b = ACS/Surgery & 1c = doctor: How many surgical teams can your hospital mobilise within 0.5 h? | (numeric) | Staff |
| 6b | If 1b = ACS/Surgery & 1c = doctor: How many surgical teams can your hospital mobilise within 2 h? | (numeric) | Staff |
| 6c | If 1b = ACS/Surgery & 1c = doctor: How many surgical teams can your hospital mobilise within 8 h? | (numeric) | Staff |
| 6d | If 1b = ACS/Surgery: During the last week, how many theatres, designed and equipped for and available to receive injured patients, did you have available? | (numeric) | Space |
| 7a | How many CT machines do you have access to within 1 h from patient admission (at your hospital or nearby) for urgent cases? | (numeric) | Stuff |
| 7b | During the last week, how often was the CT machine(s) operational/available when you needed it? | (categorical: Always/Sometimes/Rarely/Never) | Stuff |
| 8a | How many X-ray machines do you have access to within 1 h from patient admission (at your hospital or nearby) for urgent cases? | (numeric) | Stuff |
| 8b | During the last week, how often was the X-ray machine(s) operational/available when you needed it? | (categorical: Always/Sometimes/Rarely/Never) | Stuff |
| 9a | During the last week, was oxygen available when needed? | (categorical: Always/Sometimes/Rarely/Never) | Stuff |
| 9b | During the last week, was pulse oximetry available when needed? | (categorical: Always/Sometimes/Rarely/Never) | Stuff |
| 9c | During the last week, was blood pressure monitors available when needed? | (categorical: Always/Sometimes/Rarely/Never) | Stuff |
| 10a | Does your facility have O-negative blood components (“emergency blood”) stored in the emergency room for trauma use? | (categorical, Yes/No) | Stuff |
| 10b | If 10a = No; What is the average time (hours) does it take to get O-neg blood for emergency use? | (numeric) | Stuff |
| 10c | Do you have a blood bank on the hospital site? | (categorical, Yes/No) | Stuff |
| 11 | Do you have an MCI store or a storage room with extra equipment for MCI in the department? | (categorical, Yes/No) | Stuff/System |
| 12a | If 1b = ED, is there a physician available in the emergency department 24/7? | (categorical, Yes/No) | Staff |
| 12b | If 1b = ED, is there a specialist emergency physician available on call 24/7 (not in the hospital)? | (categorical, Yes/No) | Staff/System |
| 13a | Do you have capacity to monitor and treat patients with intensive care (in an ICU)? | (categorical, Yes/No) | Space/System |
| 13b | If 13a = yes, how many ICU beds do you have? | (numeric) | Space |
| 13b | Is there an ICU-physician available in the hospital 24/7? | (categorical, Yes/No) | Staff |
| 13c | Is there an ICU-physician available on call 24/7 (not in the hospital)? | (categorical, Yes/No) | Staff/System |
| 13d | Can you increase the number of ICU nurses and physicians during MCIs? | (categorical: Yes with specialist nurses/physicians; Yes, but not with specialists; No) | Staff/System |
| 14a | Can trauma-patients be monitored and treated with intermediate-care? | (categorical, Yes/No) | Space/System |
| 14b | If 14a = yes, how many HDU beds do you have? | (numeric) | Space |
| 15 | Is there anything else you would like to add? | Free text | N/A |
Appendix A.2
| Model Variables | Model |
|---|---|
| Number of manageable patients (numeric, log-transformed) | Outcome variable |
| Hospital (categorical) | Fixed-effect predictor |
| Professional role (categorical; (chief nurse as the reference category, general practitioner or specialist physician) | Random intercept (confounder) |
| Imaging access ((dichotomised to yes/no, with “yes” representing 24/7 access to x-ray and computer tomography (CT)) | Random intercept (confounder) |
| Access to critical resources (dichotomised to yes/no, with “yes” representing continuous access to monitors, oxygen, pulse oximetry, facility O-negative blood and/or an on-site blood bank) | Random intercept (confounder) |
| Access to ICU (dichotomised to yes/no, with “yes” indicating the existence of an ICU department in the hospital) | Random intercept (confounder) |
| Model Settings | |
| Modeling choice | General Least Square random-effects |
| Covariance structure | Exchangeable within group correlation structure (Stata default) |
Appendix A.3
| Frequency (%) or Mean (95% CI) | p-Value | ||
|---|---|---|---|
| Tertiary Hospitals | Secondary Hospitals | ||
| Staff | |||
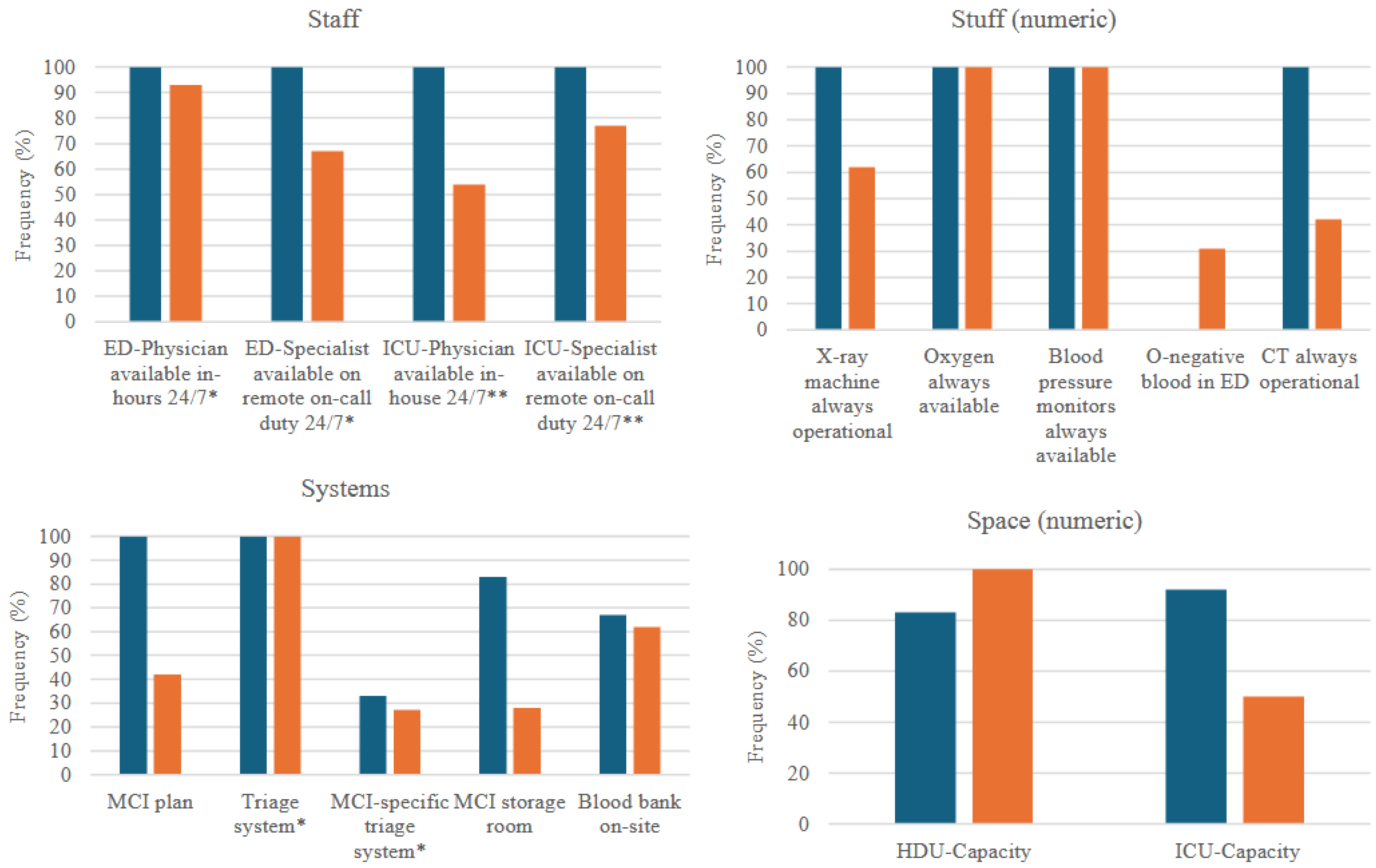
| ED-Physician available in-hours 24/7 * | 3/3 (100%) | 14/15 (93%) | 1.0 |
| ED-Specialist available on remote on-call duty 24/7 * | 3/3 (100%) | 10/15 (67%) | 0.5 |
| ICU-Physician available in-house 24/7 ** | 6/6 (100%) | 7/13 (54%) | 0.1 |
| ICU-Specialist available on remote on-call duty 24/7 ** | 6/6 (100%) | 10/13 (77%) | 0.5 |
| Stuff | |||
| Number of CT-machines | 2 (1–2) | 0.6 (0.4–0.8) | 0.01 |
| CT always operational | 6/6 (100%) | 11/26 (42%) | 0.02 |
| Number of X-ray machines | 2 (1–3) | 2 (1–2) | 0.4 |
| X-ray machine always operational | 6/6 (100%) | 16/26 (62%) | 0.1 |
| Oxygen always available | 6/6 (100%) | 26/26 (100%) | 1.0 |
| Blood pressure monitors always available | 6/6 (100%) | 26/26 (100%) | 1.0 |
| O-negative blood in ED | 0/6 (0%) | 8/26 (31%) | 0.3 |
| Time to blood delivery (h) | 3 (0.7–7) | 1 (0.4–3) | 0.5 |
| Systems | |||
| MCI plan | 6/6 (100%) | 11/26 (42%) | 0.04 |
| Triage system * | 3/3 (100%) | 15/15 (100%) | 1.0 |
| MCI-specific triage system * | 1/3 (33%) | 4/15 (27%) | 0.7 |
| MCI storage room | 5/6 (83%) | 7/25 (28%) | 0.02 |
| Blood bank on-site | 4/6 (67%) | 16/26 (62%) | 1.0 |
| Space | |||
| Number of operating rooms *** | 9 (5–13) | 1 (0.5–3) | 0.01 |
| HDU Capacity | 5/6 (83%) | 24/26 (92%) | 0.5 |
| ICU-Capacity | 6/6 (100%) | 13/26 (50%) | 0.1 |
References
- Barbera, J.; McIntyre, A. Jane’s Mass Casualty Handbook: Hospital. Emergency Preparedness and Response; Jane’s’ Information Group, Ltd.: Surrey, UK, 2013. [Google Scholar]
- Kelen, G.D.; McCarthy, M.L. The Science of Surge. Acad. Emerg. Med. 2006, 13, 1089–1094. [Google Scholar] [CrossRef]
- Barbisch, D.F.; Koenig, K.L. Understanding Surge Capacity: Essential Elements. Acad. Emerg. Med. 2006, 13, 1098–1102. [Google Scholar] [CrossRef]
- Bayram, J.D.; Zuabi, S.; Subbarao, I. Disaster Metrics: Quantitative Benchmarking of Hospital Surge Capacity in Trauma-Related Multiple Casualty Events. Disaster Med. Public Health Prep. 2011, 5, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Abir, M.; Choi, H.; Cooke, C.R.; Wang, S.C.; Davis, M.M. Effect of a Mass Casualty Incident: Clinical Outcomes and Hospital Charges for Casualty Patients Versus Concurrent Inpatients. Acad. Emerg. Med. 2012, 19, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.; Woods, D.D.; Reynolds, M.; Ting, H.-W.; Balkin, A.; Hsu, C.-W. Rethinking Preparedness Planning in Disaster Emergency Care: Lessons from a beyond-Surge-Capacity Event. World J. Emerg. Surg. WJES 2021, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Santomauro, D.F.; Aali, A.; Abate, Y.H.; Abbafati, C.; Abbastabar, H.; Abd ElHafeez, S.; Abdelmasseh, M.; Abd-Elsalam, S.; Abdollahi, A.; et al. Global Incidence, Prevalence, Years Lived with Disability (YLDs), Disability-Adjusted Life-Years (DALYs), and Healthy Life Expectancy (HALE) for 371 Diseases and Injuries in 204 Countries and Territories and 811 Subnational Locations, 1990–2021: A Systematic Analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2133–2161. [Google Scholar] [CrossRef]
- Samuel, J.C.; Akinkuotu, A.; Villaveces, A.; Charles, A.G.; Lee, C.N.; Hoffman, I.F.; Miller, W.C.; Baloyi, P.; Hoffman, M.; Brown, L.B.; et al. Epidemiology of Injuries at a Tertiary Care Center in Malawi. World J. Surg. 2009, 33, 1836–1841. [Google Scholar] [CrossRef]
- Botchey, I.M.J.; Hung, Y.W.; Bachani, A.M.; Paruk, F.; Mehmood, A.; Saidi, H.; Hyder, A.A. Epidemiology and Outcomes of Injuries in Kenya: A Multisite Surveillance Study. Surgery 2017, 162, S45–S53. [Google Scholar] [CrossRef]
- Rouhani, S.A.; Rimpel, L.; Plantin, J.J.; Calahan, C.F.; Julmisse, M.; Edmond, M.C.; Checkett, K.A.; Marsh, R.H. Mass Casualty Incident Management for Resource-Limited Settings: Lessons From Central Haiti. Disaster Med. Public Health Prep. 2022, 16, 770–776. [Google Scholar] [CrossRef]
- Petroze, R.T.; Joharifard, S.; Groen, R.S.; Niyonkuru, F.; Ntaganda, E.; Kushner, A.L.; Guterbock, T.M.; Kyamanywa, P.; Calland, J.F. Injury, Disability and Access to Care in Rwanda: Results of a Nationwide Cross-Sectional Population Study. World J. Surg. 2015, 39, 62–69. [Google Scholar] [CrossRef]
- Scott, J.W.; Nyinawankusi, J.D.; Enumah, S.; Maine, R.; Uwitonze, E.; Hu, Y.; Kabagema, I.; Byiringiro, J.C.; Riviello, R.; Jayaraman, S. Improving Prehospital Trauma Care in Rwanda Through Continuous Quality Improvement: An Interrupted Time Series Analysis. Injury 2017, 48, 1376–1381. [Google Scholar] [CrossRef]
- Notrica, M.R.; Evans, F.M.; Knowlton, L.M.; Kelly McQueen, K.A. Rwandan Surgical and Anesthesia Infrastructure: A Survey of District Hospitals. World J. Surg. 2011, 35, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Rwanda Ministry of Health. Rwanda National Surgical, Obstetrics, and Anesthesia Plan 2018–2024. 2018. Available online: https://www.moh.gov.rw/fileadmin/user_upload/Moh/Publications/Strategic_Plan/NSOAP_Rwanda-_Approved1.pdf (accessed on 13 March 2020).
- Meara, J.G.; Leather, A.J.M.; Hagander, L.; Alkire, B.C.; Alonso, N.; Ameh, E.A.; Bickler, S.W.; Conteh, L.; Dare, A.J.; Davies, J.; et al. Global Surgery 2030: Evidence and Solutions for Achieving Health, Welfare, and Economic Development. Lancet 2015, 386, 569–624. [Google Scholar] [CrossRef] [PubMed]
- Rwanda Ministry of Health. The 4-Year-Review Report of the Rwanda NSOAP Strategic Plan 2018–2024. 2023. Available online: https://moh.prod.risa.rw/index.php?eID=dumpFile&t=f&f=117766&token=43679a0e5a0ed3446d41eab885a791397932c1fa (accessed on 25 March 2025).
- Pyda, J.; Patterson, R.H.; Caddell, L.; Wurdeman, T.; Koch, R.; Polatty, D.; Card, B.; Meara, J.G.; Corlew, D.S. Towards Resilient Health Systems: Opportunities to Align Surgical and Disaster Planning. BMJ Glob. Health 2019, 4, e001493. [Google Scholar] [CrossRef] [PubMed]
- Mugabo, E.; Velin, L. Trauma Care Providers’ Perceptions of Surge Capacity in Mass-Casualty Incidents in Rwanda—A Qualitative Study; OAZIS Health: Kigali, Rwanda, 2025; submitted. [Google Scholar]
- Harris, P.A.; Taylor, R.; Thielke, R.; Payne, J.; Gonzalez, N.; Conde, J.G. Research Electronic Data Capture (REDCap)—A Metadata-Driven Methodology and Workflow Process for Providing Translational Research Informatics Support. J. Biomed. Inform. 2009, 42, 377–381. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef]
- Tilahun, L.; Desu, B.; Zeleke, M.; Dagnaw, K.; Andualem, A. Emergency and Disaster Handling Preparedness Among Front Line Health Service Providing Nurses and Associated Factors at Emergency Department, at Amhara Regional State Referral Hospitals, Ethiopia. Open Access Emerg. Med. OAEM 2021, 13, 221–232. [Google Scholar] [CrossRef]
- Stander, M.; Wallis, L.A.; Smith, W.P. Hospital Disaster Planning in the Western Cape, South Africa. Prehospital Disaster Med. 2011, 26, 283–288. [Google Scholar] [CrossRef]
- Söderin, L.; Agri, J.; Hammarberg, E.; Lennquist-Montán, K.; Montán, C. Hospital preparedness for major incidents in Sweden: A national survey with focus on mass casualty incidents. Eur. J. Trauma Emerg. Surg. 2023, 49, 635–651. [Google Scholar] [CrossRef]
- Murphy, J.P.; Kurland, L.; Rådestad, M.; Magnusson, S.; Ringqvist, T.; Rüter, A. Emergency department registered nurses overestimate their disaster competency: A cross-sectional study. Int. Emerg. Nurs. 2021, 58, 101019. [Google Scholar] [CrossRef]
- Koka, P.M.; Sawe, H.R.; Mbaya, K.R.; Kilindimo, S.S.; Mfinanga, J.A.; Mwafongo, V.G.; Wallis, L.A.; Reynolds, T.A. Disaster preparedness and response capacity of regional hospitals in Tanzania: A descriptive cross-sectional study. BMC Health Serv. Res. 2018, 18, 835. [Google Scholar] [CrossRef]
- Chokotho, L.; Jacobsen, K.H.; Burgess, D.; Labib, M.; Le, G.; Peter, N.; Lavy, C.B.D.; Pandit, H. A review of existing trauma and musculoskeletal impairment (TMSI) care capacity in East, Central, and Southern Africa. Injury 2016, 47, 1990–1995. [Google Scholar] [CrossRef]
- Kifle, F.; Boru, Y.; Tamiru, H.D.; Sultan, M.; Walelign, Y.; Demelash, A.; Beane, A.; Haniffa, R.; Gebreyesus, A.; Moore, J. Intensive Care in Sub-Saharan Africa: A National Review of the Service Status in Ethiopia. Anesth. Analg. 2022, 134, 930–937. [Google Scholar] [CrossRef]
- Sawe, H.R.; Mfinanga, J.A.; Lidenge, S.J.; Mpondo, B.C.; Msangi, S.; Lugazia, E.; Mwafongo, V.; Runyon, M.S.; Reynolds, T.A. Disease patterns and clinical outcomes of patients admitted in intensive care units of tertiary referral hospitals of Tanzania. BMC Int. Health Hum. Rights 2014, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Nisingizwe, M.P.; Ndishimye, P.; Swaibu, K.; Nshimiyimana, L.; Karame, P.; Dushimiyimana, V.; Musabyimana, J.P.; Musanabaganwa, C.; Nsanzimana, S.; Law, M.R. Effect of unmanned aerial vehicle (drone) delivery on blood product delivery time and wastage in Rwanda: A retrospective, cross-sectional study and time series analysis. Lancet Glob. Health 2022, 10, e564–e569. [Google Scholar] [CrossRef] [PubMed]
- Blimark, M.; Örtenwall, P.; Lönroth, H.; Mattsson, P.; Boffard, K.D.; Robinson, Y. Swedish emergency hospital surgical surge capacity to mass casualty incidents. Scand. J. Trauma Resusc. Emerg. Med. 2020, 28, 12. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Assessment of National Health Sector Emergency Preparedness and Response. 2008. Available online: https://www.who.int/docs/default-source/documents/global-assessment-of-national-health-sector-emergency-preparedness-and-response.pdf (accessed on 5 October 2025).
- Markin, A.; Barbero, R.; Leow, J.J.; Groen, R.S.; Perlman, G.; Habermann, E.B.; Apelgren, K.N.; Kushner, A.L.; Nwomeh, B.C. Inter-rater reliability of the PIPES tool: Validation of a surgical capacity index for use in resource-limited settings. World J. Surg. 2014, 38, 2195–2199. [Google Scholar] [CrossRef]
- Iverson, K.R.; Garringer, K.; Ahearn, O.; Alidina, S.; Citron, I.; Esseye, S.; Teshome, A.; Mukhopadhyay, S.; Burssa, D. Mixed-methods assessment of surgical capacity in two regions in Ethiopia. Br. J. Surg. 2019, 106, e81–e90. [Google Scholar] [CrossRef]
- Elective surgery system strengthening: Development, measurement, and validation of the surgical preparedness index across 1632 hospitals in 119 countries. Lancet 2022, 400, 1607–1617. [CrossRef]
| Hospital Name | Province | Hospital Classification | Catchment Population | Number of Hospital Beds * |
|---|---|---|---|---|
| University Teaching Hospital of Butare (CHUB) | Southern | Level 1 Teaching Hospital | 3.7 million | 500 |
| University Teaching Hospital of Kigali (CHUK) | Kigali | Level 1 Teaching Hospital | 6.2 million | 519 |
| Kibungo Level 2 Teaching Hospital | Eastern | Level 2 Teaching Hospital | 410,000 | 293 |
| Ruhengeri Level 2 Teaching Hospital | Northern | Level 2 Teaching Hospital | 406,557 | 328 |
| Kibuye Level 2 Teaching Hospital | Western | Level 2 Teaching Hospital | 197,491 | 206 |
| Butaro Level Two Teaching Hospital | Northern | Level 2 Teaching Hospital | 350,000 | 250 |
| Bushenge Provincial Hospital | Western | Provincial Hospital | 170,000 | 212 |
| Ruhango Provincial Hospital | Southern | Provincial Hospital | 228,992 | 192 |
| Rwamagana Level Two Teaching Hospital | Eastern | Level 2 Teaching Hospital | 369,671 | 242 |
| Kinihira Provincial Hospital | Northern | Provincial Hospital | 145,249 | 320 |
| Variable | ICC (95% CI) | Interpretation |
|---|---|---|
| MCI patient number capacity | 0.2 (0.0–0.6) | Poor reliability |
| Number of ORs | 0.0 (000–1.0) | Poor reliability |
| Number of CTs | 0.8 (0.5–1.0) | Moderate/good reliability |
| Number of X-ray machines | 0.1 (0–0.5) | Poor reliability |
| Time to blood delivery | 0.4 (0–0.9) | Poor reliability |
| Number of ICU beds | 0.8 (0.5–1) | Good reliability |
| Number of HDU beds | 0.2 (0.0–0.6) | Poor reliability |
| Model Parameter | Coefficient (95% CI) | p-Value |
|---|---|---|
| Constant | 9 (5–18) | |
| ICU availability | 1 (0.6–2) | 0.8 |
| Imaging availability | 3 (2–5) | <0.01 |
| Critical resource availability | 0.7 (0.4–1) | 0.09 |
| Professional role (nurse = reference category) -General practitioner -Specialist physician | 0.8 (0.5–1) 0.6 (0.4–1) | 0.5 0.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velin, L.; Nkeshimana, M.; Twizeyimana, E.; Nsanzimfura, D.; Wladis, A.; Pompermaier, L. Quantitative Assessment of Surge Capacity in Rwandan Trauma Hospitals: A Survey Using the 4S Framework. Int. J. Environ. Res. Public Health 2025, 22, 1559. https://doi.org/10.3390/ijerph22101559
Velin L, Nkeshimana M, Twizeyimana E, Nsanzimfura D, Wladis A, Pompermaier L. Quantitative Assessment of Surge Capacity in Rwandan Trauma Hospitals: A Survey Using the 4S Framework. International Journal of Environmental Research and Public Health. 2025; 22(10):1559. https://doi.org/10.3390/ijerph22101559
Chicago/Turabian StyleVelin, Lotta, Menelas Nkeshimana, Eric Twizeyimana, Didier Nsanzimfura, Andreas Wladis, and Laura Pompermaier. 2025. "Quantitative Assessment of Surge Capacity in Rwandan Trauma Hospitals: A Survey Using the 4S Framework" International Journal of Environmental Research and Public Health 22, no. 10: 1559. https://doi.org/10.3390/ijerph22101559
APA StyleVelin, L., Nkeshimana, M., Twizeyimana, E., Nsanzimfura, D., Wladis, A., & Pompermaier, L. (2025). Quantitative Assessment of Surge Capacity in Rwandan Trauma Hospitals: A Survey Using the 4S Framework. International Journal of Environmental Research and Public Health, 22(10), 1559. https://doi.org/10.3390/ijerph22101559







