Open Data Are Urgently Needed for One Health-Based Investigations: The Example of the 2024 Salmonella Umbilo Multi-Country Outbreak
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection from Accessible Portals
- The National Agriculture Information System (Sistema Informativo Agricolo Nazionale-SIAN) database of the Italian Ministry of Agriculture, Food Sovereignty and Forestry [10].
- The VETINFO portal, the Italian veterinary portal of the Italian Ministry of Health, developed by Istituto Zooprofilattico Sperimentale of Abruzzo and Molise (IZS AM) “Giuseppe Caporale”. This portal also integrates SIMAN–the National Information System for Animal Disease Notification, which is a centralized platform for the mandatory reporting of animal disease outbreaks in Italy, accessible only to authorized veterinary personnel [11].
2.2. Field Investigations and On-Site Observations
- Audit of the greenhouse owner focused on:
- Evaluation of irrigation water quality, with verification of potential microbial contamination.
- Environmental assessment of the surrounding area through field visits to identify possible sources of contamination, with a focus on drainage channels.
- Sampling of vegetables and water.
- Inspection of the area surrounding the greenhouses led to the discovery of an unauthorized and mismanaged manure storage tank, apparently intended for a nearby but unidentified livestock farm. Anticipating this key finding is essential for introducing the subsequent methods.
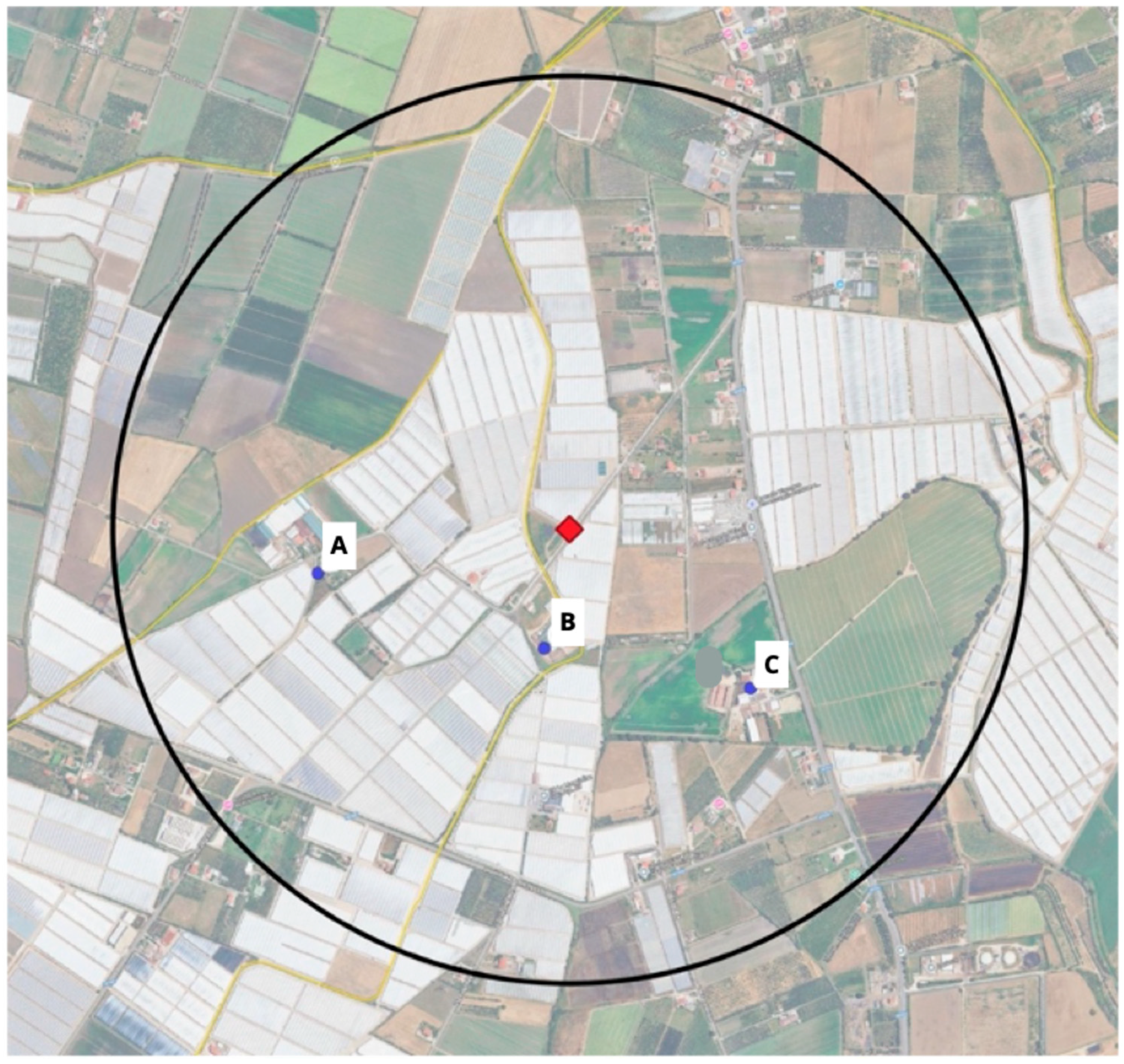
2.3. Georeferencing and Geospatial Mapping
- GPS coordinates of the rocket salad production site and irrigation channels.
- GPS coordinates of the unauthorized manure storage tank and surrounding wastewater areas.
- Locations of the nearest farms registered in the VETINFO portal, delineating a buffer zone of 1-km radius around the mismanaged manure storage tank.
2.4. Veterinary Fieldwork and Official Microbiological/Genomic Analyses
3. Results
- The S. Umbilo-infected buffalo farm;
- The other two establishments where enteritis in buffalo calves was detected;
- The unauthorized and mismanaged manure storage tank and irrigation channels.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Teklemariam, A.D.; Al-Hindi, R.R.; Albiheyri, R.S.; Alharbi, M.G.; Alghamdi, M.A.; Filimban, A.A.R.; Al Mutiri, A.S.; Al-Alyani, A.M.; Alseghayer, M.S.; Almaneea, A.M.; et al. Human Salmonellosis: A Continuous Global Threat in the Farm-to-Fork Food Safety Continuum. Foods 2023, 12, 1756. [Google Scholar] [CrossRef]
- EFSA and ECDC (European Food Safety Authority and European Centre for Disease Prevention and Control). The European Union One Health 2023 Zoonoses report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef] [PubMed]
- RASFF—The European Commission Rapid Alert System for Feed and Food. Available online: https://food.ec.europa.eu/food-safety/rasff_en (accessed on 10 June 2025).
- Rosner, B.M.; Simon, S.; Nielsen, S.; Köberl-Jelovcan, S.; Gymoese, P.; Werber, D.; Meinen, A.; Pietsch, M.; Flieger, A.; Fischer, J.; et al. Multinational investigation of a Salmonella Umbilo outbreak reveals rocket salad and baby spinach as the likely infection vehicles, Europe, 2024. Euro Surveill. 2024, 29, 2400728. [Google Scholar] [CrossRef]
- Lapage, S.P.; Taylor, J.; Nicewonger, C.R.; Phillips, A.G. New serotypes of Salmonella identified before 1964 at the Salmonella reference laboratory, Colindale. Int. J. Syst. Evol. Microbiol. 1996, 16, 253–298. [Google Scholar] [CrossRef]
- Grimont, P.A.; Weill, F.X. Antigenic formulae of the Salmonella serovars. In WHO Collaborating Centre for Reference and Research on Salmonella; WHO: Geneva, Switzerland, 2007; Volume 9, pp. 1–166. Available online: https://www.pasteur.fr/sites/default/files/veng_0.pdf (accessed on 17 May 2025).
- Mazzeo, A.; Mascolo, C.; Maiuro, L.; Esposito, M.; Ferrara, C.; Rossi, N.; Di Chiro, V.; Rosati, S.; Sorrentino, E. Brucellosis in cattle and buffalo in southern Italian provinces: Trends in presence of territory-specific One Health measures. Front. Microb. 2025, 16, 1609336. [Google Scholar] [CrossRef]
- ECDC—The European Centre for Disease Prevention and Control. Available online: https://www.ecdc.europa.eu/en (accessed on 2 June 2025).
- EFSA—The European Food Safety Authority. Available online: https://open.efsa.europa.eu/ (accessed on 3 June 2025).
- SIAN. The Italian Ministry of Agriculture, Food Sovereignty and Forestry Database. Available online: https://www.sian.it/portale/ (accessed on 31 May 2025).
- VETINFO, the Italian Veterinary Portal. Available online: https://www.vetinfo.it/ (accessed on 17 May 2025).
- ISTAT. National Institute of Statistics. In GIstat—Hydrographic Network and Water Canals; Version 2023, Retrieved from ISTAT GIstat Portal; ISTAT: Rome, Italy, 2023; Available online: https://www.istat.it/ (accessed on 10 June 2025).
- European Union Centre for Disease Prevention and Control. Atlas. Available online: https://atlas.ecdc.europa.eu/ (accessed on 2 June 2025).
- Luppi, A.; Torreggiani, C.; Prosperi, A.; Arrigoni, M. Manuale Operativo per la Gestione di Casi di Salmonellosi nell’Allevamento della Bovina da Latte. Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna and Servizio Sanitario Regionale dell’Emilia-Romagna. 2023. Available online: https://www.izsler.it/wp-content/uploads/sites/2/2023/09/Manuale-Operativo-Salmonellosi-nellallevamento-Bovino.pdf (accessed on 2 July 2025).
- Mazzeo, A.; Tremonte, P.; Lombardi, S.J.; Caturano, C.; Correra, A.; Sorrentino, E. From the Intersection of Food-Borne Zoonoses and EU Green Policies to an In-Embryo One Health Financial Model. Foods 2022, 11, 2736. [Google Scholar] [CrossRef]
- CAMPANIA REGION. Mandatory Eradication Programme for Infectious Diseases in Cattle and Buffalo in Campania (DGRC n. 104/2022). Available online: https://oev.izsmportici.it/dgrc-104-2022-approvazione-del-programma-obbligatorio-di-eradicazione-delle-malattie-infettive-delle-specie-bovina-e-bufalina-in-regione-campania/ (accessed on 2 July 2025).
- Italian Ministry of Health. Decree of 2 May 2024—Adoption of the Mandatory National Programmes for the Eradication of Brucellosis and Tuberculosis in Cattle and Brucellosis in Sheep and Goats; Official Gazette of the Italian Republic: Rome, Italy, 2024; pp. 17–20. Available online: https://www.izsler.it/tbcentro/wp-content/uploads/sites/19/2024/12/interno_IZS_Brucellosi.pdf (accessed on 18 June 2025).
- Italian Ministry of Health. Order No. 1/2025, of the National Extraordinary Commissioner for the Control and Eradication of Bovine, Buffalo, Ovine, and Caprine Brucellosis, and Bovine and Buffalo Tuberculosis. Strengthening Measures for the Eradication of Water Buffalo Brucellosis in the Province of Caserta; Official Gazette, General Series No. 60 of 13 March 2025; Italian Ministry of Health: Rome, Italy, 2025; Available online: https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2025-03-13&atto.codiceRedazionale=25A01570&elenco30giorni=false (accessed on 18 June 2025).
- Bilei, S.; Salinetti, A.P.; Tolli, R.; Di Giampietro, G.; Marrocco, M.G. Salmonella Rapporto regionale sulla sorveglianza di laboratorio—Anno 2004 Centro di Riferimento Regionale per gli Enterobatteri Patogeni Istituto Zooprofilattico Sperimentale delle Regioni Lazio e Toscana Via Appia Nuova, 1411 00178 Roma. Available online: https://www.izslt.it/crep/wp-content/uploads/sites/6/2017/03/crep_report_2004.pdf (accessed on 19 June 2025).
- Gambi, L.; Ravaioli, V.; Rossini, R.; Tranquillo, V.; Boscarino, A.; Mattei, S.; D’incau, M.; Tosi, G.; Fiorentini, L.; Donato, A.D. Prevalence of Different Salmonella enterica Subspecies and Serotypes in Wild Carnivores in Emilia-Romagna Region, Italy. Animals 2022, 12, 3368. [Google Scholar] [CrossRef]
- Peruzy, M.F.; La Tela, I.; Carullo, M.R.; Ioele, S.; Proroga, Y.T.R.; Balestrieri, A.; Murru, N. Occurrence and distribution of Salmonella serovars associated with human infection isolated from irrigation waters and food-producing animals in southern Italy: Eleven-year monitoring (2011–2021). Ital. J. Food Saf. 2023, 12, 11538. [Google Scholar] [CrossRef]
- Borriello, G.; Lucibelli, M.G.; Pesciaroli, M.; Carullo, M.R.; Graziani, C.; Ammendola, S.; Battistoni, A.; Ercolini, D.; Pasquali, P.; Galiero, G. Diversity of Salmonella spp. serovars isolated from the intestines of water buffalo calves with gastroenteritis. BMC Vet. Res. 2012, 8, 201. [Google Scholar] [CrossRef]
- Sagoo, S.K.; Little, C.L.; Ward, L.; Gillespie, I.A.; Mitchell, R.T. Microbiological study of ready-to-eat salad vegetables from retail establishments uncovers a national outbreak of salmonellosis. J. Food Prot. 2003, 66, 403–409. [Google Scholar] [CrossRef]
- Guerin, P.J.; De Jong, B.; Heir, E.; Hasseltvedt, V.; Kapperud, G.; Sturmo, K.; Gondrosen, B.; Lassen, J.; Andersson, Y.; Aavitsland, P. Outbreak of Salmonella Livingstone infection in Norway and Sweden due to contaminated processed fish products. Epidemiol. Infect. 2004, 132, 889–895. [Google Scholar] [CrossRef]
- Bouallègue-Godet, O.; Salem, Y.B.; Fabre, L.; Demartin, M.; Grimont, P.A.D.; Mzoughi, R.; Weill, F. Nosocomial. Outbreak Caused by Salmonella enterica Serotype Livingstone Producing CTX-M-27 Extended-Spectrum β-Lactamase in a Neonatal Unit in Sousse, Tunisia. J Clin Microbiol. 2005, 43, 1037–1044. [Google Scholar] [CrossRef]
- Hu, Q.; Coburn, B.; Deng, W.; Li, Y.; Shi, X.; Lan, Q.; Wang, B.; Coombes, B.K.; Finlay, B.B. Salmonella enterica serovar Senftenberg human clinical isolates lacking SPI-1. J. Clin. Microbiol. 2008, 46, 1330–1336. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. 92 Salmonella Senftenberg cases reported in 11 EU/EEA Countries. 2023. Available online: https://www.ecdc.europa.eu/en/news-events/92-salmonella-senftenberg-cases-reported-11-eueea-countries (accessed on 16 July 2025).
- Keisling, C.; Hatfield, J.; Moore, D.; Graves, S.; Smith, B.; Wagner, J.; Casey, R.; Young, E.L.; Oakeson, K.; Lanier, W. Notes from the Field: Rapid Linkage of a Salmonella Livingstone Outbreak to a Restaurant, Using Open-Ended Interviews and Patient Purchase Histories—Utah, 2023–2024. MMWR 2024, 73, 536–537. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, A.; Tremonte, P.; Rossi, N.; Ferrara, C.; Mascolo, C.; Lombardi, S.J.; Sorrentino, E. Modulation of the One Health Approach to Tackle Brucellosis in Buffaloes and Cattle in Two Italian Territories with Different Characteristics. J. Buffalo Sci. 2023, 12, 55–69. [Google Scholar] [CrossRef]
- Oh, H.; Choi, Y.; Lee, J. Antibiotic-Resistant Salmonella in Animal Products Jeopardize Human Health. Food Sci. Anim. Resour. 2025, 45, 409. [Google Scholar] [CrossRef]
- Ibrahim, H.S.; Abdelhamed, G.S.; Elgohary, A.H.; Fathi, M. Relationship between virulence and antibiotic resistant genes in some Gram negative bacteria causing diarrhea in calves. J. Adv. Vet. Res. 2025, 15, 6–12. [Google Scholar]
- Dafale, N.A.; Srivastava, S.; Purohit, H.J. Zoonosis: An Emerging Link to Antibiotic Resistance Under "One Health Approach". Indian J. Microbiol. 2020, 60, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Sekhwal, M.K.; Li, L.; Pierre, T.; Matthews, T.; Luley, E.; Tewari, D.; Kuchipudi, S.V.; Jayarao, B.; Byukusenge, M. Molecular Epidemiology of Salmonella enterica Serotype Dublin Isolated from 2011 to 2022 from Veal and Dairy Cattle in Pennsylvania. Microorganisms 2025, 13, 400. [Google Scholar] [CrossRef] [PubMed]
- Campioni, F.; Vilela, F.P.; Cao, G.; Kastanis, G.; dos Prazeres Rodrigues, D.; Costa, R.G.; Tiba-Casas, M.R.; Yin, L.; Allard, M.; Falcão, J.P. Whole genome sequencing analyses revealed that Salmonella enterica serovar Dublin strains from Brazil belonged to two predominant clades. Sci. Rep. 2022, 12, 10555. [Google Scholar] [CrossRef]
- Velasquez-Munoz, A.; Castro-Vargas, R.; Cullens-Nobis, F.M.; Mani, R.; Abuelo, A. Review: Salmonella Dublin in dairy cattle. Front. Vet. Sci. 2024, 10, 1331767. [Google Scholar] [CrossRef] [PubMed]
- Holschbach, C.L.; Breuer, R.M.; Pohly, A.E.; Crawford, C.; Aulik, N.A. Multi-drug resistant Salmonella ser. Dublin cultured from cryopreserved Holstein semen. Vet. Rec. Case Rep. 2024, 12, e791. [Google Scholar] [CrossRef]
- National Reference Centre (NRC) for Salmonella—Istituto Zooprofilattico Sperimentale Delle Venezie. Protocollo per la Gestione di un Focolaio di Salmonellosi negli Allevamenti di Bovine da Latte. Available online: https://www.izsvenezie.it/documenti/temi/salmonellosi/protocollo-focolai-salmonella-bovine-latte.pdf (accessed on 3 July 2025).
- National Control Plan for Salmonellosis in Poultry 2025-2027 (PNCSA)—Italian Ministry of Heath. Available online: https://www.izsvenezie.it/documenti/temi/salmonellosi/normativa/piani-di-controllo/piani-di-controllo/piano-nazionale-salmonellosi-2025.pdf (accessed on 29 June 2025).
- Zizza, A.; Fallucca, A.; Guido, M.; Restivo, V.; Roveta, M.; Trucchi, C. Foodborne Infections and Salmonella: Current Primary Prevention Tools and Future Perspectives. Vaccines 2025, 13, 29. [Google Scholar] [CrossRef] [PubMed]
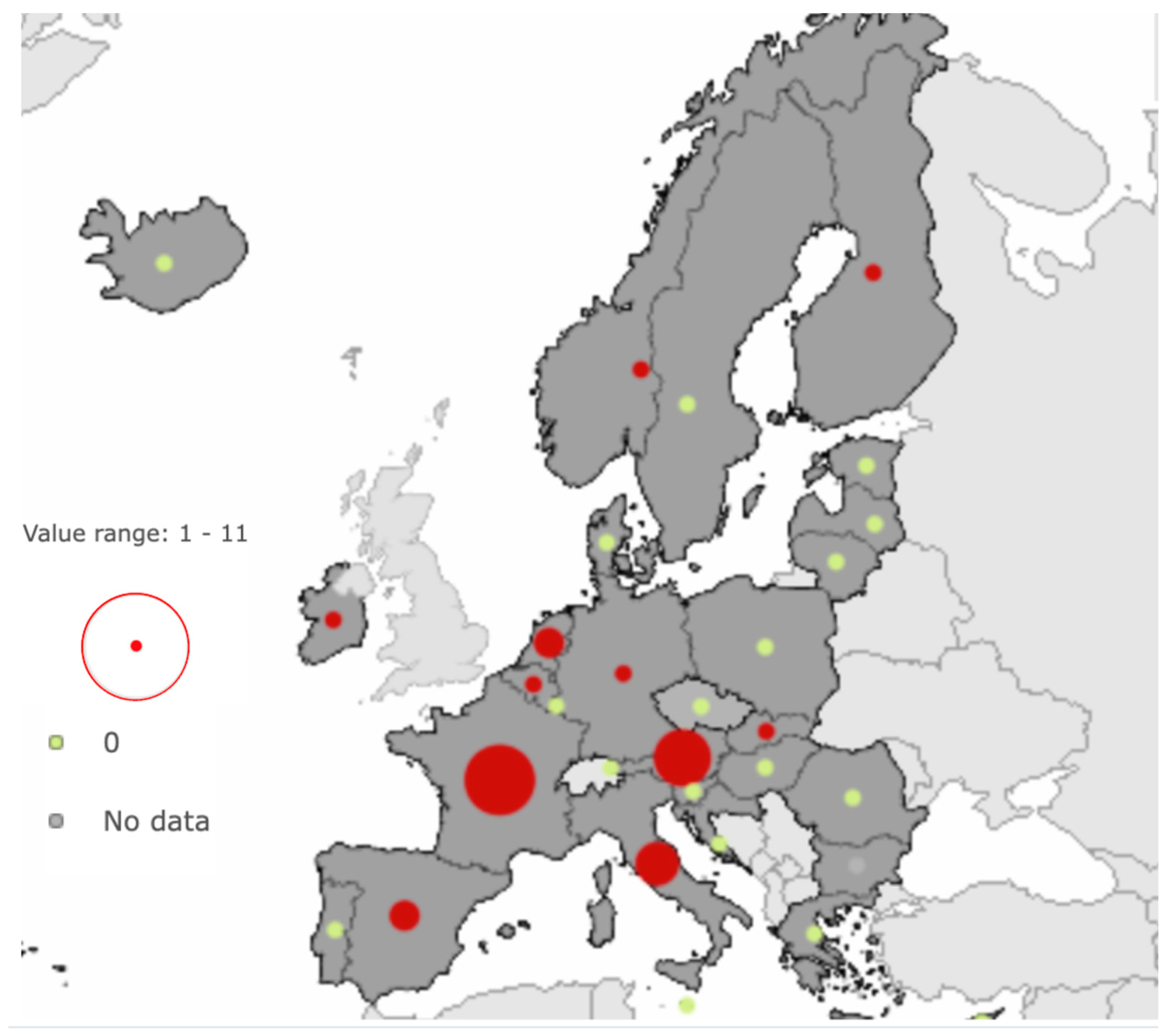
| EU/EEA MEMBER STATES | 2020 | 2021 | 2022 | 2023 |
|---|---|---|---|---|
| No. of Human Cases | No. of Human Cases | No. of Human Cases | No. of Human Cases | |
| AUSTRIA | 4 | 0 | 2 | 4 |
| BELGIUM | 1 | 1 | 0 | 1 |
| CROATIA | 0 | 0 | 1 | 0 |
| CZECHIA | 0 | 1 | 0 | 0 |
| FINLAND | 1 | 0 | 0 | 1 |
| FRANCE | 5 | 5 | 5 | 5 |
| GERMANY | 0 | 2 | 3 | 1 |
| IRELAND | 1 | 0 | 0 | 1 |
| ITALY | 3 | 1 | 0 | 3 |
| MALTA | 0 | 0 | 1 | 0 |
| NETHERLANDS | 2 | 1 | 2 | 2 |
| NORWAY | 1 | 0 | 0 | 1 |
| SLOVAKIA | 1 | 0 | 0 | 1 |
| SLOVENIA | 0 | 0 | 4 | 0 |
| SPAIN | 0 | 4 | 0 | 0 |
| SWEDEN | 0 | 0 | 5 | 0 |
| TOTAL | 19 | 15 | 23 | 20 |
| Salmonella Serotypes | Infection in Buffaloes | RASSF Alerts | Location of Salmonella-Positive Greenhouses and/or Buffalo Farms in Province of Salerno |
|---|---|---|---|
| S. Umbilo | YES | RASFF Alert No. 2024.7033 ROCKET SALAD related to the 2024 multi-country outbreak | EBOLI Site of origin of the multi-country outbreak 2024 |
| S. Umbilo | YES | RASFF Alert No. 2024.7478 LEAF SPINACH related to the 2024 multi-country outbreak | EBOLI Site of origin of the multi-country outbreak 2024 |
| S. Livingstone | YES | RASFF Alert No. 2024.8024 ROCKET SALAD not related to human outbreaks | EBOLI Site of origin of the multi-country outbreak 2024 |
| S. Senftenberg | YES | NO RASFF ALERT | EBOLI Site of origin of the multi-country outbreak 2024 |
| S. Napoli | Not included in the epidemiological investigation | RASFF Alert No. 2024.8347 ROCKET SALAD | BATTIPAGLIA and PONTECAGNANO FAIANO |
| S. Stanleyville | Not included in the epidemiological investigation | RASFF Alert No. 2024.8772 ROCKET SALAD | PONTECAGNANO FAIANO |
| S. Napoli | Not included in the epidemiological investigation | RASFF Alert No. 2025.3978 ROCKET SALAD | BELLIZZI |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazzeo, A.; Mascolo, C.; Esposito, M.; Maiuro, L.; Rosati, S.; Sorrentino, E. Open Data Are Urgently Needed for One Health-Based Investigations: The Example of the 2024 Salmonella Umbilo Multi-Country Outbreak. Int. J. Environ. Res. Public Health 2025, 22, 1478. https://doi.org/10.3390/ijerph22101478
Mazzeo A, Mascolo C, Esposito M, Maiuro L, Rosati S, Sorrentino E. Open Data Are Urgently Needed for One Health-Based Investigations: The Example of the 2024 Salmonella Umbilo Multi-Country Outbreak. International Journal of Environmental Research and Public Health. 2025; 22(10):1478. https://doi.org/10.3390/ijerph22101478
Chicago/Turabian StyleMazzeo, Alessandra, Celestina Mascolo, Marco Esposito, Lucia Maiuro, Sebastiano Rosati, and Elena Sorrentino. 2025. "Open Data Are Urgently Needed for One Health-Based Investigations: The Example of the 2024 Salmonella Umbilo Multi-Country Outbreak" International Journal of Environmental Research and Public Health 22, no. 10: 1478. https://doi.org/10.3390/ijerph22101478
APA StyleMazzeo, A., Mascolo, C., Esposito, M., Maiuro, L., Rosati, S., & Sorrentino, E. (2025). Open Data Are Urgently Needed for One Health-Based Investigations: The Example of the 2024 Salmonella Umbilo Multi-Country Outbreak. International Journal of Environmental Research and Public Health, 22(10), 1478. https://doi.org/10.3390/ijerph22101478









