The Burden of Lip and Oral Cavity Cancer Among Women Across 204 Countries and Territories in the Context of the Framework Convention on Tobacco Control: An Interrupted Time Series Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.1.1. MPOWER Scores
2.1.2. Lip and Oral Cavity Cancer Rates
2.1.3. Socio-Demographic Index (SDI)
2.2. Study Design
2.3. Statistical Analysis
- If the APC and its 95% CI are positive, the trend or slope is increasing.
- If the APC and its 95% CI are negative, the trend or slope is decreasing.
- If the 95% CI crosses zero, the trend or slope is considered stable, indicating no significant change.
3. Results
Analysis by Socio-Demographic Index
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LOC | Lip and oral cavity cancer |
| WHO-FCTC | World Health Organization Framework Convention on Tobacco Control |
| DALYs | Disability-adjusted life years |
| SDI | Socio-demographic Index |
| APC | Annual Percent Change |
| 95% CI | 95% Confidence Interval |
| IQR | Interquartile range |
| ST | Smokeless tobacco |
| PAF | Population attributable fraction |
References
- Warnakulasuriya, S.; Kerr, A.R. Oral cancer screening: Past, present, and future. J. Dent. Res. 2021, 100, 1313–1320. [Google Scholar] [CrossRef]
- GBD 2019 Lip, Oral, and Pharyngeal Cancer Collaborators. The global, regional, and national burden of adult lip, oral, and pharyngeal cancer in 204 countries and territories: A systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009, 45, 309–316. [Google Scholar] [CrossRef]
- Akashanand; Zahiruddin, Q.S.; Jena, D.; Ballal, S.; Kumar, S.; Bhat, M.; Sharma, S.; Kumar, M.R.; Rustagi, S.; Gaidhane, A.M.; et al. Burden of oral cancer and associated risk factors at national and state levels: A systematic analysis from the Global Burden of Disease in India, 1990–2021. Oral Oncol. 2024, 159, 107063. [Google Scholar] [CrossRef]
- Bosetti, C.; Carioli, G.; Santucci, C.; Bertuccio, P.; Gallus, S.; Garavello, W.; Negri, E.; La Vecchia, C. Global trends in oral and pharyngeal cancer incidence and mortality. Int. J. Cancer 2020, 147, 1040–1049. [Google Scholar] [CrossRef]
- Rai, P.; Ng, A.; Intekhab, I.; Sim, Y.F.; Lai, C.W.M.; Loh, J.S.P. Oral cancer in Asia: A systematic review. Adv. Oral Maxillofac. Surg. 2022, 8, 100366. [Google Scholar] [CrossRef]
- Ramadan, S.; Mokhtari, T.E.; Al-Qurayshi, Z.; Rich, J.T.; Harbison, R.A.; Zolkind, P.; Jackson, R.S.; Pipkorn, P.; Kang, S.Y.; Mazul, A.L.; et al. Trends in incidence of oral cavity squamous cell carcinoma in the United States, 2001–2019. Surg. Oncol. Insights 2024, 1, 100055. [Google Scholar] [CrossRef]
- Sartori, L.R.M.; Nóbrega, K.H.S.; Schuch, H.S.; Cademartori, M.G.; de Arruda, J.A.A.; Martins, M.D.; Schuch, L.F.; Vasconcelos, A.C.U. Temporal trends of women with oral cavity, base of tongue and lip cancers in Brazil: An ecological study covering mortality data from 1980 to 2018. Community Dent. Oral Epidemiol. 2023, 51, e12731. [Google Scholar] [CrossRef]
- Bosetti, C.; Negri, E.; Franceschi, S.; Conti, E.; Levi, F.; Tomei, F.; La Vecchia, C. Risk factors for oral and pharyngeal cancer in women: A study from Italy and Switzerland. Br. J. Cancer 2000, 82, 204–207. [Google Scholar] [CrossRef]
- Shrestha, G.; Chang, C.-P.; Pun, C.B.; Gautam, D.K.; Siwakoti, B.; Sapkota, A.; Hashibe, M. Differences in risk factors for head and neck cancer among men and women in Nepal: A case-control study. Cancer Epidemiol. 2022, 82, 102319. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Living with oral cancer: Epidemiology with particular reference to prevalence and lifestyle changes that influence survival. Oral Oncol. 2010, 46, 407–410. [Google Scholar] [CrossRef]
- Paraje, G.; Muñoz, M.F.; Wu, D.C.; Jha, P. Reductions in smoking due to ratification of the Framework Convention for Tobacco Control in 171 countries. Nat. Med. 2024, 30, 683–689. [Google Scholar] [CrossRef]
- WHO. WHO Report on the Global Tobacco Epidemic, 2023: Protect People from Tobacco Smoke. 2023. Available online: http://www.who.int/publications (accessed on 1 March 2025).
- da Silva, B.M.; Hagen, L.M.; da Cunha, A.R.; Hugo, F.N.; Amenábar, J.M. Incidence, mortality and oral cancer disability-adjusted life years in 204 countries and territories before and after the adoption of FCTC/WHO: Interrupted time series study. Tob. Control, 2025; advance online publication. [Google Scholar] [CrossRef]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- Hategeka, C.; Ruton, H.; Karamouzian, M.; Lynd, L.D.; Law, M.R. Use of interrupted time series methods in the evaluation of health system quality improvement interventions: A methodological systematic review. BMJ Glob. Health 2020, 5, e003567. [Google Scholar] [CrossRef]
- WHO. Global Health Observatory. Available online: http://www.who.int/data/gho (accessed on 1 March 2025).
- Institute for Health Metrics and Evaluation (IHME). GBD 2021 Results. Available online: http://vizhub.healthdata.org/gbd-results/ (accessed on 1 March 2025).
- Institute for Health Metrics and Evaluation (IHME). Global Health Data Exchange (GHDx). Available online: http://ghdx.healthdata.org/ (accessed on 1 March 2025).
- Antunes, J.L.F.; Cardoso, M.R.A. Using time series analysis in epidemiological studies. Epidemiol. Serv. Saude 2015, 24, 565–576. [Google Scholar] [CrossRef]
- Antunes, J.L.; Waldman, E.A. Trends and spatial distribution of deaths of children aged 12–60 months in São Paulo, Brazil, 1980–98. Bull. World Health Organ. 2002, 80, 391–398. [Google Scholar]
- Flor, L.S.; Reitsma, M.B.; Gupta, V.; Ng, M.; Gakidou, E. The effects of tobacco control policies on global smoking prevalence. Nat. Med. 2021, 27, 239–243. [Google Scholar] [CrossRef]
- Lyle, G.; Hendrie, D. Global smoking-related deaths averted due to MPOWER policies implemented at the highest level between 2007 and 2020. Global. Health 2024, 20, 40. [Google Scholar] [CrossRef]
- Ngo, A.; Cheng, K.W.; Chaloupka, F.J.; Shang, C. The effect of MPOWER scores on cigarette smoking prevalence and consumption. Prev. Med. 2017, 105, 10–14. [Google Scholar] [CrossRef]
- Ren, Z.H.; Hu, C.Y.; He, H.R.; Li, Y.J.; Lyu, J. Global and regional burdens of oral cancer from 1990 to 2017: Results from the Global Burden of Disease Study. Cancer Commun. 2020, 40, 81–92. [Google Scholar] [CrossRef]
- Wu, J.; Chen, H.; Liu, Y.; Yang, R.; An, N. The global, regional, and national burden of oral cancer, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. J. Cancer Res. Clin. Oncol. 2025, 151, 53. [Google Scholar] [CrossRef]
- Hernández-Morales, A.; González-López, B.S.; Scougall-Vilchis, R.J.; Bermeo-Escalona, J.R.; Velázquez-Enríquez, U.; Islas-Zarazúa, R.; Márquez-Rodríguez, S.; Sosa-Velasco, T.A.; Medina-Solís, C.E.; Maupomé, G. Lip and oral cavity cancer incidence and mortality rates associated with smoking and chewing tobacco use and the human development index in 172 countries worldwide: An ecological study 2019–2020. Healthcare 2023, 11, 1063. [Google Scholar] [CrossRef]
- GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Mackay, J.; Amos, A. Women and tobacco. Respirology 2003, 8, 123–130. [Google Scholar] [CrossRef]
- Pathania, V. Women and the smoking epidemic: Turning the tide. Bull. World Health Organ. 2011, 89, 162. [Google Scholar] [CrossRef]
- GBD 2019 Respiratory Tract Cancers Collaborators. Global, regional, and national burden of respiratory tract cancers and associated risk factors from 1990 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Respir. Med. 2021, 9, 1030–1049. [Google Scholar] [CrossRef]
- Kuang, Z.; Wang, J.; Liu, K.; Wu, J.; Ge, Y.; Zhu, G.; Cao, L.; Ma, X.; Li, J. Global, regional, and national burden of tracheal, bronchus, and lung cancer and its risk factors from 1990 to 2021: Findings from the Global Burden of Disease Study 2021. EClinicalMedicine 2024, 75, 102804. [Google Scholar] [CrossRef]
- Sun, R.; Dou, W.; Liu, W.; Li, J.; Han, X.; Li, S.; Wu, X.; Wang, F.; Xu, X.; Li, J. Global, regional, and national burden of oral cancer and its attributable risk factors from 1990 to 2019. Cancer Med. 2023, 12, 13811–13820. [Google Scholar] [CrossRef]
- Rahmanian, S.D.; Diaz, P.T.; Wewers, M.E. Tobacco use and cessation among women: Research and treatment-related issues. J. Womens Health 2011, 20, 349–357. [Google Scholar] [CrossRef]
- Scharf, D.; Shiffman, S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction 2004, 99, 1462–1469. [Google Scholar] [CrossRef]
- Bohadana, A.; Nilsson, F.; Rasmussen, T.; Martinet, Y. Gender differences in quit rates following smoking cessation with combination nicotine therapy: Influence of baseline smoking behavior. Nicotine Tob. Res. 2003, 5, 111–116. [Google Scholar] [CrossRef]
- Allen, A.M.; Oncken, C.; Hatsukami, D. Women and smoking: The effect of gender on the epidemiology, health effects, and cessation of smoking. Curr. Addict. Rep. 2014, 1, 53–60. [Google Scholar] [CrossRef]
- DeVito, E.E.; Herman, A.I.; Waters, A.J.; Valentine, G.W.; Sofuoglu, M. Subjective, physiological, and cognitive responses to intravenous nicotine: Effects of sex and menstrual cycle phase. Neuropsychopharmacology 2014, 39, 1431–1440. [Google Scholar] [CrossRef]
- Copeland, A.L.; Spears, C.A.; Baillie, L.E.; McVay, M.A. Fear of fatness and drive for thinness in predicting smoking status in college women. Addict. Behav. 2015, 54, 1–6. [Google Scholar] [CrossRef]
- Kuo, C.-W.; Lin, C.-F.; Chen, C.-Y.; Wang, R.-H.; Chou, C.-Y.; Cheng, H.-J.; Wu, J.-S.; Chen, C.-W.; Shieh, C.-C.; Yu, T. Body-weight gain in women during smoking cessation is a sex-specific predictor of 6-month abstinence: A retrospective cohort study. Front. Public Health 2022, 10, 872220. [Google Scholar] [CrossRef]
- Lopez, E.N.; Drobes, D.J.; Thompson, J.K.; Brandon, T.H. Effects of a body image challenge on smoking motivation among college females. Health Psychol. 2008, 27, S243–S251. [Google Scholar] [CrossRef]
- Dieleman, L.A.; van Peet, P.G.; Vos, H.M.M. Gender differences within the barriers to smoking cessation and the preferences for interventions in primary care: A qualitative study using focus groups in The Hague, The Netherlands. BMJ Open 2021, 11, e042623. [Google Scholar] [CrossRef]
- Siddiqi, K.; Husain, S.; Vidyasagaran, A.; Readshaw, A.; Mishu, M.P.; Sheikh, A. Global burden of disease due to smokeless tobacco consumption in adults: An updated analysis of data from 127 countries. BMC Med. 2020, 18, 222. [Google Scholar] [CrossRef]
- Rumgay, H.; Nethan, S.T.; Shah, R.; Vignat, J.; Ayo-Yusuf, O.; Chaturvedi, P.; Guerra, E.N.S.; Gupta, P.C.; Gupta, R.; Liu, S.; et al. Global burden of oral cancer in 2022 attributable to smokeless tobacco and areca nut consumption: A population attributable fraction analysis. Lancet Oncol. 2024, 25, 1413–1423. [Google Scholar] [CrossRef]
- Chugh, A.; Arora, M.; Jain, N.; Vidyasagaran, A.; Readshaw, A.; Sheikh, A.; Eckhardt, J.; Siddiqi, K.; Chopra, M.; Mishu, M.P.; et al. The global impact of tobacco control policies on smokeless tobacco use: A systematic review. Lancet Glob. Health 2023, 11, e953–e968. [Google Scholar] [CrossRef]
- Mehrotra, R.; Yadav, A.; Sinha, D.N.; Parascandola, M.; John, R.M.; Ayo-Yusuf, O.; Nargis, N.; Hatsukami, D.K.; Warnakulasuriya, S.; Straif, K.; et al. Smokeless tobacco control in 180 countries across the globe: Call to action for full implementation of WHO FCTC measures. Lancet Oncol. 2019, 20, e208–e217. [Google Scholar] [CrossRef]
- Bouvard, V.; Nethan, S.T.; Singh, D.; Warnakulasuriya, S.; Mehrotra, R.; Chaturvedi, A.K.; Chen, T.H.-H.; Ayo-Yusuf, O.A.; Gupta, P.C.; Kerr, A.R.; et al. IARC Perspective on Oral Cancer Prevention. N. Engl. J. Med. 2022, 387, 1934–1936. [Google Scholar] [CrossRef]
- GBD 2019 Chewing Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of chewing tobacco use in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e482–e499. [Google Scholar] [CrossRef]
- Conway, D.; Purkayastha, M.; Chestnutt, I. The changing epidemiology of oral cancer: Definitions, trends, and risk factors. Br. Dent. J. 2018, 225, 867–873. [Google Scholar] [CrossRef]
- Dar-Odeh, N.; Arabiat, D.; Abu-Hammad, A.; Alshamasneh, L.; Meer, R.; Al-Fatafta, D.; Abu-Hammad, O. Recent trends in risk factors and clinical presentation of oral cancer in women: A systematic review. J. Adv. Oral Res. 2025; advance online publication. [Google Scholar] [CrossRef]
- Greaves, L. The Meanings of Smoking to Women and Their Implications for Cessation. Int. J. Environ. Res. Public Health 2015, 12, 1449–1465. [Google Scholar] [CrossRef]
- Greaves, L.; Tungohan, E. Engendering tobacco control: Using an international public health treaty to reduce smoking and empower women. Tob. Control 2007, 16, 148–150. [Google Scholar] [CrossRef]
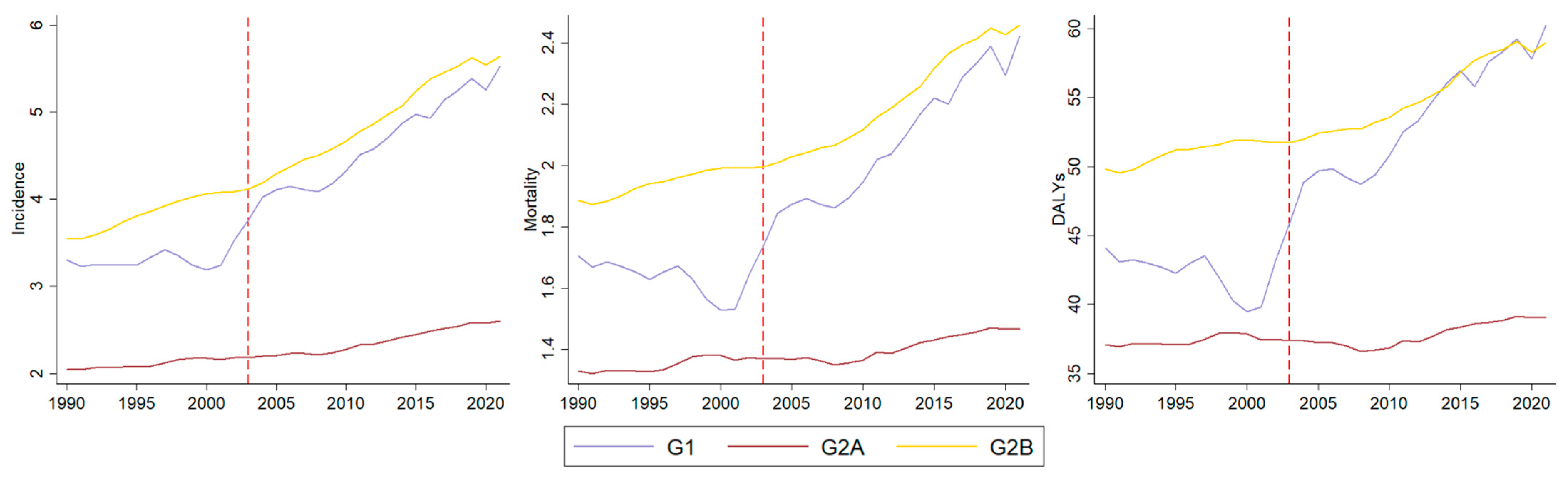
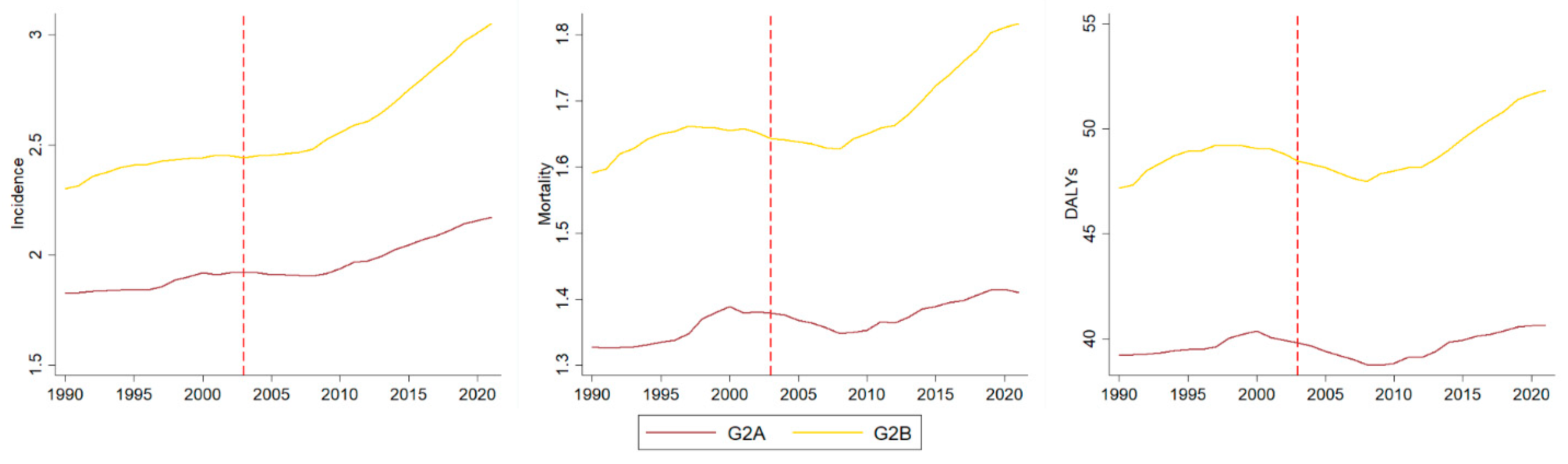
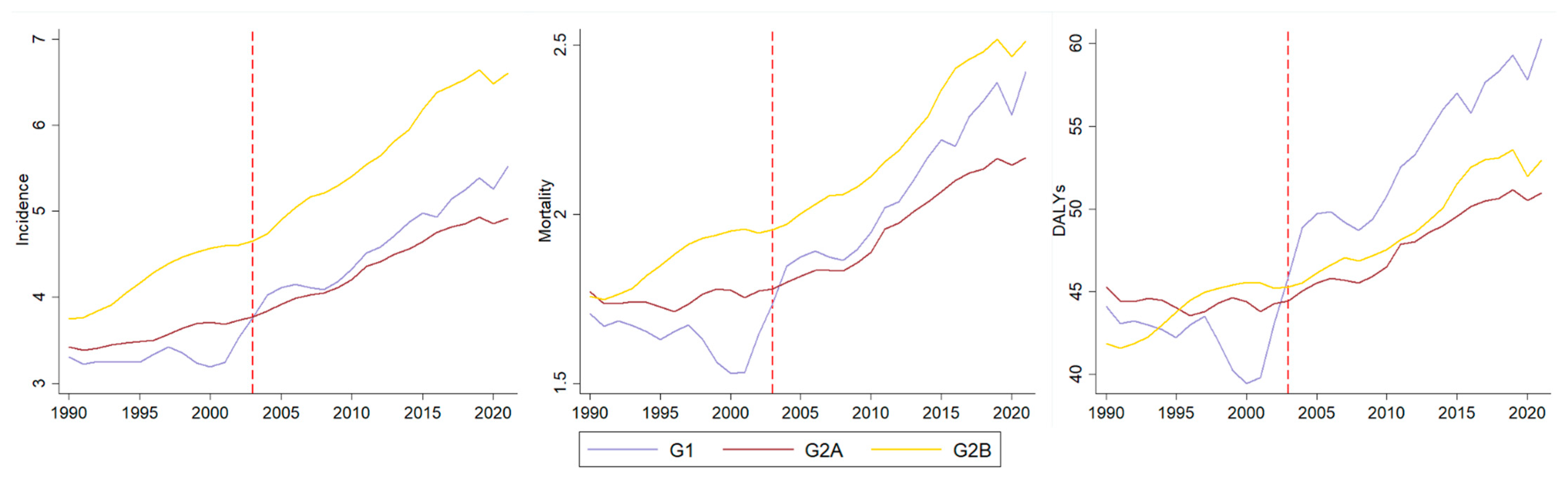
| APC | Group | Incidence [95% CI] | Mortality [95% CI] | DALYs [95% CI] | |||
|---|---|---|---|---|---|---|---|
| Trend | G1 | 0.78 [−0.07 ± 1.64] | Stability | −0.14 [−0.86 ± 0.58] | Stability | 0.01 [−0.90 ± 0.94] | Stability |
| G2A | 0.48 [0.19 ± 0.77] | Increasing | 0.22 [−0.08 ± 0.51] | Stability | 0.05 [−0.23 ± 0.33] | Stability | |
| G2B | 1.23 [0.92 ± 1.54] | Increasing | 0.42 [0.12 ± 0.72] | Increasing | 0.28 [0.03 ± 0.53] | Increasing | |
| Slope | G1 | 1.65 [0.42 ± 2.91] | Increasing | 2.26 [1.20 ± 3.33] | Increasing | 1.81 [0.46 ± 3.18] | Increasing |
| G2A | 0.48 [0.06 ± 0.90] | Increasing | 0.16 [−0.26 ± 0.58] | Stability | 0.18 [−0.22 ± 0.59] | Stability | |
| G2B | 0.50 [0.05 ± 0.95] | Increasing | 0.71 [0.29 ± 1.14] | Increasing | 0.43 [0.06 ± 0.79] | Increasing | |
| APC | Group | Incidence [95% CI] | Mortality [95% CI] | DALYs [95% CI] | |||
|---|---|---|---|---|---|---|---|
| Trend | G2A | 0.38 [0.08 ± 0.69] | Increasing | 0.30 [0.06 ± 0.53] | Increasing | 0.13 [−0.12 ± 0.38] | Stability |
| G2B | 0.51 [0.14 ± 0.88] | Increasing | 0.30 [−0.02 ± 0.62] | Stability | 0.27 [−0.05 ± 0.59] | Stability | |
| Slope | G2A | 0.28 [−0.15 ± 0.70] | Stability | −0.17 [−0.51 ± 0.16] | Stability | −0.04 [−0.38 ± 0.30] | Stability |
| G2B | 0.64 [0.14 ± 1.15] | Increasing | 0.20 [−0.24 ± 0.63] | Stability | 0.04 [−0.39 ± 0.48] | Stability | |
| APC | Group | Incidence [95% CI] | Mortality [95% CI] | DALYs [95% CI] | |||
|---|---|---|---|---|---|---|---|
| Trend | G1 | 0.78 [−0.07 ± 1.64] | Stability | −0.14 [−0.86 ± 0.58] | Stability | 0.01 [−0.90 ± 0.94] | Stability |
| G2A | 0.83 [0.55 ± 1.11] | Increasing | 0.01 [−0.26 ± 0.28] | Stability | −0.14 [−0.33 ± 0.05] | Stability | |
| G2B | 1.82 [1.36 ± 2.28] | Increasing | 0.84 [0.43 ± 1.25] | Increasing | 0.65 [0.28 ± 1.03] | Increasing | |
| Slope | G1 | 1.65 [0.42 ± 2.91] | Increasing | 2.26 [1.20 ± 3.33] | Increasing | 1.81 [0.46 ± 3.18] | Increasing |
| G2A | 0.72 [0.32 ± 1.13] | Increasing | 1.16 [0.77 ± 1.56] | Increasing | 0.97 [0.70 ± 1.25] | Increasing | |
| G2B | 0.11 [−0.55 ± 0.76] | Stability | 0.53 [−0.06 ± 1.13] | Stability | 0.19 [−0.34 ± 0.73] | Stability | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hagen, L.M.; Gasparini, L.R.; da Silva, B.M.; Cunha, A.R.d.; Hugo, F.N.; Amenábar, J.M. The Burden of Lip and Oral Cavity Cancer Among Women Across 204 Countries and Territories in the Context of the Framework Convention on Tobacco Control: An Interrupted Time Series Analysis. Int. J. Environ. Res. Public Health 2025, 22, 1464. https://doi.org/10.3390/ijerph22101464
Hagen LM, Gasparini LR, da Silva BM, Cunha ARd, Hugo FN, Amenábar JM. The Burden of Lip and Oral Cavity Cancer Among Women Across 204 Countries and Territories in the Context of the Framework Convention on Tobacco Control: An Interrupted Time Series Analysis. International Journal of Environmental Research and Public Health. 2025; 22(10):1464. https://doi.org/10.3390/ijerph22101464
Chicago/Turabian StyleHagen, Laila Menezes, Larissa Rodrigues Gasparini, Bruna Machado da Silva, Amanda Ramos da Cunha, Fernando Neves Hugo, and José Miguel Amenábar. 2025. "The Burden of Lip and Oral Cavity Cancer Among Women Across 204 Countries and Territories in the Context of the Framework Convention on Tobacco Control: An Interrupted Time Series Analysis" International Journal of Environmental Research and Public Health 22, no. 10: 1464. https://doi.org/10.3390/ijerph22101464
APA StyleHagen, L. M., Gasparini, L. R., da Silva, B. M., Cunha, A. R. d., Hugo, F. N., & Amenábar, J. M. (2025). The Burden of Lip and Oral Cavity Cancer Among Women Across 204 Countries and Territories in the Context of the Framework Convention on Tobacco Control: An Interrupted Time Series Analysis. International Journal of Environmental Research and Public Health, 22(10), 1464. https://doi.org/10.3390/ijerph22101464






