Physical Activity, Sedentary Time, and Psychosocial Functioning among Adults with Cancer: A Scoping Review
Abstract
1. Background
2. Methods
2.1. Inclusion and Exclusion Criteria
2.2. Data Sources and Search Strategy
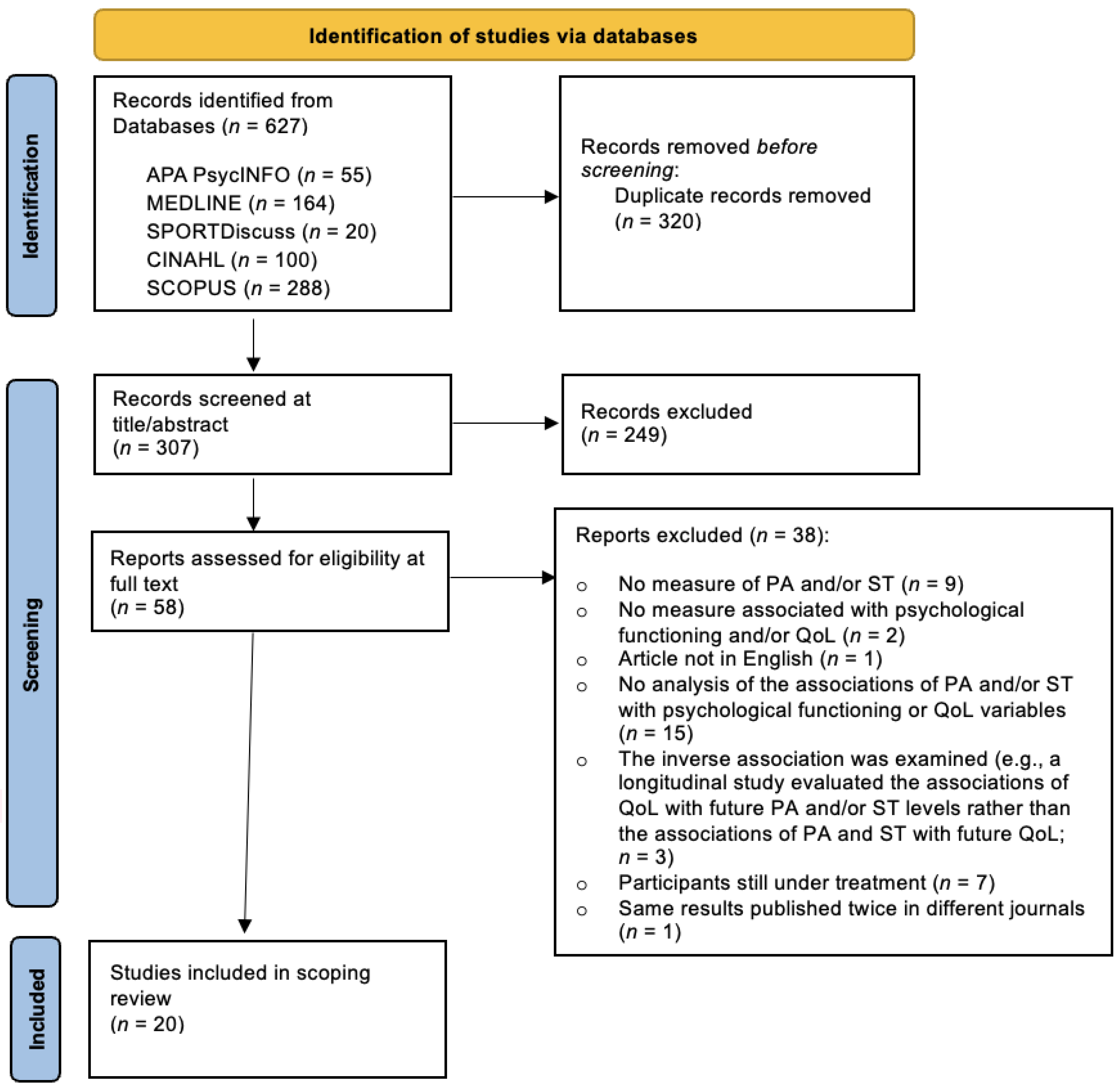
2.3. Study Selection Process
2.4. Data Extraction
3. Results
3.1. Descriptive Characteristics of Studies
3.2. Targeted Population
3.3. Assessment of PA and ST
| First Author, Year of Publication | Study Design | PA and ST Measures | Psychological Functioning and/or QoL Measures | Participants | ||||
|---|---|---|---|---|---|---|---|---|
| Sample Size and Country | Sex | Mean Age (±SD) | Cancer and Treatment Types | Mean Time since End of Treatment (±SD) | ||||
| Di Blasio et al., 2022 [44] | Cross-sectional | SenseWear Pro3 or SenseWear Mini armband accelerometer (worn on a wrist for 7 days) | Anxiety and depression: HADS | 219 (Italy) | Women | 50.98 (±6.28) Range: 30–60 | Breast Surgery (all) Hormone therapy (all) | Not reported but 6 to 48 months after breast surgery and current hormone therapy a |
| Doré et al., 2022 [17] | Longitudinal over 48 months (Every 3 months during the first year, and then after 24 and 48 months) | ActiGraph GT3X accelerometer (worn on a hip for 7 days every 3 months during the first year, and then after 24 and 48 months) | Depression: CES-D | 199 (Canada) | Women | 55.0 (±11.0) Range: 28–79 | Breast (Stages I–III) Lymph or axillary node dissection (n = 116) Lumpectomy (n = 119) Single mastectomy (n = 56) Double mastectomy (n = 34) Chemotherapy (n = 128) Radiotherapy (n = 176) Hormonal therapy (n = 101) | 3.5 months (±2.3) |
| D’Silva et al., 2018 [12] | Cross-sectional | ActiGraph GT3X accelerometer (worn on a hip for 7 days) | QoL: FACT-Lung | 127 (Canada) | 73 women and 54 men | 71.4 (±9) | Lung (Stages I–III) Surgery (n = 82) Surgery and adjuvant chemotherapy (n = 32) Radical (n = 16) Palliative (n = 10 b) None (n = 3 c) | Not reported but mean time since diagnosis = 76.4 months (±47.0) |
| Floor Kenkhuis et al., 2021 [45] | Longitudinal over 24 months (6 weeks, 6 months, 12 months, and 24 months post-treatment) | PA: SQUASH ST: triaxial MOX accelerometer (worn on a thigh for 7 days at 6 weeks, and 6, 12, and 24 months post-treatment) | QoL: EORTC QLQ-C30 | 397 (The Netherlands) | 270 men and 126 women | 67.0 (±9.1) | Colorectal (Stages I–III) Surgery (n = 354) Chemotherapy (n = 155) Radiotherapy (n = 101) Stoma (n = 110) | Not reported but 6 weeks to 24 months |
| Gaskin et al., 2016 [32] | Cross-sectional | ActiGraph GT1M accelerometer (worn on a hip for 7 days) | QoL: EORTC QLQ-C30 + EORTC QLQ- PR25 Anxiety: MAX-PC Depression: CES-D | 98 (Australia) | Men | 67.3 (±8.0) | Prostate (Stages I–III) Surgery only (n = 37) Radiotherapy only (n = 14) Surgery and radiotherapy (n = 27) Hormonotherapy with surgery and/or radiotherapy (n = 20) | 26.1 weeks (±10.1) |
| Hartman et al., 2017 [46] | Cross-sectional | ActiGraph GT3X+ accelerometer (worn on a hip for 7 days) | QoL: SF-36 | 134 (USA) | Women | 62.6 (±6.6) | Breast (Stages I–III) Surgery (all) Chemotherapy (n = 65) Endocrine therapy (n = 93) | Not reported but mean time since diagnosis = 2.1 years (±1.2) |
| Hidde et al., 2022 [47] | Cross-sectional | activPAL accelerometer (worn on a wrist for 7 days) | QoL: FACT-G | 73 (USA) | 55 women and 18 men | 53 (±13.0) | Breast (n = 22) Colorectal (n = 24) Leukemia/Lymphoma (n = 7) Other (n = 20) (Stages 0–IV) Surgery (n = 66) Chemotherapy (n = 54) Radiation (n = 38) Other (n = 12) | Between 16.3 months and 31.2 months depending on the treatment |
| Nurnazahiah et al., 2022 [48] | Cross-sectional | ActivPAL3TM accelerometer (worn on a thigh for 7 days) | QoL: EORTC QLQ–C30 | 83 (Malaysia) | Women | 52.8 (±7.8) | Breast (Stages I–III) Surgery (n = 83) Chemotherapy (n = 83) Radiotherapy (n = 73) | Not reported but mean time since diagnosis = 6.84 years (±4.13) |
| Phillips et al., 2015 [49] | Longitudinal over 6 months (Baseline and 6 months later) | ActiGraph accelerometer (worn on a hip for 7 days at baseline only) | QoL: FACT-B Anxiety and depression: HADS | 358 (USA) | Women | 56.4 (±9.0) | Breast (Stages 0–IV) Surgery + radiotherapy + chemotherapy (n = 142) Surgery + radiotherapy (n = 101) Surgery + chemotherapy (n = 60) Surgery only (n = 55) | Not reported but mean time since diagnosis = 81.7 months (±67.7) |
| Rees-Punia et al., 2020 [25] | Cross-sectional | Questions created specifically for the aim of the study | Global mental health: PROMIS | 7966 (USA) | 3395 women and 4571 men | 78.3 (±5.4) | Colorectal (n = 810) Lung (n = 282) Breast (n = 1708) Endometrial (n = 308) Prostate (n = 3089) Other (n = 1769) * Treatment types were neither presented nor detailed in the study. | Not reported but mean time since diagnosis = 2.9 years (±1.2) in the first group (1–5 years after diagnosis) and 7.5 years (±1.4) in the second group (6–10 years after diagnosis) |
| Roekel et al., 2016 [50] | Cross-sectional | triaxial MOX accelerometer (worn on a thigh for 7 days) | QoL: EORTC QLQ-C30 Anxiety and depression: HADS | 145 (The Netherlands) | 54 women and 91 men | 70.0 (±8.7) | Colorectal (Stages I–III) Surgery (n = 139) Chemotherapy (n = 75) Radiotherapy (n = 55) | Not reported but mean time since diagnosis = 5.7 years (±1.9) |
| Schofield et al., 2018 [51] | Cross-sectional | ActiGraph GT3X+ accelerometer (worn on a hip for 7 days) | QoL: SF-36 | 20 (Australia) | Women | 63.2 (±8.9) | Ovarian (Stages III–IV) Surgery (all) Chemotherapy (all) | 5.3 months |
| Trinh et al., 2015 [52] | Cross-sectional | ActiGraph GT3X accelerometer (worn for 7 days; no information regarding where the accelerometer was worn available in the article) | Depression: CES-10 | 195 (Canada) | Women | 55.0 (±11.0) | Breast (Stages I–III) Lumpectomy (n = 117) Single mastectomy (n = 54) Double mastectomy (n = 31) Chemotherapy (n = 125) Radiotherapy (n = 174) Hormonal therapy (n = 98) | 3.5 months (±2.4) |
| Vallance et al., 2014 [53] | Cross-sectional | ActiGraph GT3X+ accelerometer (worn on a hip for 7 days) | QoL: FACT-C | 178 (Canada and Australia) | 79 women and 99 men | 64.3 (±10.3) | Colon (Stages I–III) Chemotherapy (n = 80) Others d | Not reported but mean time since diagnosis = 18.9 months (±4.4) |
| Vallance et al., 2015 [54] | Cross-sectional | ActiGraph GT3X+ accelerometer (worn on a hip for 7 days) | Depression: PHQ-9 State anxiety: SAI Satisfaction with life: SWLS | 180 (Canada and Australia) | 81 women and 99 men | 64.3 (±10.3) | Colon (Stages I–IV) Chemotherapy (n = 81) Others d | Not reported but mean time since diagnosis = 18.8 months (±4.4) |
| Vallance et al., 2017 [55] | Cross-sectional | ActiGraph GT3X accelerometer (worn on a hip for 7 days) | QoL: FACT-G | 156 (Australia) | 76 women and 80 men | 62.2 (±12.9) Range: 22–82 | Non-Hodgkin lymphoma Chemotherapy only (n = 48) Other treatment only (n = 15) Chemotherapy and 1 other treatment (n = 59) Chemotherapy and 2+ other treatments (n = 18) None (n = 15 e) | Not reported but mean time since diagnosis = 35.1 months (±11.6) |
| Vallance et al., 2018 [56] | Cross-sectional | ActiGraph GT3X accelerometer (worn on a hip for 7 days) | Depression: PHQ-9 State anxiety: SAI Psychological growth: PTGI Satisfaction with life: SWLS | 127 (Canada) | 73 women and 54 men | 71.49 (±9.0) | Lung (Stages I–IV) Surgery (n = 82) Radical (n = 32) Palliative (n = 10) None (n = 3 f) | Not reported but mean time since diagnosis = 76.4 months (±47.0) |
| Welch et al., 2019 [57] | Cross-sectional | ActiGraph GT1M and GT3X accelerometer (worn on a hip for 7 days) | QoL: FACT-B Anxiety and depression: HADS | 753 (USA) | Women | 56.4 (±9.5) | Breast Chemotherapy (n = 462) Radiation (n = 513) Chemotherapy + radiation (n = 306) | Not reported but mean time since diagnosis = 7.0 years (±5.9) |
| Wrosch et al., 2013 [58] | Longitudinal over 3 months (Baseline and 3 months later) | Adapted version of the Leisure Time Exercise Questionnaire | Emotional well-being: POMS | 176 (Canada) | Women | 54.86 (±10.83) | Breast Lumpectomy (n = 131) Lymph node dissection (n = 118) Single mastectomy (n = 60) Double mastectomy (n = 45) Chemotherapy (n = 136) Radiotherapy (n = 153) Hormone therapy (n = 108) | 2.89 months (±2.86) |
| Yan et al., 2021 [59] | Cross-sectional | IPAQ | QoL: EORTC QLQ-C30 | 1546 (China) | 1131 women and 415 men | Not reported but 53.56% were 60 years old and above | Not reported (all g) | Not reported, but participants had to have completed initial treatment h |
3.4. Cross-Sectional Studies (n = 16)
3.4.1. Associations of PA and ST with Anxiety and Depression Symptoms
3.4.2. Associations of PA and ST with Psychological Constructs other Than Anxiety and Depression
3.4.3. Associations of PA and ST with QoL
3.5. Longitudinal Studies (n = 4)
3.5.1. Associations of PA and ST with Anxiety and Depression Symptoms
3.5.2. Associations of PA and ST with Psychological Constructs other Than Anxiety and Depression
3.5.3. Associations of PA and ST with QoL
4. Discussion
4.1. Gaps in the Literature
4.2. Future Avenues
4.3. Strengths and Limitations of the Current Scoping Review
4.4. Clinical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Société Canadienne du Cancer. Vue D’Ensemble des Statistiques sur le Cancer. 2019. Available online: https://cancer.ca/fr/research/cancer-statistics/cancer-statistics-at-a-glance (accessed on 30 August 2023).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA: Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Eyl, R.E.; Koch-Gallenkamp, L.; Jansen, L.; Walter, V.; Carr, P.; Hoffmeister, M.; Chang-Claude, J.; Brenner, H.; Arndt, V. Potential determinants of physical inactivity among long-term colorectal cancer survivors. J. Cancer Surviv. 2018, 12, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Fitch, M.; Zomer, S.; Lockwood, G.; Louzado, C.; Shaw Moxam, R.; Rahal, R.; Green, E. Experiences of adult cancer survivors in transitions. Support. Care Cancer 2019, 27, 2977–2986. [Google Scholar] [CrossRef]
- Ninot, G.; Flori, N.; Huteau, M.E.; Stoebner-Delbarre, A.; Senesse, P. Exercise and cancer: Evidence of efficacy during and after treatments. Bull. Cancer 2020, 107, 474–489. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, J.K.; Vincent, A.J. Exercise improves quality of life in patients with cancer: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016, 50, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.Y.T.; Ho, J.W.C.; Hui, B.P.H.; Lee, A.M.; Macfarlane, D.J.; Leung, S.S.K.; Cerin, E.; Chan, W.Y.Y.; Leung, I.P.F.; Lam, S.H.S.; et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ 2012, 344, 17. [Google Scholar] [CrossRef]
- American Cancer Society. Physical Activity and the Person with Cancer. 2022. Available online: https://www.cancer.org/cancer/survivorship/be-healthy-after-treatment/physical-activity-and-the-cancer-patient (accessed on 30 August 2023).
- World Health Organization. Physical Activity. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/physical-activity (accessed on 30 August 2023).
- Tremblay, M.S.; Aubert, S.; Barnes, J.D.; Saunders, T.J.; Carson, V.; Latimer-Cheung, A.E.; Chastin, S.F.; Altenburg, T.M.; Chinapaw, M.J. Sedentary behavior research network (SBRN)–terminology consensus project process and outcome. Int. J. Behav. Nutr. Phys. Act. 2017, 14, 75. [Google Scholar] [CrossRef]
- Patterson, R.; McNamara, E.; Tainio, M.; de Sá, T.H.; Smith, A.D.; Sharp, S.J.; Edwards, P.; Woodcock, J.; Brage, S.; Wijndaele, K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: A systematic review and dose response meta-analysis. Eur. J. Epidemiol. 2018, 33, 811–829. [Google Scholar] [CrossRef]
- D’Silva, A.; Gardiner, P.A.; Boyle, T.; Bebb, D.G.; Johnson, S.T.; Vallance, J.K. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among lung cancer survivors: A quantile regression approach. Lung Cancer 2018, 119, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Van Roekel, E.H.; Winkler, E.A.; Bours, M.J.; Lynch, B.M.; Willems, P.J.; Meijer, K.; Kant, I.; Beets, G.L.; Sanduleanu, S.; Healy, G.N. Associations of sedentary time and patterns of sedentary time accumulation with health-related quality of life in colorectal cancer survivors. Prev. Med. Rep. 2016, 4, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, M.-G. Effects of exercise interventions on breast cancer patients during adjuvant therapy: A systematic review and meta-analysis of randomized controlled trials. Cancer Nurs. 2020, 43, 115–125. [Google Scholar] [CrossRef]
- Lynch, B.M.; Leitzmann, M.F. An evaluation of the evidence relating to physical inactivity, sedentary behavior, and cancer incidence and mortality. Curr. Epidemiol. Rep. 2017, 4, 221–231. [Google Scholar] [CrossRef]
- Blair, C.K.; Morey, M.C.; Desmond, R.A.; Cohen, H.J.; Sloane, R.; Snyder, D.C.; Demark-Wahnefried, W. Light-intensity activity attenuates functional decline in older cancer survivors. Med. Sci. Sports Exerc. 2014, 46, 1375. [Google Scholar] [CrossRef]
- Doré, I.; Plante, A.; Peck, S.S.; Bedrossian, N.; Sabiston, C.M. Physical activity and sedentary time: Associations with fatigue, pain, and depressive symptoms over 4 years post-treatment among breast cancer survivors. Support. Care Cancer 2022, 30, 785–792. [Google Scholar] [CrossRef]
- Sweegers, M.; Boyle, T.; Vallance, J.; Chinapaw, M.; Brug, J.; Aaronson, N.; D’silva, A.; Kampshoff, C.; Lynch, B.; Nollet, F. Which cancer survivors are at risk for a physically inactive and sedentary lifestyle? Results from pooled accelerometer data of 1447 cancer survivors. Int. J. Behav. Nutr. Phys. Act. 2019, 16, 66. [Google Scholar] [CrossRef]
- Blough, J.; Loprinzi, P.D. Experimentally investigating the joint effects of physical activity and sedentary behavior on depression and anxiety: A randomized controlled trial. J. Affect. Disord. 2018, 239, 258–268. [Google Scholar] [CrossRef]
- Biddle, S.J.; Henson, J.; Davies, M.J.; Khunti, K.; Sutton, S.; Yates, T.; Edwardson, C.L. Device-assessed total and prolonged sitting time: Associations with anxiety, depression, and health-related quality of life in adults. J. Affect. Disord. 2021, 287, 107–114. [Google Scholar] [CrossRef]
- Chan, R.; Crichton, M.; Crawford-Williams, F.; Agbejule, O.; Yu, K.; Hart, N.; de Abreu Alves, F.; Ashbury, F.; Eng, L.; Fitch, M. The efficacy, challenges, and facilitators of telemedicine in post-treatment cancer survivorship care: An overview of systematic reviews. Ann. Oncol. 2021, 32, 1552–1570. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.; Jiang, H.; Howell, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2016, 10, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Sabiston, C.M.; Wrosch, C.; Fong, A.J.; Brunet, J.; Gaudreau, P.; O’Loughlin, J.; Meterissian, S. Life after breast cancer: Moving on, sitting down or standing still? A prospective study of Canadian breast cancer survivors. BMJ Open 2018, 8, e021770. [Google Scholar] [CrossRef] [PubMed]
- Ganz, P.A.; Stanton, A.L. Psychosocial and physical health in post-treatment and extended cancer survivorship. In Clinical Psycho-Oncology: An International Perspective; John Wiley & Sons: West Sussex, UK, 2012; pp. 237–247. [Google Scholar]
- Rees-Punia, E.; Patel, A.V.; Nocera, J.R.; Chantaprasopsuk, S.; Demark-Wahnefried, W.; Leach, C.R.; Smith, T.G.; Cella, D.; Gapstur, S.M. Self-reported physical activity, sitting time, and mental and physical health among older cancer survivors compared with adults without a history of cancer. Cancer 2020, 127, 115–123. [Google Scholar] [CrossRef]
- Balhareth, A.; Aldossary, M.Y.; McNamara, D. Impact of physical activity and diet on colorectal cancer survivors’ quality of life: A systematic review. World J. Surg. Oncol. 2019, 17, 153. [Google Scholar] [CrossRef]
- Jones, T.L.; Sandler, C.X.; Spence, R.R.; Hayes, S.C. Physical activity and exercise in women with ovarian cancer: A systematic review. Gynecol. Oncol. 2020, 158, 803–811. [Google Scholar] [CrossRef]
- Biller, V.S.; Leitzmann, M.F.; Sedlmeier, A.M.; Berger, F.F.; Ortmann, O.; Jochem, C. Sedentary behaviour in relation to ovarian cancer risk: A systematic review and meta-analysis. Eur. J. Epidemiol. 2021, 36, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.; Lee, D.-W.; Kim, H.-R.; Kang, M.-Y. Sedentary work and breast cancer risk: A systematic review and meta-analysis. J. Occup. Health 2021, 63, e12239. [Google Scholar] [CrossRef]
- Ekelund, U.; Brown, W.J.; Steene-Johannessen, J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.E.; Lee, I.-M. Do the associations of sedentary behaviour with cardiovascular disease mortality and cancer mortality differ by physical activity level? A systematic review and harmonised meta-analysis of data from 850 060 participants. Br. J. Sports Med. 2019, 53, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Société Canadienne du Cancer. Types de Traitements. Available online: https://cancer.ca/fr/treatments/treatment-types (accessed on 4 October 2023).
- Gaskin, C.J.; Craike, M.; Mohebbi, M.; Salmon, J.; Courneya, K.S.; Broadbent, S.; Livingston, P.M. Associations of objectively measured moderate-to-vigorous physical activity and sedentary behavior with quality of life and psychological well-being in prostate cancer survivors. Cancer Causes Control 2016, 27, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008, 31, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, M.T.; Healy, G.N.; Dunstan, D.W.; Zderic, T.W.; Owen, N. Too little exercise and too much sitting: Inactivity physiology and the need for new recommendations on sedentary behavior. Curr. Cardiovasc. Risk Rep. 2008, 2, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Owen, N.; Healy, G.N.; Matthews, C.E.; Dunstan, D.W. Too much sitting: The population-health science of sedentary behavior. Exerc. Sport. Sci. Rev. 2010, 38, 105. [Google Scholar] [CrossRef] [PubMed]
- Ro, E.; Clark, L.A. Psychosocial functioning in the context of diagnosis: Assessment and theoretical issues. Psychol. Assess. 2009, 21, 313. [Google Scholar] [CrossRef] [PubMed]
- Froehlich-Grobe, K.; Jones, D.; Businelle, M.S.; Kendzor, D.E.; Balasubramanian, B.A. Impact of disability and chronic conditions on health. Disabil. Health J. 2016, 9, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Lockwood, C.; Dos Santos, K.B.; Pap, R. Practical guidance for knowledge synthesis: Scoping review methods. Asian Nurs. Res. 2019, 13, 287–294. [Google Scholar] [CrossRef]
- Hackenbroich, S.; Kranke, P.; Meybohm, P.; Weibel, S. Include or not to include conference. Syst. Rev. 2022, 11, 178. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.; Horsley, T.; Weeks, L. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.D.; Marnie, C.; Tricco, A.C.; Pollock, D.; Munn, Z.; Alexander, L.; McInerney, P.; Godfrey, C.M.; Khalil, H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid. Synth. 2020, 18, 2119–2126. [Google Scholar] [CrossRef] [PubMed]
- Vanhelst, J. Physical activity assessment using accelerometry. Revue D’epidemiologie et de Sante Publique 2019, 67, 126–134. [Google Scholar] [CrossRef]
- Gresham, G.; Schrack, J.; Gresham, L.M.; Shinde, A.M.; Hendifar, A.E.; Tuli, R.; Rimel, B.; Figlin, R.; Meinert, C.L.; Piantadosi, S. Wearable activity monitors in oncology trials: Current use of an emerging technology. Contemp. Clin. Trials 2018, 64, 13–21. [Google Scholar] [CrossRef]
- Di Blasio, A.; Di Dalmazi, G.; Morano, T.; Bucci, I.; Verrocchio, S.; Grossi, S.; Cianchetti, E.; Valentini, P.; Cugusi, L.; Gobbo, S.; et al. Relationships between daily physical activity combinations and psychophysical health status of Italian breast cancer survivors. Home Health Care Serv. Q. 2022, 41, 200–218. [Google Scholar] [CrossRef]
- Floor Kenkhuis, M.; Van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.G.; Keulen, E.T.P.; Van Duijnhoven, F.J.B.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal Associations of Sedentary Behavior and Physical Activity with Quality of Life in Colorectal Cancer Survivors. Med. Sci. Sports Exerc. 2021, 53, 2298–2308. [Google Scholar] [CrossRef]
- Hartman, S.J.; Marinac, C.R.; Bellettiere, J.; Godbole, S.; Natarajan, L.; Patterson, R.E.; Kerr, J. Objectively measured sedentary behavior and quality of life among survivors of early stage breast cancer. Support. Care Cancer 2017, 25, 2495–2503. [Google Scholar] [CrossRef]
- Hidde, M.C.; Lyden, K.; Henry, K.; Leach, H.J. Reallocating time to physical activity and sleep: Associations with quality of life in cancer survivors. Support. Care Cancer 2022, 30, 7527–7534. [Google Scholar] [CrossRef]
- Nurnazahiah, A.; Shahril, M.R.; Nor Syamimi, Z.; Ahmad, A.; Sulaiman, S.; Lua, P.L. Relationship of objectively measured physical activity and sedentary behaviour with health-related quality of life among breast cancer survivors. Health Qual. Life Outcomes 2020, 18, 222. [Google Scholar] [CrossRef]
- Phillips, S.M.; Awick, E.A.; Conroy, D.E.; Pellegrini, C.A.; Mailey, E.L.; McAuley, E. Objectively measured physical activity and sedentary behavior and quality of life indicators in survivors of breast cancer. Cancer 2015, 121, 4044–4052. [Google Scholar] [CrossRef] [PubMed]
- Roekel, E.; Bours, M.; Breedveld-Peters, J.; Willems, P.; Meijer, K.; Kant, I.; den Brandt, P.; Beets, G.; Sanduleanu, S.; Weijenberg, M.; et al. Modeling how substitution of sedentary behavior with standing or physical activity is associated with health-related quality of life in colorectal cancer survivors. Cancer Causes Control 2016, 27, 513–525. [Google Scholar] [CrossRef]
- Schofield, C.; Newton, R.U.; Cohen, P.A.; Galvão, D.A.; McVeigh, J.A.; Mohan, G.R.; Tan, J.; Salfinger, S.G.; Straker, L.M.; Peddle-McIntyre, C.J. Health-related quality of life and pelvic floor dysfunction in advanced-stage ovarian cancer survivors: Associations with objective activity behaviors and physiological characteristics. Support. Care Cancer 2018, 26, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Trinh, L.; Amireault, S.; Lacombe, J.; Sabiston, C.M. Physical and psychological health among breast cancer survivors: Interactions with sedentary behavior and physical activity. Psychooncology 2015, 24, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Vallance, J.K.; Boyle, T.; Courneya, K.S.; Lynch, B.M. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer 2014, 120, 2919–2926. [Google Scholar] [CrossRef] [PubMed]
- Vallance, J.K.; Boyle, T.; Courneya, K.S.; Lynch, B.M. Accelerometer-assessed physical activity and sedentary time among colon cancer survivors: Associations with psychological health outcomes. J. Cancer Surviv. 2015, 9, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Vallance, J.K.; Buman, M.P.; Lynch, B.M.; Boyle, T. Reallocating time to sleep, sedentary, and active behaviours in non-Hodgkin lymphoma survivors: Associations with patient-reported outcomes. Ann. Hematol. 2017, 96, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Vallance, J.K.; Bebb, G.D.; Boyle, T.; Johnson, S.T.; Gardiner, P.A.; D’Silva, A. Psychosocial health is associated with objectively assessed sedentary time and light intensity physical activity among lung cancer survivors. Ment. Health Phys. Act. 2018, 14, 61–65. [Google Scholar] [CrossRef]
- Welch, W.A.; Ehlers, D.; Gavin, K.L.; Aguinaga, S.; Cottrell, A.; Nielsen, A.; Solk, P.; McAuley, E.; Phillips, S.M. Effects of reallocating sedentary time with physical activity on quality of life indicators in breast cancer survivors. Psychooncology 2019, 28, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Wrosch, C.; Sabiston, C.M. Goal adjustment, physical and sedentary activity, and well-being and health among breast cancer survivors. Psychooncology 2013, 22, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Che, B.; Lv, B.; Wu, P.; Lu, X.; Zhang, Y.; Wang, J.; Yu, J. The association between physical activity, sedentary time and health-related quality of life in cancer survivors. Health Qual. Life Outcomes 2021, 19, 213. [Google Scholar] [CrossRef] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public. Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef]
- Karimi, M.; Brazier, J. Health, health-related quality of life, and quality of life: What is the difference? Pharmacoeconomics 2016, 34, 645–649. [Google Scholar] [CrossRef]
- Mitchell, A.J.; Meader, N.; Symonds, P. Diagnostic validity of the Hospital Anxiety and Depression Scale (HADS) in cancer and palliative settings: A meta-analysis. J. Affect. Disord. 2010, 126, 335–348. [Google Scholar] [CrossRef]
- Keyes, C.L.; Shmotkin, D.; Ryff, C.D. Optimizing well-being: The empirical encounter of two traditions. J. Personal. Soc. Psychol. 2002, 82, 1007. [Google Scholar] [CrossRef]
- Trudel-Fitzgerald, C.; Millstein, R.A.; Von Hippel, C.; Howe, C.J.; Tomasso, L.P.; Wagner, G.R.; VanderWeele, T.J. Psychological well-being as part of the public health debate? Insight into dimensions, interventions, and policy. BMC Public Health 2019, 19, 1712. [Google Scholar] [CrossRef]
- Ryff, C.D.; Keyes, C.L.M. The structure of psychological well-being revisited. J. Personal. Soc. Psychol. 1995, 69, 719. [Google Scholar] [CrossRef]
- Anderson, E.; Shivakumar, G. Effects of exercise and physical activity on anxiety. Front. Psychiatry 2013, 4, 27. [Google Scholar] [CrossRef]
- Dinas, P.; Koutedakis, Y.; Flouris, A. Effects of exercise and physical activity on depression. Ir. J. Med. Sci. 2011, 180, 319–325. [Google Scholar] [CrossRef]
- Ekelund, U.; Steene-Johannessen, J.; Brown, W.J.; Fagerland, M.W.; Owen, N.; Powell, K.E.; Bauman, A.; Lee, I.-M. Does physical activity attenuate, or even eliminate, the detrimental association of sitting time with mortality? A harmonised meta-analysis of data from more than 1 million men and women. Lancet 2016, 388, 1302–1310. [Google Scholar] [CrossRef] [PubMed]
- Dishman, R.K.; Washburn, R.A.; Schoeller, D.A. Measurement of Physical Activity. Quest 2001, 53, 295–309. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.E.; Hardt, J.; Gorber, S.C.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Cardilli, L.; Reed, J.L.; Saunders, T.J.; Kite, C.; Douillette, K.; Fournier, K.; Buckley, J.P. A comparison of self-reported and device measured sedentary behaviour in adults: A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Lischetzke, T.; Könen, T. Daily diary methodology. In Encyclopedia of Quality of Life and Well-Being Research; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–8. [Google Scholar]
- Shiffman, S.; Stone, A.A.; Hufford, M.R. Ecological momentary assessment. Annu. Rev. Clin. Psychol. 2008, 4, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.C.; Depp, C.A.; Wetherell, J.L.; Lenze, E.J. Ecological momentary assessment versus standard assessment instruments for measuring mindfulness, depressed mood, and anxiety among older adults. J. Psychiatr. Res. 2016, 75, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.E.; Vowles, K.E.; Smith, B.W.; Gan, G.N.; Edelman, M.J. Daily diary study of hope, stigma, and functioning in lung cancer patients. Health Psychol. 2018, 37, 218. [Google Scholar] [CrossRef]
- Kampshoff, C.S.; Verdonck-de Leeuw, I.M.; van Oijen, M.G.; Sprangers, M.A.; Buffart, L.M. Ecological momentary assessments among patients with cancer: A scoping review. Eur. J. Cancer Care 2019, 28, e13095. [Google Scholar] [CrossRef]
- Zhai, L.; Zhang, Y.; Zhang, D. Sedentary behaviour and the risk of depression: A meta-analysis. Br. J. Sports Med. 2015, 49, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Teychenne, M.; Ball, K.; Salmon, J. Sedentary behavior and depression among adults: A review. Int. J. Behav. Med. 2010, 17, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Ferroni, E. The Physiological and Psychological Effects of Indoor Versus Outdoor Aerobic Exercise in Female Cancer Survivors: A Literature Review. Master’s Thesis, Northern Michigan University, Marquette, MI, USA, 2020. [Google Scholar]
- Bouillet, T.; Joly, F.; Saghatchian, M.; Guéroult-Accolas, L.; Tahar, J.-M.; Descotes, J.-M.; Krakowski, I. Activité Physique Adaptée et cancer métastatique: Quels besoins et quelles attentes? Bull. Cancer 2022, 109, 1287–1297. [Google Scholar] [CrossRef] [PubMed]
| Concept | Search Number | Terms |
|---|---|---|
| Post-treatment | 1 | (“After treatment*” OR “After-Treatment*” OR Recovery OR “Post-treatment*” OR Posttreatment* OR “Post Treatment” OR Surviv* OR “Follow-Up” OR Aftercare OR “After Care”) N4 a Cancer* |
| Physical activity (PA) | 2 | Sport* OR “Physical Activit*” OR “Physical-activit*” OR Exercise* OR “Physical Exercise*” OR Training OR “Physical training” OR Fitness OR Workout* OR Aerobic |
| Sedentary time (ST) | 3 | Sedentar* |
| Psychological functioning/QoL | 4 | “Quality Of Life” OR “Life Quality” OR QoL OR HRQoL OR “Well Being” OR “Well-being” OR Affect* OR Emotion* OR Mood* OR Feeling* OR “Mental-health” OR “Mental Health” OR Anxiet* OR Depress* OR Stress OR Distress* |
| Combined search | 5 | 1 AND 2 AND 3 AND 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Côté, A.; Miquelon, P.; Trudel-Fitzgerald, C. Physical Activity, Sedentary Time, and Psychosocial Functioning among Adults with Cancer: A Scoping Review. Int. J. Environ. Res. Public Health 2024, 21, 225. https://doi.org/10.3390/ijerph21020225
Côté A, Miquelon P, Trudel-Fitzgerald C. Physical Activity, Sedentary Time, and Psychosocial Functioning among Adults with Cancer: A Scoping Review. International Journal of Environmental Research and Public Health. 2024; 21(2):225. https://doi.org/10.3390/ijerph21020225
Chicago/Turabian StyleCôté, Arianne, Paule Miquelon, and Claudia Trudel-Fitzgerald. 2024. "Physical Activity, Sedentary Time, and Psychosocial Functioning among Adults with Cancer: A Scoping Review" International Journal of Environmental Research and Public Health 21, no. 2: 225. https://doi.org/10.3390/ijerph21020225
APA StyleCôté, A., Miquelon, P., & Trudel-Fitzgerald, C. (2024). Physical Activity, Sedentary Time, and Psychosocial Functioning among Adults with Cancer: A Scoping Review. International Journal of Environmental Research and Public Health, 21(2), 225. https://doi.org/10.3390/ijerph21020225







