Exploring the Link between Altitude of Residence and Smoking Patterns in the United States
Abstract
1. Introduction
2. Methods
2.1. Smoking Rate Data
2.2. Mean County Altitude Data
2.3. Potential Covariates
2.4. Data Analyses
3. Results
4. Discussion
4.1. Pharmacokinetics of Inhaled Nicotine in Cigarette Smoke
4.2. Pharmacodynamics of Nicotine and Altitude
4.3. Mechanistic Pathways Involving Neurotransmitter Metabolism
4.4. Developmental Considerations Relevant to Smoking, Altitude, and Adolescence
4.5. Limitations
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Health and Human Services. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health: Atlanta, GA, USA, 2014.
- Öberg, M.; Jaakkola, M.S.; Woodward, A.; Peruga, A.; Prüss-Ustün, A. Worldwide burden of disease from exposure to second-hand smoke: A retrospective analysis of data from 192 countries. Lancet 2011, 377, 139–146. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2013: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Jamal, A.; King, B.A.; Neff, L.J.; Whitmill, J.; Babb, S.D.; Graffunder, C.M. Current Cigarette Smoking Among Adults—United States, 2005–2015. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, M.E.; Loretan, C.G.; Wang, T.W.; Jamal, A.; Homa, D.M. Tobacco product use among adults—United States, 2020. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 397. [Google Scholar] [CrossRef] [PubMed]
- Jamal, A.; Homa, D.M.; O’Connor, E.; Babb, S.D.; Caraballo, R.S.; Singh, T.; Hu, S.S.; King, B.A. Current cigarette smoking Among Adults—United States, 2005–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 1233–1240. [Google Scholar] [CrossRef]
- GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990–2015: A systematic analysis from the Global Burden of Disease Study 2015. Lancet 2017, 389, 1885–1906. [Google Scholar] [CrossRef]
- Soneji, S.; Barrington-Trimis, J.L.; Wills, T.A.; Leventhal, A.M.; Unger, J.B.; Gibson, L.A.; Yang, J.; Primack, B.A.; Andrews, J.A.; Miech, R.A.; et al. Association between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2017, 171, 788–797. [Google Scholar] [CrossRef]
- Siahpush, M.; Wakefield, M.A.; Spittal, M.J.; Durkin, S.J.; Scollo, M.M. Taxation reduces social disparities in adult smoking prevalence. Am. J. Prev. Med. 2009, 36, 285–291. [Google Scholar] [CrossRef]
- Momperousse, D.; Delnevo, C.D.; Lewis, M.J. Exploring the seasonality of cigarette-smoking behaviour. Tob. Control 2007, 16, 69–70. [Google Scholar] [CrossRef]
- Merrill, R.M. Explaining the inverse association between altitude and obesity. J. Obes. 2020, 2020, 1946723. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, C.; Tchernis, R.; Ukert, B. The effect of smoking on obesity: Evidence from a randomized trial. J. Health Econ. 2018, 57, 31–44. [Google Scholar] [CrossRef] [PubMed]
- DelMastro, K.; Hellem, T.; Kim, N.; Kondo, D.; Sung, Y.H.; Renshaw, P.F. Incidence of major depressive episode correlates with elevation of substate region of residence. J. Affect. Disord. 2011, 129, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, K.K.; Kim, N.; Kondo, D.G.; Renshaw, P.F. Cocaine use in the past year is associated with altitude of residence. J. Addict. Med. 2012, 6, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.S.; Coon, H.; Kim, N.; Renshaw, P.F.; Kondo, D.G. Altitude is a risk factor for completed suicide in bipolar disorder. Med. Hypotheses 2014, 82, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Huber, R.S.; Kim, T.S.; Kim, N.; Kuykendall, M.D.; Sherwood, S.N.; Renshaw, P.F.; Kondo, D.G. Association Between Altitude and Regional Variation of ADHD in Youth. J. Atten. Disord. 2018, 22, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Sabic, H.; Kious, B.; Boxer, D.; Fitzgerald, C.; Riley, C.; Scholl, L.; McGlade, E.; Yurgelun-Todd, D.; Renshaw, P.F.; Kondo, D.G. Effect of Altitude on Veteran Suicide Rates. High Alt. Med. Biol. 2019, 20, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Mickelson, J.B.; Brenner, B.E.; Haws, C.A.; Yurgelun-Todd, D.A.; Renshaw, P.F. Altitude, gun ownership, rural areas, and suicide. Am. J. Psychiatry 2011, 168, 49–54. [Google Scholar] [CrossRef]
- Kim, T.S.; Kondo, D.G.; Kim, N.; Renshaw, P.F. Altitude may contribute to regional variation in methamphetamine use in the United States: A population database study. Psychiatry Investig. 2014, 11, 430–436. [Google Scholar] [CrossRef][Green Version]
- Simeonov, K.P.; Himmelstein, D.S. Lung cancer incidence decreases with elevation: Evidence for oxygen as an inhaled carcinogen. PeerJ 2015, 3, e705. [Google Scholar] [CrossRef]
- Gourgoulianis, K.I.; Brelas, N.; Hatziparasides, G.; Papayianni, M.; Molyvdas, P.A. The influence of altitude in bronchial asthma. Arch. Med. Res. 2001, 32, 429–431. [Google Scholar] [CrossRef] [PubMed]
- Menezes, A.M.; Perez-Padilla, R.; Jardim, J.R.; Muino, A.; Lopez, M.V.; Valdivia, G.; Montes de Oca, M.; Talamo, C.; Hallal, P.C.; Victora, C.G.; et al. Chronic obstructive pulmonary disease in five Latin American cities (the PLATINO study): A prevalence study. Lancet 2005, 366, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Bialous, S.A.; Sarna, L. Lung Cancer and Tobacco: What Is New? Nurs. Clin. N. Am. 2017, 52, 53–63. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. What Are the Risk Factors for Lung Cancer? Available online: https://www.cdc.gov/cancer/lung/basic_info/risk_factors.htm (accessed on 31 January 2024).
- University of Wisconsin Population Health Institute; Robert Wood Johnson Foundation. County Health Rankings & Roadmaps. National Data & Documentation 2010–2023: 2023 County Health Rankings; University of Wisconsin Population Health Institute; Robert Wood Johnson Foundation: Madison, WI, USA, 2023. [Google Scholar]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System, Numbers of Variables, Surveillance States, and Records by Year (1984–2016). Available online: https://www.cdc.gov/brfss/annual_data/all_years/states_data.htm (accessed on 31 January 2024).
- National Geospatial-Intelligence Agency and National Aeronautics and Space Administration. Shuttle Radar Topography Mission (SRTM) Dataset; U.S. Geological Survey (USGS): Reston, VA, USA, 2000.
- Nighbor, T.D.; Doogan, N.J.; Roberts, M.E.; Cepeda-Benito, A.; Kurti, A.N.; Priest, J.S.; Johnson, H.K.; Lopez, A.A.; Stanton, C.A.; Gaalema, D.E. Smoking prevalence and trends among a US national sample of women of reproductive age in rural versus urban settings. PLoS ONE 2018, 13, e0207818. [Google Scholar] [CrossRef] [PubMed]
- Lampert, T. Smoking, physical inactivity, and obesity: Associations with social status. Dtsch. Arztebl. Int. 2010, 107, 1. [Google Scholar] [PubMed]
- Ahmadi, F.; Alavi, S.; Sadeghipur, H.R. Evaluation of the effectiveness of physical activity on the quality of life among tobacco consumers. Mil. Caring Sci. J. 2022, 9, 171–179. [Google Scholar]
- Kock, L.; Brown, J.; Cox, S.; McNeill, A.; Robson, D.; Shahab, L.; Tattan-Birch, H.; Brose, L.S. Association of psychological distress with smoking cessation, duration of abstinence from smoking, and use of non-combustible nicotine-containing products: A cross-sectional population survey in Great Britain. Addict. Behav. 2023, 138, 107570. [Google Scholar] [CrossRef]
- Zvolensky, M.J.; Jardin, C.; Wall, M.M.; Gbedemah, M.; Hasin, D.; Shankman, S.A.; Gallagher, M.W.; Bakhshaie, J.; Goodwin, R.D. Psychological distress among smokers in the United States: 2008–2014. Nicotine Tob Res. 2018, 20, 707–713. [Google Scholar] [CrossRef]
- Lasser, K.; Boyd, J.W.; Woolhandler, S.; Himmelstein, D.U.; McCormick, D.; Bor, D.H. Smoking and mental illness: A population-based prevalence study. JAMA 2000, 84, 2606–2610. [Google Scholar] [CrossRef]
- Shiffman, S.; Balabanis, M. Do drinking and smoking go together? Alcohol Health Res. World 1996, 20, 107. [Google Scholar]
- Barbeau, E.M.; Krieger, N.; Soobader, M.J. Working class matters: Socioeconomic disadvantage, race/ethnicity, gender, and smoking in NHIS 2000. Am. J. Public Health 2004, 94, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Gilman, S.E.; Martin, L.T.; Abrams, D.B.; Kawachi, I.; Kubzansky, L.; Loucks, E.B.; Rende, R.; Rudd, R.; Buka, S.L. Educational attainment and cigarette smoking: A causal association? Int. J. Epidemiol. 2008, 37, 615–624. [Google Scholar] [CrossRef]
- Hiscock, R.; Bauld, L.; Amos, A.; Fidler, J.A.; Munafo, M. Socioeconomic status and smoking: A review. Ann. N. Y. Acad. Sci. 2012, 1248, 107–123. [Google Scholar] [CrossRef]
- Kaleta, D.; Makowiec-Dabrowska, T.; Dziankowska-Zaborszczyk, E.; Fronczak, A. Predictors of smoking initiation--results from the Global Adult Tobacco Survey (GATS) in Poland 2009–2010. Ann. Agric. Environ. Med. 2013, 20, 756–766. [Google Scholar]
- DeNavas-Walt, C.; Proctor, B.D. Income and Poverty in the United States: 2014; U.S. Census Bureau: Washington, DC, USA, 2015. Available online: https://www.census.gov/content/dam/Census/library/publications/2015/demo/p60-252.pdf (accessed on 31 January 2024).
- Mowery, P.D.; Dube, S.R.; Thorne, S.L.; Garrett, B.E.; Homa, D.M.; Henderson, P.N. Disparities in smoking-related mortality among American Indians/Alaska Natives. Am. J. Prev. Med. 2015, 49, 738–744. [Google Scholar] [CrossRef] [PubMed]
- CDC. Conclusion and Future Directions: CDC Health Disparities and Inequalities Report—United States, 2013; CDC: Atlanta, GA, USA, 2013; Volume 62, p. 184.
- Nguyen-Grozavu, F.T.; Pierce, J.P.; Sakuma, K.-L.K.; Leas, E.C.; McMenamin, S.B.; Kealey, S.; Benmarhnia, T.; Emery, S.L.; White, M.M.; Fagan, P. Widening disparities in cigarette smoking by race/ethnicity across education level in the United States. Prev. Med. 2020, 139, 106220. [Google Scholar] [CrossRef] [PubMed]
- Siahpush, M.; Singh, G.K.; Jones, P.R.; Timsina, L.R. Racial/ethnic and socioeconomic variations in duration of smoking: Results from 2003, 2006 and 2007 Tobacco Use Supplement of the Current Population Survey. J. Public Health 2010, 32, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Balogh, E.; Faubl, N.; Riemenschneider, H.; Balázs, P.; Bergmann, A.; Cseh, K.; Horváth, F.; Schelling, J.; Terebessy, A.; Wagner, Z. Cigarette, waterpipe and e-cigarette use among an international sample of medical students. Cross-sectional multicenter study in Germany and Hungary. BMC Public Health 2018, 18, 591. [Google Scholar] [CrossRef] [PubMed]
- Lushniak, B.D.; Samet, J.M.; Pechacek, T.F.; Norman, L.A.; Taylor, P.A. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General; CDC: Atlanta, GA, USA, 2014.
- WHO. WHO Report on the Global Tobacco Epidemic, 2023: Protect People from Tobacco Smoke; WHO: Geneva, Switzerland, 2023. [Google Scholar]
- McRobbie, H.; Bullen, C.; Glover, M.; Whittaker, R.; Wallace-Bell, M.; Fraser, T. New Zealand smoking cessation guidelines. New Z. Med. J. (Online) 2008, 121, 57–70. [Google Scholar]
- Merrill, R.M.; Frutos, A. Reduced lung cancer mortality with lower atmospheric pressure. Dose-Response 2018, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weir, E.K.; Archer, S.L. The mechanism of acute hypoxic pulmonary vasoconstriction: The tale of two channels. FASEB J. 1995, 9, 183–189. [Google Scholar] [CrossRef]
- Siques, P.; Brito, J.; Pena, E. Reactive Oxygen Species and Pulmonary Vasculature During Hypobaric Hypoxia. Front. Physiol. 2018, 9, 865. [Google Scholar] [CrossRef]
- Weir, E.K.; López-Barneo, J.; Buckler, K.J.; Archer, S.L. Acute oxygen-sensing mechanisms. N. Engl. J. Med. 2005, 353, 2042–2055. [Google Scholar] [CrossRef]
- Hashimoto, F.; McWilliams, B.; Qualls, C. Pulmonary ventilatory function decreases in proportion to increasing altitude. Wilderness Environ. Med. 1997, 8, 214–217. [Google Scholar] [CrossRef]
- Ziaee, V.; Alizadeh, R.; Movafegh, A. Pulmonary function parameters changes at different altitudes in healthy athletes. Iran. J. Allergy Asthma Immunol. 2008, 7, 79–84. [Google Scholar]
- Fischer, R.; Lang, S.M.; Bergner, A.; Huber, R.M. Monitoring of expiratory flow rates and lung volumes during a high altitude expedition. Eur. J. Med. Res. 2005, 10, 469–474. [Google Scholar] [PubMed]
- Imperato, A.; Mulas, A.; Di Chiara, G. Nicotine preferentially stimulates dopamine release in the limbic system of freely moving rats. Eur. J. Pharmacol. 1986, 132, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Iversen, L.L. Smoking...harmful to the brain. Nature 1996, 382, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Tapper, A.R.; McKinney, S.L.; Nashmi, R.; Schwarz, J.; Deshpande, P.; Labarca, C.; Whiteaker, P.; Marks, M.J.; Collins, A.C.; Lester, H.A. Nicotine activation of alpha4* receptors: Sufficient for reward, tolerance, and sensitization. Science 2004, 306, 1029–1032. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, A.K.; Jackson, A.; Bagdas, D.; Imad Damaj, M. Reversal of Nicotine Withdrawal Signs Through Positive Allosteric Modulation of alpha4beta2 Nicotinic Acetylcholine Receptors in Male Mice. Nicotine Tob. Res. 2018, 20, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Jorenby, D.E.; Hays, J.T.; Rigotti, N.A.; Azoulay, S.; Watsky, E.J.; Williams, K.E.; Billing, C.B.; Gong, J.; Reeves, K.R.; Varenicline Phase 3 Study, G. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: A randomized controlled trial. JAMA 2006, 296, 56–63. [Google Scholar] [CrossRef]
- Coe, J.W.; Brooks, P.R.; Vetelino, M.G.; Wirtz, M.C.; Arnold, E.P.; Huang, J.; Sands, S.B.; Davis, T.I.; Lebel, L.A.; Fox, C.B.; et al. Varenicline: An alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J. Med. Chem. 2005, 48, 3474–3477. [Google Scholar] [CrossRef]
- Campanucci, V.A.; Krishnaswamy, A.; Cooper, E. Mitochondrial reactive oxygen species inactivate neuronal nicotinic acetylcholine receptors and induce long-term depression of fast nicotinic synaptic transmission. J. Neurosci. 2008, 28, 1733–1744. [Google Scholar] [CrossRef] [PubMed]
- Haefely, W.; Burkard, W.P.; Cesura, A.M.; Kettler, R.; Lorez, H.P.; Martin, J.R.; Richards, J.G.; Scherschlicht, R.; Da Prada, M. Biochemistry and pharmacology of moclobemide, a prototype RIMA. Psychopharmacology 1992, 106 (Suppl. 1), S6–S14. [Google Scholar] [CrossRef]
- Youdim, M.B.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Bacher, I.; Houle, S.; Xu, X.; Zawertailo, L.; Soliman, A.; Wilson, A.A.; Selby, P.; George, T.P.; Sacher, J.; Miler, L.; et al. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch. Gen. Psychiatry 2011, 68, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.S.; Volkow, N.D.; Wang, G.J.; Pappas, N.; Logan, J.; MacGregor, R.; Alexoff, D.; Shea, C.; Schlyer, D.; Wolf, A.P.; et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature 1996, 379, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Hogg, R.C. Contribution of Monoamine Oxidase Inhibition to Tobacco Dependence: A Review of the Evidence. Nicotine Tob. Res. 2016, 18, 509–523. [Google Scholar] [CrossRef]
- Royal College of Physicians. Nicotine Without Smoke: Tobacco Harm Reduction; Royal College of Physicians: London, UK, April 2016; ISBN 978-1-86016-600-6. [Google Scholar]
- Benowitz, N.L.; Hukkanen, J.; Jacob, P., 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handb. Exp. Pharmacol. 2009, 192, 29–60. [Google Scholar] [CrossRef]
- Gottlieb, S.; Zeller, M. A Nicotine-Focused Framework for Public Health. New Engl. J. Med. 2017, 377, 1111–1114. [Google Scholar] [CrossRef]
- Warner, K.E.; Schroeder, S.A. FDA’s Innovative Plan to Address the Enormous Toll of Smoking. JAMA 2017, 318, 1755–1756. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. FDA Announces Comprehensive Regulatory Plan to Shift Trajectory of Tobacco Related Disease, Death. [FDA News Release]; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2017.
- Donny, E.C.; Denlinger, R.L.; Tidey, J.W.; Koopmeiners, J.S.; Benowitz, N.L.; Vandrey, R.G.; al’Absi, M.; Carmella, S.G.; Cinciripini, P.M.; Dermody, S.S.; et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. New Engl. J. Med. 2015, 373, 1340–1349. [Google Scholar] [CrossRef]
- Higgins, S.T.; Heil, S.H.; Sigmon, S.C.; Tidey, J.W.; Gaalema, D.E.; Hughes, J.R.; Stitzer, M.L.; Durand, H.; Bunn, J.Y.; Priest, J.S.; et al. Addiction Potential of Cigarettes With Reduced Nicotine Content in Populations With Psychiatric Disorders and Other Vulnerabilities to Tobacco Addiction. JAMA Psychiatry 2017, 74, 1056–1064. [Google Scholar] [CrossRef]
- Todorović, I.; Cheng, F.; Stojisavljević, S.; Marinković, S.; Kremenović, S.; Savić, P.; Golić-Jelić, A.; Stojaković, N.; Stoisavljević-Šatara, S.; Igić, R. Prevalence of Cigarette Smoking and Influence of Associated Factors among Students of the University of Banja Luka: A Cross-Sectional Study. Medicina 2022, 58, 502. [Google Scholar] [CrossRef]
- Natividad, L.A.; Torres, O.V.; Friedman, T.C.; O’Dell, L.E. Adolescence is a period of development characterized by short- and long-term vulnerability to the rewarding effects of nicotine and reduced sensitivity to the anorectic effects of this drug. Behav. Brain Res. 2013, 257, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Schassburger, R.L.; Pitzer, E.M.; Smith, T.T.; Rupprecht, L.E.; Thiels, E.; Donny, E.C.; Sved, A.F. Adolescent Rats Self-Administer Less Nicotine Than Adults at Low Doses. Nicotine Tob. Res. 2016, 18, 1861–1868. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, R.N.; Colby, S.M.; Tidey, J.W.; Jackson, K.M.; Cioe, P.A.; Krishnan-Sarin, S.; Hatsukami, D. Adolescent smokers’ response to reducing the nicotine content of cigarettes: Acute effects on withdrawal symptoms and subjective evaluations. Drug Alcohol Depend. 2018, 188, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, R.N.; Tidey, J.W.; Cao, Q.; Colby, S.M.; McClernon, F.J.; Koopmeiners, J.S.; Hatsukami, D.; Donny, E.C. Age Moderates Smokers’ Subjective Response to Very-Low Nicotine Content Cigarettes: Evidence from a Randomized Controlled Trial. Nicotine Tob. Res. 2019, 21, 962–969. [Google Scholar] [CrossRef]
- Jerzyński, T.; Stimson, G.V.; Shapiro, H.; Król, G. Estimation of the global number of e-cigarette users in 2020. Harm Reduct. J. 2021, 18, 1–10. [Google Scholar] [CrossRef]

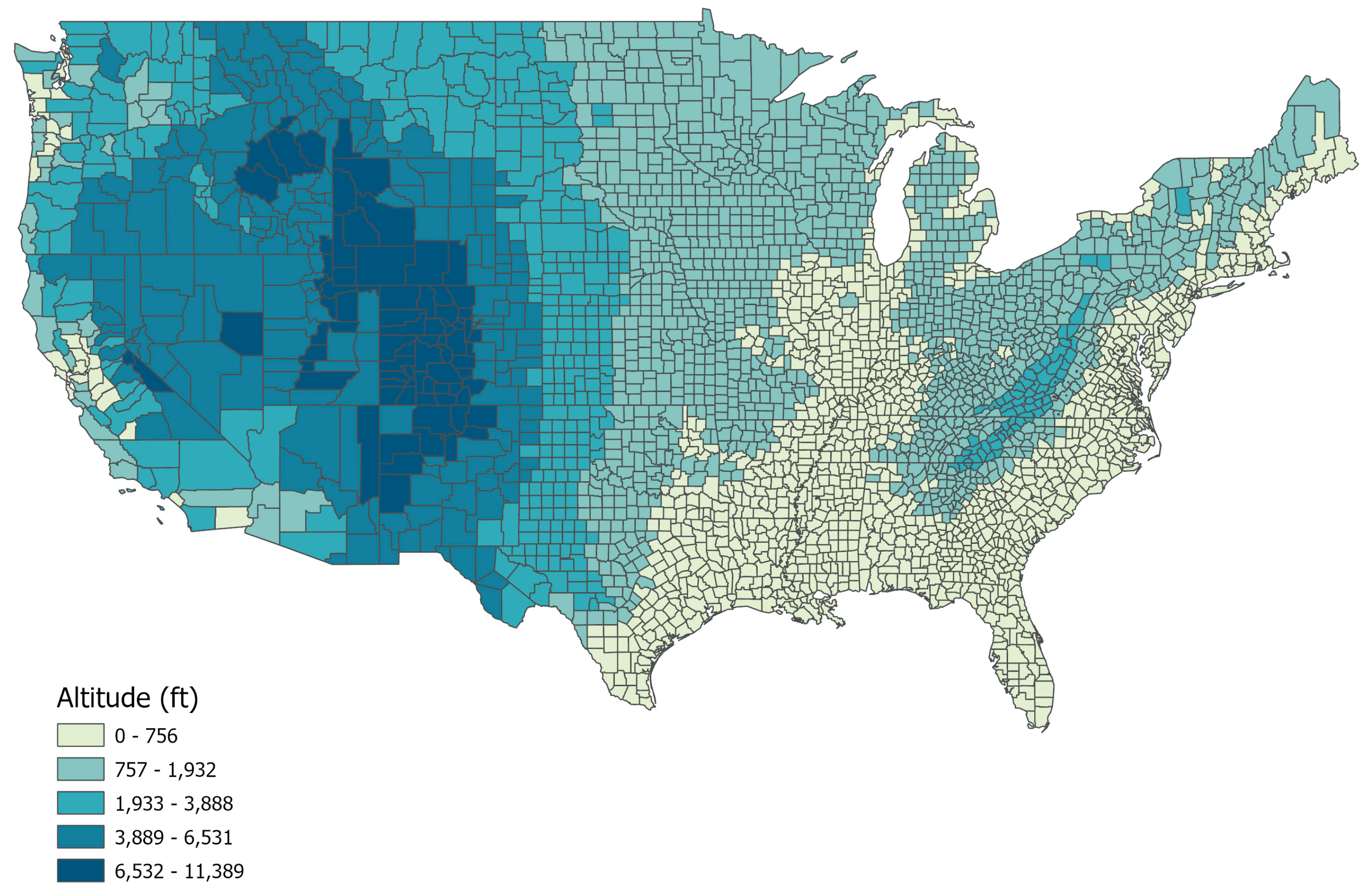
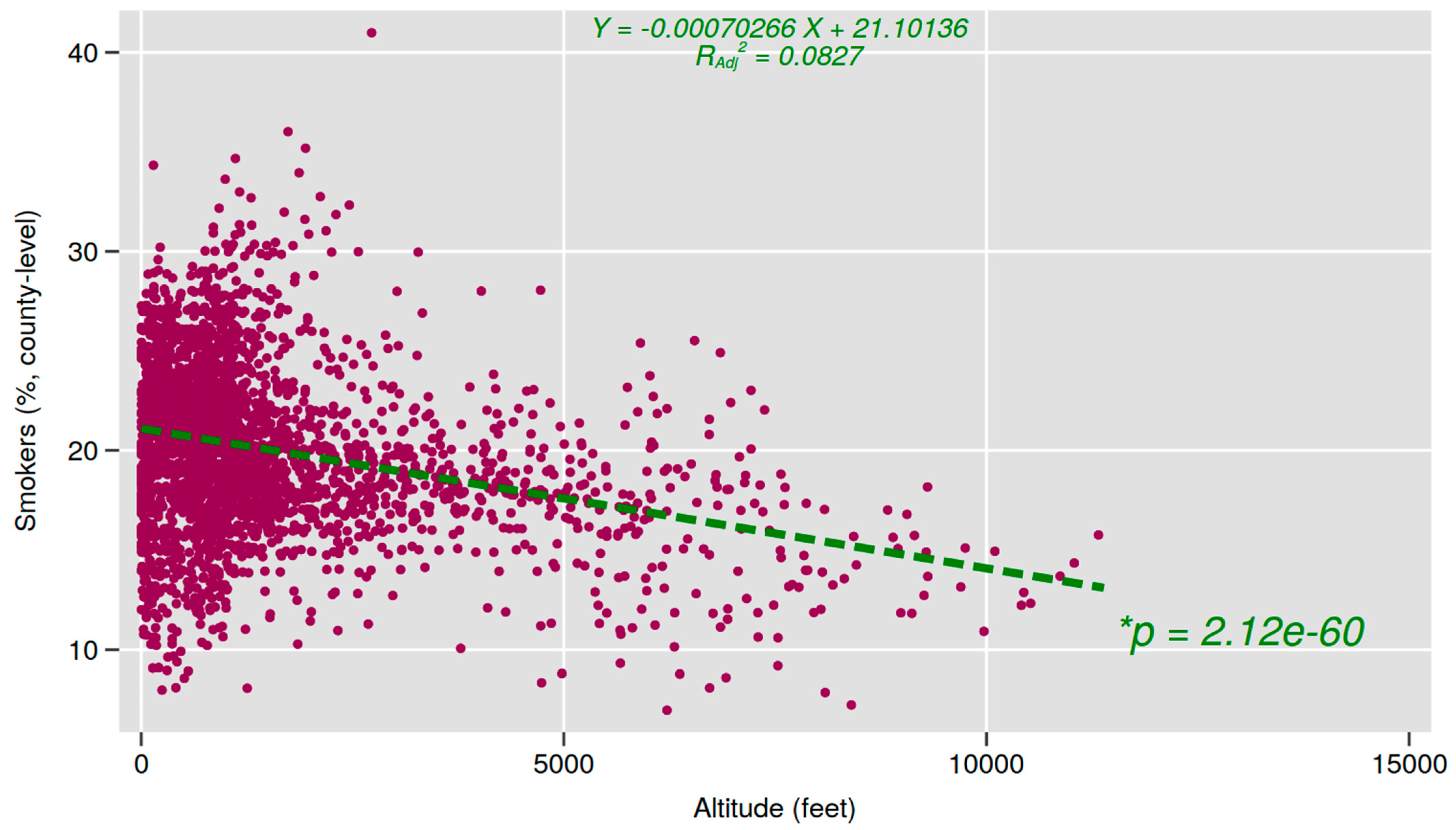
| No. | Mean | Median | SD | Min | Max | Pearson Correlation with % Smoking | p Value | |
|---|---|---|---|---|---|---|---|---|
| Environmental | ||||||||
| Altitude (ft) | 3106 | 1449.21 | 912.01 | 1665.62 | 2.63 | 11388.98 | −0.291 | ** |
| Demographic | ||||||||
| % Female | 3106 | 49.59 | 49.96 | 2.28 | 24.56 | 57.05 | −0.054 | * |
| % American Indian/Alaskan native | 3106 | 2.17 | 0.71 | 6.61 | 0.00 | 85.68 | 0.207 | ** |
| % African American | 3106 | 9.13 | 2.41 | 14.27 | 0.00 | 85.62 | 0.165 | ** |
| % Non-Hispanic white | 3106 | 75.51 | 82.60 | 19.92 | 2.68 | 97.59 | 0.069 | ** |
| % High school degree | 3106 | 87.94 | 89.21 | 5.87 | 49.67 | 99.40 | −0.525 | ** |
| % Rural | 3106 | 63.93 | 66.52 | 33.56 | 0.00 | 100.00 | 0.435 | ** |
| Health | ||||||||
| % Physically inactive | 3106 | 25.74 | 25.20 | 5.18 | 11.30 | 47.20 | 0.779 | ** |
| % Excessive drinking | 3106 | 19.07 | 18.85 | 3.22 | 8.19 | 28.93 | −0.371 | ** |
| % Frequent mental distress | 3106 | 15.76 | 15.70 | 2.02 | 10.10 | 23.30 | 0.779 | ** |
| Socioeconomic | ||||||||
| % Poverty | 3106 | 13.75 | 12.80 | 5.40 | 3.00 | 43.90 | 0.661 | ** |
| % Unemployed | 3106 | 4.62 | 4.40 | 1.70 | 0.89 | 17.30 | 0.180 | ** |
| State excise tax | 3106 | 1.34 | 1.20 | 0.95 | 0.17 | 4.50 | -0.323 | ** |
| β | Lower | Upper | p Value | |
|---|---|---|---|---|
| Mean county altitude (× 10 3, or per 1000 feet) | −0.143 | −0.178 | −0.108 | ** |
| % Female | −0.109 | −0.132 | −0.087 | ** |
| % American Indian/Alaskan native | 0.176 | 0.167 | 0.186 | ** |
| % African American | 0.049 | 0.043 | 0.055 | ** |
| % Non-Hispanic white | 0.113 | 0.108 | 0.118 | ** |
| % High school degree | −0.070 | −0.085 | −0.055 | ** |
| % Rural | 0.003 | 0.001 | 0.005 | * |
| % Physically inactive | 0.410 | 0.389 | 0.431 | ** |
| % Excessive drinking | 0.078 | 0.057 | 0.098 | ** |
| % Frequent mental distress | 0.518 | 0.479 | 0.558 | ** |
| % Poverty | 0.084 | 0.067 | 0.100 | ** |
| % Unemployed | 0.122 | 0.086 | 0.158 | ** |
| State excise tax | −0.199 | −0.260 | −0.138 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boxer, D.J.-M.; Sung, Y.-H.; Nunez, N.A.; Fitzgerald, C.E.; Renshaw, P.F.; Kondo, D.G. Exploring the Link between Altitude of Residence and Smoking Patterns in the United States. Int. J. Environ. Res. Public Health 2024, 21, 226. https://doi.org/10.3390/ijerph21020226
Boxer DJ-M, Sung Y-H, Nunez NA, Fitzgerald CE, Renshaw PF, Kondo DG. Exploring the Link between Altitude of Residence and Smoking Patterns in the United States. International Journal of Environmental Research and Public Health. 2024; 21(2):226. https://doi.org/10.3390/ijerph21020226
Chicago/Turabian StyleBoxer, Danielle Jeanne-Marie, Young-Hoon Sung, Nicolas A. Nunez, Colleen Elizabeth Fitzgerald, Perry Franklin Renshaw, and Douglas Gavin Kondo. 2024. "Exploring the Link between Altitude of Residence and Smoking Patterns in the United States" International Journal of Environmental Research and Public Health 21, no. 2: 226. https://doi.org/10.3390/ijerph21020226
APA StyleBoxer, D. J.-M., Sung, Y.-H., Nunez, N. A., Fitzgerald, C. E., Renshaw, P. F., & Kondo, D. G. (2024). Exploring the Link between Altitude of Residence and Smoking Patterns in the United States. International Journal of Environmental Research and Public Health, 21(2), 226. https://doi.org/10.3390/ijerph21020226







