Lifestyle Factors and Associations with Individual and Comorbid Cardiometabolic and Pulmonary Disease Among U.S. Adults
Abstract
1. Introduction
2. Materials and Methods
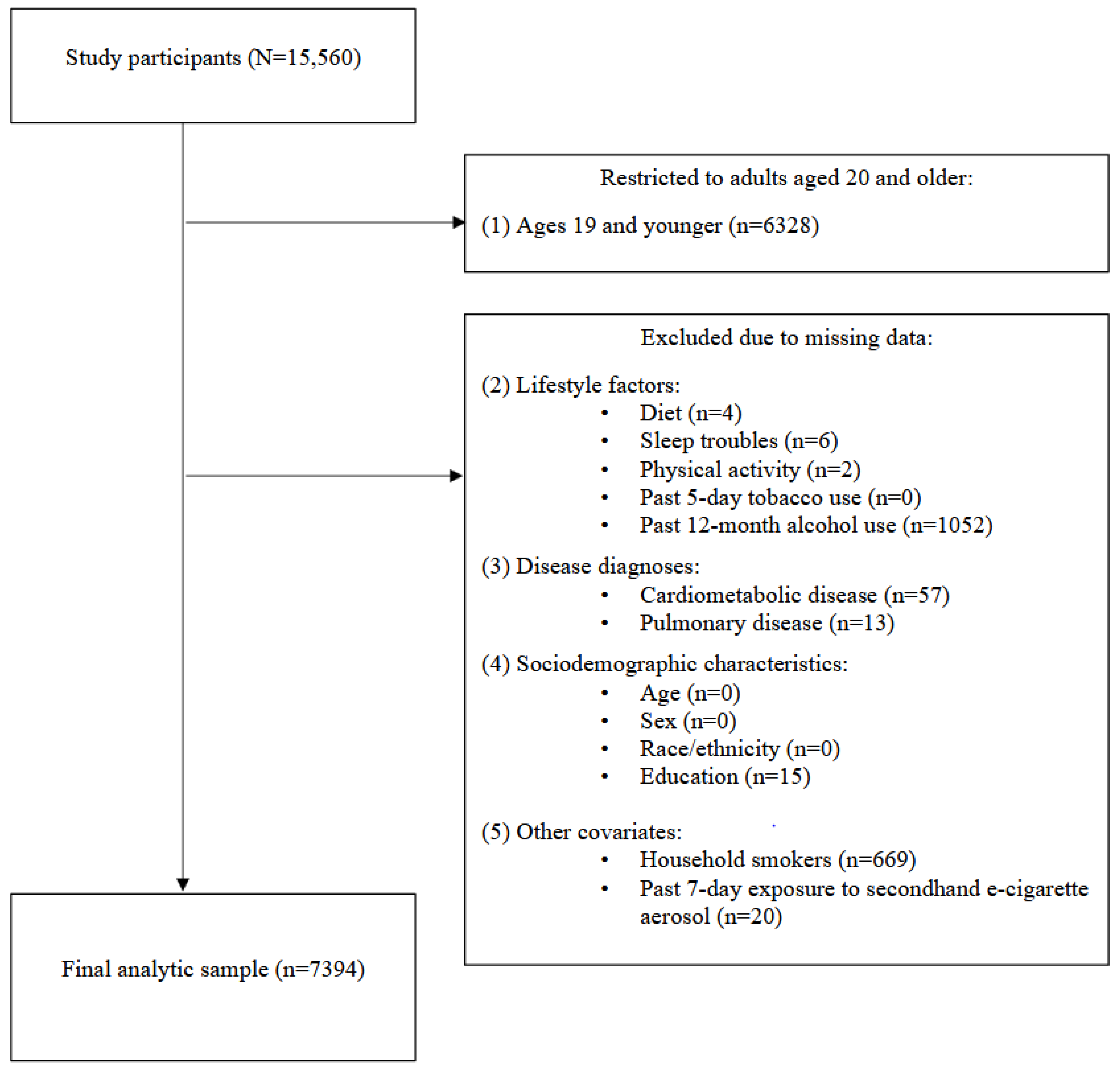
2.1. Participants
2.2. Primary Measures
2.2.1. Cardiometabolic and Pulmonary Disease
2.2.2. Lifestyle Factors
2.2.3. Sociodemographic and Environmental Characteristics
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
3.2. Lifestyle Factors and Cardiometabolic Disease
3.3. Lifestyle Factors and Pulmonary Disease
3.4. Lifestyle Factors and Comorbid CMD and Pulmonary Disease
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agbonlahor, O.; DeJarnett, N.; Hart, J.L.; Bhatnagar, A.; McLeish, A.C.; Walker, K.L. Racial/ethnic discrimination and cardiometabolic diseases: A systematic review. J. Racial Ethn. Health Disparities 2024, 11, 783–807. [Google Scholar] [CrossRef] [PubMed]
- Cebron Lipovec, N.; Beijers, R.J.; van den Borst, B.; Doehner, W.; Lainscak, M.; Schols, A.M. The prevalence of metabolic syndrome in chronic obstructive pulmonary disease: A systematic review. COPD 2016, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.S.; Aday, A.W.; Almarzooq, Z.I.; Anderson, C.A.; Arora, P.; Avery, C.L.; Baker-Smith, C.M.; Gibbs, B.B.; Beaton, A.Z.; Boehme, A.K.; et al. 2024 Heart disease and stroke statistics: A report of US and global data from the American Heart Association. Circulation 2024, 149, e347–e913. [Google Scholar] [CrossRef] [PubMed]
- Sciurba, F.C. Physiologic similarities and differences between COPD and asthma. Chest 2004, 126 (Suppl. S2), 117S–124S; discussion 159S–161S. [Google Scholar] [CrossRef]
- Wang, S.; Teng, H.; Zhang, L.; Wu, L. Association between dietary antioxidant intakes and chronic respiratory diseases in adults. World Allergy Organ. J. 2024, 17, 100851. [Google Scholar] [CrossRef]
- Fine, K.S.; Wilkins, J.T.; Sawicki, K.T. Circulating branched chain amino acids and cardiometabolic disease. J. Am. Heart Assoc. 2024, 13, e031617. [Google Scholar] [CrossRef]
- Liu, Y.; Carlson, S.A.; Watson, K.B.; Xu, F.; Greenlund, K.J. Trends in the prevalence of chronic obstructive pulmonary disease among adults aged ≥18 years—United States, 2011–2021. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 1250–1256. [Google Scholar] [CrossRef]
- Ghamari, S.H.; Mohebi, F.; Abbasi-Kangevari, M.; Peiman, S.; Rahimi, B.; Ahmadi, N.; Farzi, Y.; Seyfi, S.; Shahbal, N.; Modirian, M.; et al. Patient experience with chronic obstructive pulmonary disease: A nationally representative demonstration study on quality and cost of healthcare services. Front. Public Health 2023, 11, 1112072. [Google Scholar] [CrossRef]
- Viglino, D.; Martin, M.; Piché, M.E.; Brouillard, C.; Després, J.P.; Alméras, N.; Tan, W.C.; Coats, V.; Bourbeau, J.; Pépin, J.L.; et al. Metabolic profiles among COPD and controls in the CanCOLD population-based cohort. PLoS ONE 2020, 15, e0231072. [Google Scholar] [CrossRef]
- Miller, J.; Edwards, L.D.; Agustí, A.; Bakke, P.; Calverley, P.M.; Celli, B.; Coxson, H.O.; Crim, C.; Lomas, D.A.; Miller, B.E.; et al. Comorbidity, systemic inflammation and outcomes in the ECLIPSE cohort. Respir. Med. 2013, 107, 1376–1384. [Google Scholar] [CrossRef]
- Agbonlahor, O.; Osasuyi, O.; Mustapha, T. Health care provider lifestyle modification advice for adults with hypertension in the United States. Eur. J. Environ. Public Health 2023, 7, em0133. [Google Scholar] [CrossRef] [PubMed]
- Rosoff, D.B.; Davey Smith, G.; Mehta, N.; Clarke, T.K.; Lohoff, F.W. Evaluating the relationship between alcohol consumption, tobacco use, and cardiovascular disease: A multivariable mendelian randomization study. PLoS Med. 2020, 17, e1003410. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.F.; Hirschtick, J.L.; Fleischer, N.L.; Arenberg, D.A.; Barnes, G.D.; Levy, D.T.; Sanchez-Romero, L.M.; Jeon, J.; Meza, R. Cigarettes, ENDS use, and chronic obstructive pulmonary disease incidence: A prospective longitudinal study. Am. J. Prev. Med. 2023, 65, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, Y.; Zhao, M.; Yu, X.; Zhang, C.; Magnussen, C.G.; Xi, B. Association of the American Heart Association’s new “Life’s Essential 8” with all-cause and cardiovascular disease-specific mortality: Prospective cohort study. BMC Med. 2023, 21, 116. [Google Scholar] [CrossRef]
- Shi, S.; Huang, H.; Huang, Y.; Zhong, V.W.; Feng, N. Lifestyle behaviors and cardiometabolic diseases by race and ethnicity and social risk factors among US young adults, 2011 to 2018. J. Am. Heart Assoc. 2023, 12, e028926. [Google Scholar] [CrossRef]
- Ussery, E.N.; Fulton, J.E.; Galuska, D.A.; Katzmarzyk, P.T.; Carlson, S.A. Joint prevalence of sitting time and leisure-time physical activity among US adults, 2015–2016. JAMA 2018, 320, 2036–2038. [Google Scholar] [CrossRef]
- Liu, J.J.; Micha, R.; Li, Y.; Mozaffarian, D. Trends in food sources and diet quality among US children and adults, 2003–2018. JAMA Netw. Open 2021, 4, e215262. [Google Scholar] [CrossRef]
- Gutkind, S.; Fink, D.S.; Shmulewitz, D.; Stohl, M.; Hasin, D. Psychosocial and health problems associated with alcohol use disorder and cannabis use disorder in U.S. adults. Drug Alcohol Depend. 2021, 229 Pt B, 109137. [Google Scholar] [CrossRef]
- Di, H.; Guo, Y.; Daghlas, I.; Wang, L.; Liu, G.; Pan, A.; Liu, L.; Shan, Z. Evaluation of sleep habits and disturbances among US adults, 2017–2020. JAMA Netw. Open 2022, 5, e2240788. [Google Scholar] [CrossRef]
- NCHS. National Health and Nutrition Examination Survey (NHANES) 2017–March 2020 Pre-Pandemic; National Center for Health Statistics: Hyattsville, MD, USA, 2020. Available online: https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/default.aspx?Cycle=2017-20 (accessed on 23 August 2024).
- Glechner, A.; Keuchel, L.; Affengruber, L.; Titscher, V.; Sommer, I.; Matyas, N.; Wagner, G.; Kien, C.; Klerings, I.; Gartlehner, G. Effects of lifestyle changes on adults with prediabetes: A systematic review and meta-analysis. Prim. Care Diabetes 2018, 12, 393–408. [Google Scholar] [CrossRef]
- NCHS. NHANES Survey Methods and Analytic Guidelines; National Center for Health Statistics: Hyattsville, MD, USA, 2020. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 23 August 2024).
- Paing, P.Y.; Littman, A.J.; Reese, J.A.; Sitlani, C.M. Association of achievement of the American Heart Association’s Life’s Essential 8 goals with incident cardiovascular diseases in the SHFS. J. Am. Heart Assoc. 2024, 13, e032918. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, S.T.; Singh-Manoux, A.; Pentti, J.; Madsen, I.E.; Sabia, S.; Alfredsson, L.; Bjorner, J.B.; Borritz, M.; Burr, H.; Goldberg, M.; et al. Association of healthy lifestyle with years lived without major chronic diseases. JAMA Intern. Med. 2020, 80, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, X.; Wei, L.; Yang, K.; Jiao, M. The impact of high-risk lifestyle factors on all-cause mortality in the US non-communicable disease population. BMC Public Health 2023, 23, 422. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Ouyang, Y.; Yang, Y.; Liu, Z.; Zhao, M. Impact of sleep problems on the cardiometabolic risks: An integrated epidemiological and metabolomics study. Diabetol. Metab. Syndr. 2024, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S. Sleep and cardiometabolic health-not so strange bedfellows. Lancet Diabetes Endocrinol. 2023, 11, 532–534. [Google Scholar] [CrossRef]
- Brainard, J.; Gobel, M.; Scott, B.; Koeppen, M.; Eckle, T. Health implications of disrupted circadian rhythms and the potential for daylight as therapy. Anesthesiology 2015, 122, 1170–1175. [Google Scholar] [CrossRef]
- Lei, Y.; Zou, K.; Xin, J.; Wang, Z.; Liang, K.; Zhao, L.; Ma, X. Sedentary behavior is associated with chronic obstructive pulmonary disease: A generalized propensity score-weighted analysis. Medicine 2021, 100, e25336. [Google Scholar] [CrossRef]
- Otieno, P.; Asiki, G.; Wekesah, F.; Wilunda, C.; Sanya, R.E.; Wami, W.; Agyemang, C. Multimorbidity of cardiometabolic diseases: A cross-sectional study of patterns, clusters and associated risk factors in sub-Saharan Africa. BMJ Open 2023, 13, e064275. [Google Scholar] [CrossRef]
- Oates, G.R.; Jackson, B.E.; Partridge, E.E.; Singh, K.P.; Fouad, M.N.; Bae, S. Sociodemographic patterns of chronic disease: How the Mid-South region compares to the rest of the country. Am. J. Prev. Med. 2017, 52, S31–S39. [Google Scholar] [CrossRef]
- Knutson, K.L. Sociodemographic and cultural determinants of sleep deficiency: Implications for cardiometabolic disease risk. Soc. Sci. Med. 2013, 79, 7–15. [Google Scholar] [CrossRef]
- Churchwell, K.; Elkind, M.S.V.; Benjamin, R.M.; Carson, A.P.; Chang, E.K.; Lawrence, W.; Mills, A.; Odom, T.M.; Rodriguez, C.J.; Rodriguez, F.; et al. Call to action: Structural racism as a fundamental driver of health disparities: A presidential advisory from the American Heart Association. Circulation 2020, 142, e454–e468. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Haisum Maqsood, M.; Yahya, T.; Amin, Z.; Acquah, I.; Valero-Elizondo, J.; Andrieni, J.; Dubey, P.; Jackson, R.K.; Daffin, M.A.; et al. Race, racism, and cardiovascular health: Applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ. Cardiovasc. Qual. Outcomes 2022, 15, e007917. [Google Scholar] [CrossRef] [PubMed]
- Carnethon, M.R.; Pu, J.; Howard, G.; Albert, M.A.; Anderson, C.A.M.; Bertoni, A.G.; Mujahid, M.S.; Palaniappan, L.; Taylor, H.A., Jr.; Willis, M.; et al. Cardiovascular health in African Americans: A scientific statement from the American Heart Association. Circulation 2017, 136, e393–e423. [Google Scholar] [CrossRef] [PubMed]
- Griffin, S.F.; Wilson, D.K.; Wilcox, S.; Buck, J.; Ainsworth, B.E. Physical activity influences in a disadvantaged African American community and the communities’ proposed solutions. Health Promot. Pract. 2008, 9, 180–190. [Google Scholar] [CrossRef]
- Appel, L.J. Lifestyle modification as a means to prevent and treat high blood pressure. J. Am. Soc. Nephrol. 2003, 14 (Suppl. S2), S99–S102. [Google Scholar] [CrossRef]
- Jones, R.C.; Hyland, M.E.; Hanney, K.; Erwin, J. A qualitative study of compliance with medication and lifestyle modification in chronic obstructive pulmonary disease (COPD). Prim. Care Respir. J. 2004, 13, 149–154. [Google Scholar] [CrossRef][Green Version]
- Zhang, D.; Liu, Y.; Cheng, C.; Wang, Y.; Xue, Y.; Li, W.; Li, X. Dose-related effect of secondhand smoke on cardiovascular disease in nonsmokers: Systematic review and meta-analysis. Int. J. Hyg. Environ. Health 2020, 228, 113546. [Google Scholar] [CrossRef]
- Su, W.C.; Juan, H.L.; Lee, J.I.; Huang, S.P.; Chen, S.C.; Geng, J.H. Secondhand smoke increases the risk of developing chronic obstructive pulmonary disease. Sci. Rep. 2024, 14, 7481. [Google Scholar] [CrossRef]
| Variables | n (%) | 95% CI |
|---|---|---|
| Age (years) | ||
| 20–34 | 1712 (27.4) | 25.2, 29.6 |
| 35–49 | 1715 (24.7) | 22.6, 27.0 |
| 50–59 | 1278 (18.2) | 16.5, 19.9 |
| 60 and older | 2689 (29.7) | 26.6, 33.2 |
| Sex | ||
| Male | 3617 (48.4) | 46.7, 50.1 |
| Female | 3777 (51.6) | 49.9, 53.3 |
| Education level completed | ||
| <High school | 1309 (10.3) | 9.2, 11.4 |
| High school/GED | 1799 (27.1) | 24.3, 30.2 |
| Associate degree | 2458 (30.5) | 28.6, 32.6 |
| College degree | 1828 (32.1) | 27.7, 36.8 |
| Race/ethnicity | ||
| Mexican American | 864 (8.2) | 6.1, 11.0 |
| Other Hispanic | 750 (7.3) | 5.9, 8.9 |
| NH White | 2656 (64.3) | 59.3, 69.0 |
| NH Black | 1951 (10.9) | 8.4, 14.2 |
| Other races or ethnicities | 1173 (9.3) | 7.5, 11.4 |
| CMD | ||
| No | 2784 (41.7) | 39.3, 44.2 |
| Yes | 4610 (58.3) | 55.8, 60.7 |
| PD | ||
| No | 6712 (91.3) | 90.1, 92.4 |
| Yes | 682 (8.7) | 7.6, 9.9 |
| Chronic disease morbidity | ||
| No disease | 2653 (39.9) | 37.4, 42.5 |
| Comorbid CMD and PD | 551 (6.9) | 5.9, 8.0 |
| Individual CMD or PD | 4190 (53.2) | 50.9, 55.5 |
| Past 5-day tobacco use | ||
| No | 5646 (77.4) | 75.0, 79.7 |
| Yes | 1748 (22.6) | 20.3, 25.0 |
| Past 12-month alcohol use | ||
| Never | 675 (6.7) | 5.9, 7.7 |
| Former | 1486 (16.2) | 14.9, 17.5 |
| Past year | 5233 (77.1) | 75.7, 78.5 |
| Diet | ||
| Good | 4927 (69.4) | 67.4, 71.3 |
| Poor | 2467 (30.6) | 28.7, 32.6 |
| Sleep troubles | ||
| No | 5223 (69.2) | 67.0, 71.2 |
| Yes | 2171 (30.8) | 28.8, 33.0 |
| Physical activity | ||
| No | 3876 (55.3) | 52.4, 58.0 |
| Yes | 3518 (44.7) | 42.0, 47.6 |
| Household smokers | ||
| No | 5045 (71.0) | 67.6, 74.3 |
| Yes | 2349 (29.0) | 25.7, 32.4 |
| Past 7-day secondhand e-cigarette exposure | ||
| No | 6386 (84.5) | 82.1, 86.7 |
| Yes | 1008 (15.5) | 13.3, 17.9 |
| Cardiometabolic Disease (CMD) a | ||
|---|---|---|
| OR b | 95% CI | |
| Past 5-day tobacco use (ref: no) | ||
| Yes | 0.83 | 0.66, 1.04 |
| Past 12-month alcohol use (ref: never) | ||
| Former alcohol use | 1.67 | 1.25, 2.24 |
| Past year alcohol use | 1.27 | 0.97, 1.66 |
| Age (ref: 60 years and older) | ||
| 20–34 years | 0.22 | 0.17, 0.28 |
| 35–49 years | 0.37 | 0.29, 0.48 |
| 50–59 years | 0.56 | 0.39, 0.80 |
| Sex (ref: male) | ||
| Female | 0.96 | 0.80, 1.15 |
| Race/ethnicity (ref: non-Hispanic White) | ||
| Mexican American | 1.18 | 0.91, 1.53 |
| Other Hispanic | 0.94 | 0.77, 1.15 |
| Non-Hispanic Black | 1.29 | 1.02, 1.62 |
| Other races/ethnicities c | 1.11 | 0.91, 1.35 |
| Education completed (ref: less than high school) | ||
| High school/GED | 1.37 | 1.06, 1.77 |
| Associate degree | 1.58 | 1.26, 1.99 |
| College degree | 1.12 | 0.87, 1.44 |
| Sleep troubles (ref: no) | ||
| Yes | 2.47 | 2.17, 2.82 |
| Physical activity (ref: no) | ||
| Yes | 0.75 | 0.62, 0.91 |
| Diet (ref: good) | ||
| Poor | 1.80 | 1.48, 2.18 |
| Household smokers (ref: no) | ||
| Yes | 0.90 | 0.69, 1.18 |
| Past 7-day secondhand e-cigarette exposure (ref: no) | ||
| Yes | 1.12 | 0.81, 1.55 |
| Pulmonary Disease (PD) a | ||
|---|---|---|
| OR b | 95% CI | |
| Past 5-day tobacco use (ref: no) | ||
| Yes | 2.36 | 1.58, 3.53 |
| Past 12-month alcohol use (ref: never) | ||
| Former alcohol use | 2.90 | 1.84, 4.59 |
| Past year alcohol use | 1.61 | 1.06, 2.43 |
| Age (ref: 60 years and older) | ||
| 20–34 years | 0.15 | 0.08, 0.27 |
| 35–49 years | 0.25 | 0.16, 0.38 |
| 50–59 years | 0.40 | 0.26, 0.62 |
| Sex (ref: male) | ||
| Female | 1.20 | 0.83, 1.73 |
| Race/ethnicity (ref: non-Hispanic White) | ||
| Mexican American | 0.35 | 0.21, 0.57 |
| Other Hispanic | 0.70 | 0.50, 0.99 |
| Non-Hispanic Black | 0.69 | 0.48, 1.01 |
| Other races/ethnicities c | 1.00 | 0.76, 1.32 |
| Education completed (ref: less than high school) | ||
| High school/GED | 1.23 | 0.92, 1.64 |
| Associate degree | 0.89 | 0.62, 1.28 |
| College degree | 0.51 | 0.31, 0.83 |
| Sleep troubles (ref: no) | ||
| Yes | 2.29 | 1.86, 2.82 |
| Physical activity (ref: no) | ||
| Yes | 0.77 | 0.62, 0.96 |
| Diet (ref: good) | ||
| Poor | 1.20 | 0.94, 1.52 |
| Household smokers (ref: no) | ||
| Yes | 1.57 | 1.13, 2.19 |
| Past 7-day secondhand e-cigarette exposure (ref: no) | ||
| Yes | 1.41 | 0.89, 2.23 |
| Comorbid CMD and PD a | Individual CMD or PD a | Comorbid vs. Individual CMD or PD b | ||||
|---|---|---|---|---|---|---|
| OR c | 95% CI | OR c | 95% CI | OR c | 95% CI | |
| Past 5-day tobacco use (ref: no) | ||||||
| Yes | 1.78 | 1.09, 2.90 | 0.88 | 0.66, 1.16 | 2.03 | 1.34, 3.07 |
| Past 12-month alcohol use (ref: never) | ||||||
| Former alcohol use | 4.52 | 2.77, 7.39 | 1.61 | 1.20, 2.18 | 2.80 | 1.88, 4.18 |
| Past year alcohol use | 1.93 | 1.15, 3.25 | 1.28 | 0.96, 1.70 | 1.51 | 0.97, 2.35 |
| Age (ref: 60 years and older) | ||||||
| 20–34 years | 0.05 | 0.02, 0.10 | 0.22 | 0.17, 0.27 | 0.21 | 0.10, 0.48 |
| 35–49 years | 0.13 | 0.08, 0.21 | 0.35 | 0.27, 0.46 | 0.37 | 0.23, 0.60 |
| 50–59 years | 0.25 | 0.13, 0.46 | 0.56 | 0.39, 0.79 | 0.44 | 0.28, 0.70 |
| Sex (ref: male) | ||||||
| Female | 1.12 | 0.74, 1.71 | 0.97 | 0.81, 1.16 | 1.15 | 0.83, 1.60 |
| Race/ethnicity (ref: non-Hispanic White) | ||||||
| Mexican American | 0.44 | 0.24, 0.79 | 1.18 | 0.91, 1.53 | 0.37 | 0.22, 0.63 |
| Other Hispanic | 0.70 | 0.50, 0.98 | 0.93 | 0.76, 1.14 | 0.75 | 0.52, 1.07 |
| Non-Hispanic Black | 0.94 | 0.57, 1.55 | 1.25 | 1.01, 1.57 | 0.75 | 0.52, 1.09 |
| Other races/ethnicities d | 1.01 | 0.70, 1.47 | 1.15 | 0.92, 1.43 | 0.88 | 0.60, 1.30 |
| Education completed (ref: <high school) | ||||||
| High school/GED | 1.54 | 1.03, 2.30 | 1.42 | 1.07, 1.88 | 1.09 | 0.77, 1.53 |
| Associate degree | 1.30 | 0.82, 2.07 | 1.62 | 1.26, 2.07 | 0.80 | 0.54, 1.19 |
| College degree | 0.54 | 0.28, 1.03 | 1.15 | 0.89, 1.50 | 0.47 | 0.27, 0.82 |
| Sleep troubles (ref: no) | ||||||
| Yes | 5.12 | 4.10, 6.38 | 2.40 | 2.09, 2.75 | 2.13 | 1.66, 2.74 |
| Physical activity (ref: no) | ||||||
| Yes | 0.59 | 0.43, 0.80 | 0.77 | 0.62, 0.94 | 0.77 | 0.57, 1.04 |
| Diet (ref: good) | ||||||
| Poor | 1.98 | 1.43, 2.75 | 1.80 | 1.46, 2.22 | 1.10 | 0.87, 1.42 |
| Household smokers (ref: no) | ||||||
| Yes | 1.42 | 0.86, 2.35 | 0.90 | 0.68, 1.18 | 1.59 | 1.08, 2.33 |
| Past 7-day secondhand e-cigarette exposure (ref: no) | ||||||
| Yes | 1.56 | 0.83, 2.94 | 1.11 | 0.82, 1.49 | 1.41 | 0.86, 2.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbonlahor, O.; Mattingly, D.T.; Richardson, M.K.; Hart, J.L.; McLeish, A.C.; Walker, K.L. Lifestyle Factors and Associations with Individual and Comorbid Cardiometabolic and Pulmonary Disease Among U.S. Adults. Int. J. Environ. Res. Public Health 2024, 21, 1674. https://doi.org/10.3390/ijerph21121674
Agbonlahor O, Mattingly DT, Richardson MK, Hart JL, McLeish AC, Walker KL. Lifestyle Factors and Associations with Individual and Comorbid Cardiometabolic and Pulmonary Disease Among U.S. Adults. International Journal of Environmental Research and Public Health. 2024; 21(12):1674. https://doi.org/10.3390/ijerph21121674
Chicago/Turabian StyleAgbonlahor, Osayande, Delvon T. Mattingly, Maggie K. Richardson, Joy L. Hart, Alison C. McLeish, and Kandi L. Walker. 2024. "Lifestyle Factors and Associations with Individual and Comorbid Cardiometabolic and Pulmonary Disease Among U.S. Adults" International Journal of Environmental Research and Public Health 21, no. 12: 1674. https://doi.org/10.3390/ijerph21121674
APA StyleAgbonlahor, O., Mattingly, D. T., Richardson, M. K., Hart, J. L., McLeish, A. C., & Walker, K. L. (2024). Lifestyle Factors and Associations with Individual and Comorbid Cardiometabolic and Pulmonary Disease Among U.S. Adults. International Journal of Environmental Research and Public Health, 21(12), 1674. https://doi.org/10.3390/ijerph21121674






