Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
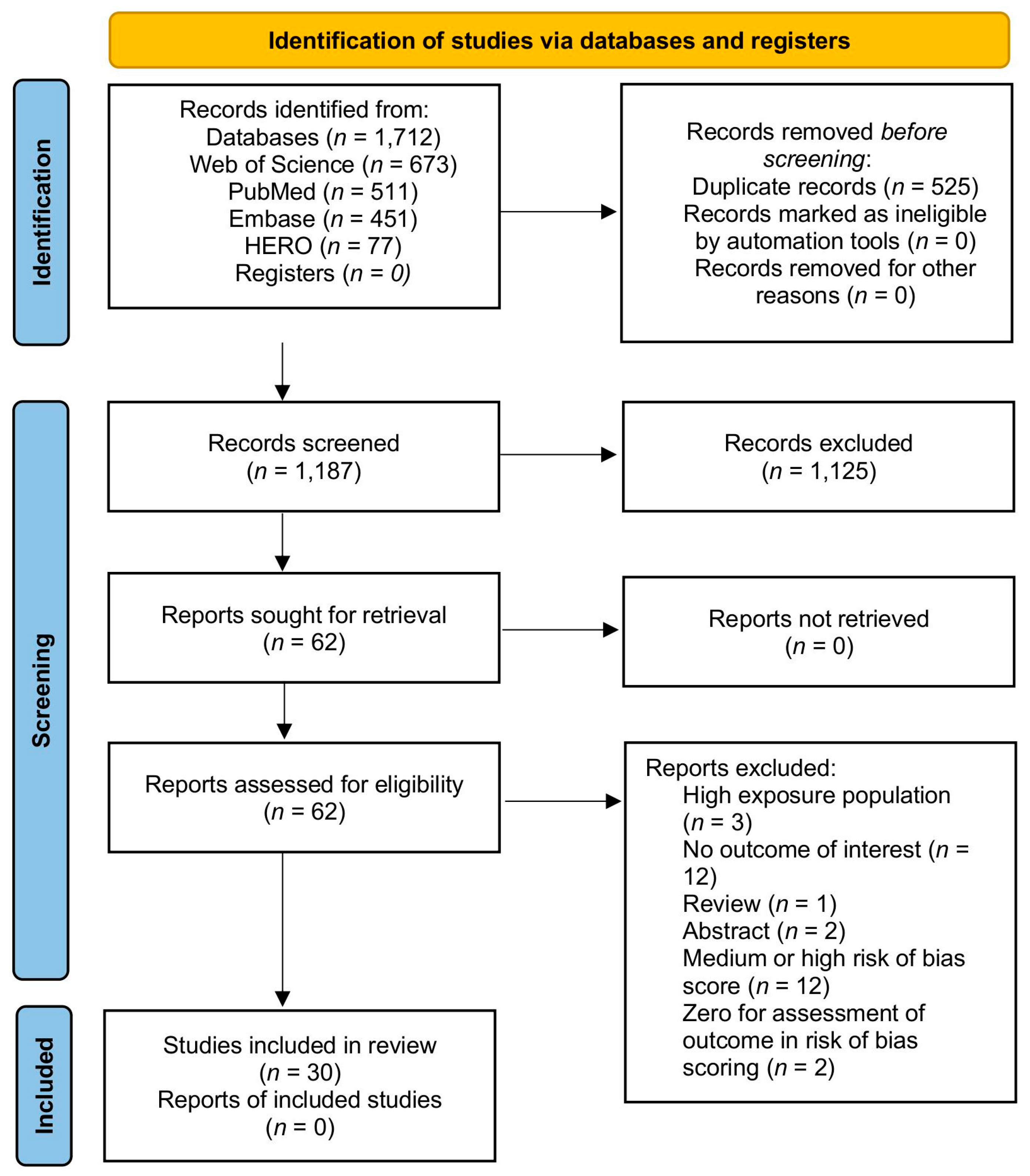
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items
2.7. Study Risk of Bias Assessment
2.8. Effect Measures
2.9. Synthesis Methods
2.10. Reporting Bias Assessment and Certainty Assessment
3. Results and Discussion
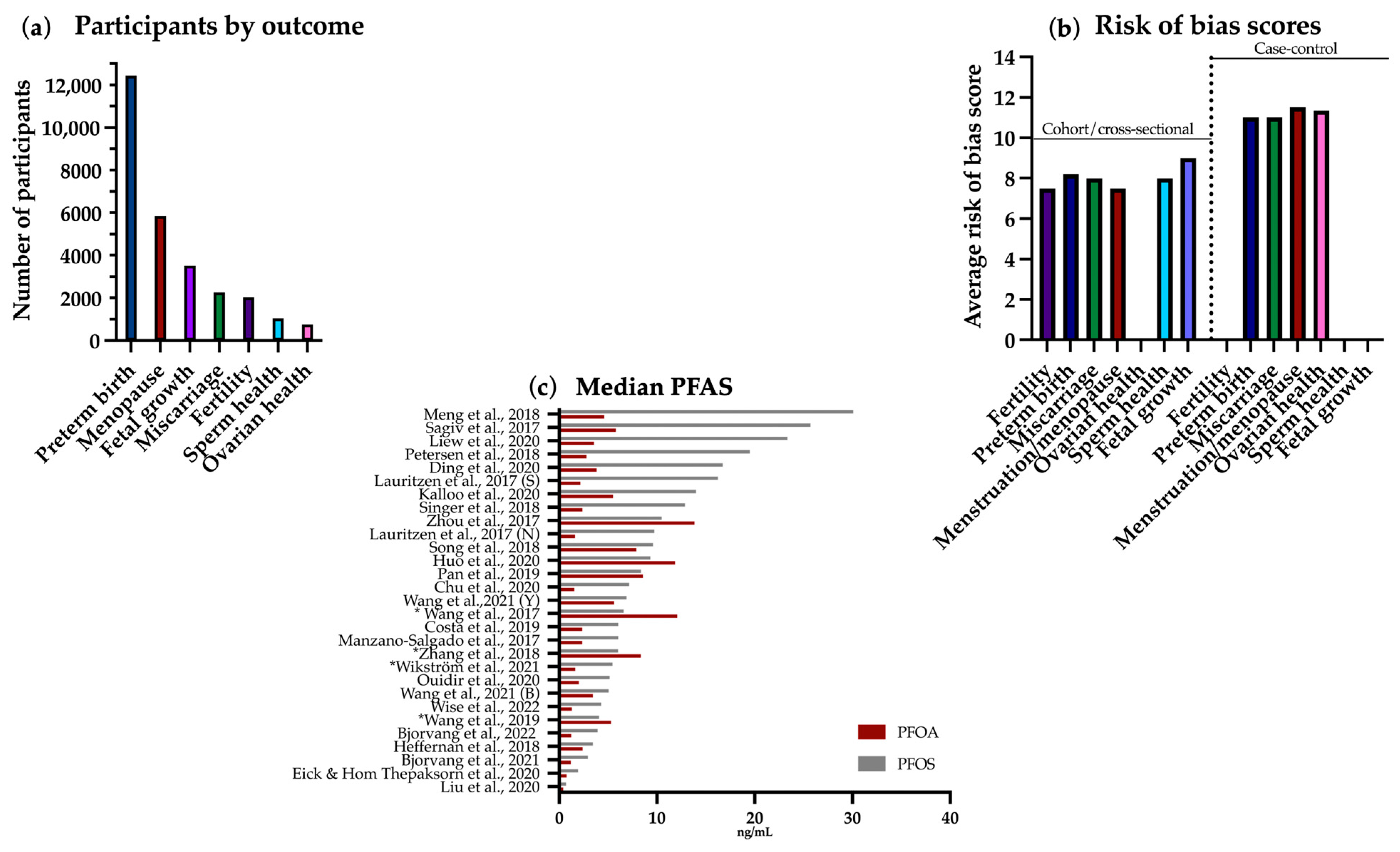
3.1. Included Studies: Overview and Characteristics
3.2. Fertility
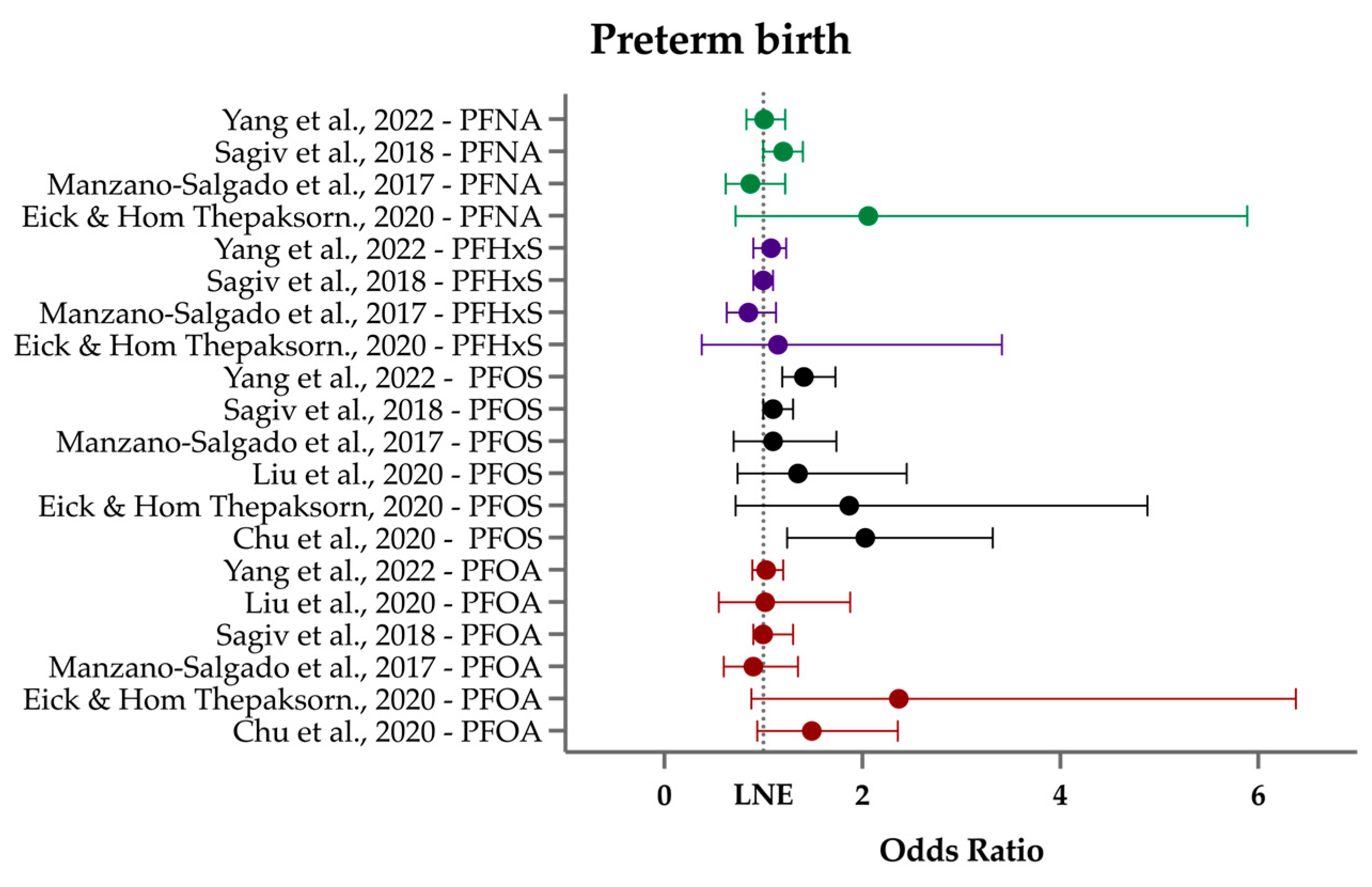
3.3. Preterm Birth/Gestational Age at Birth
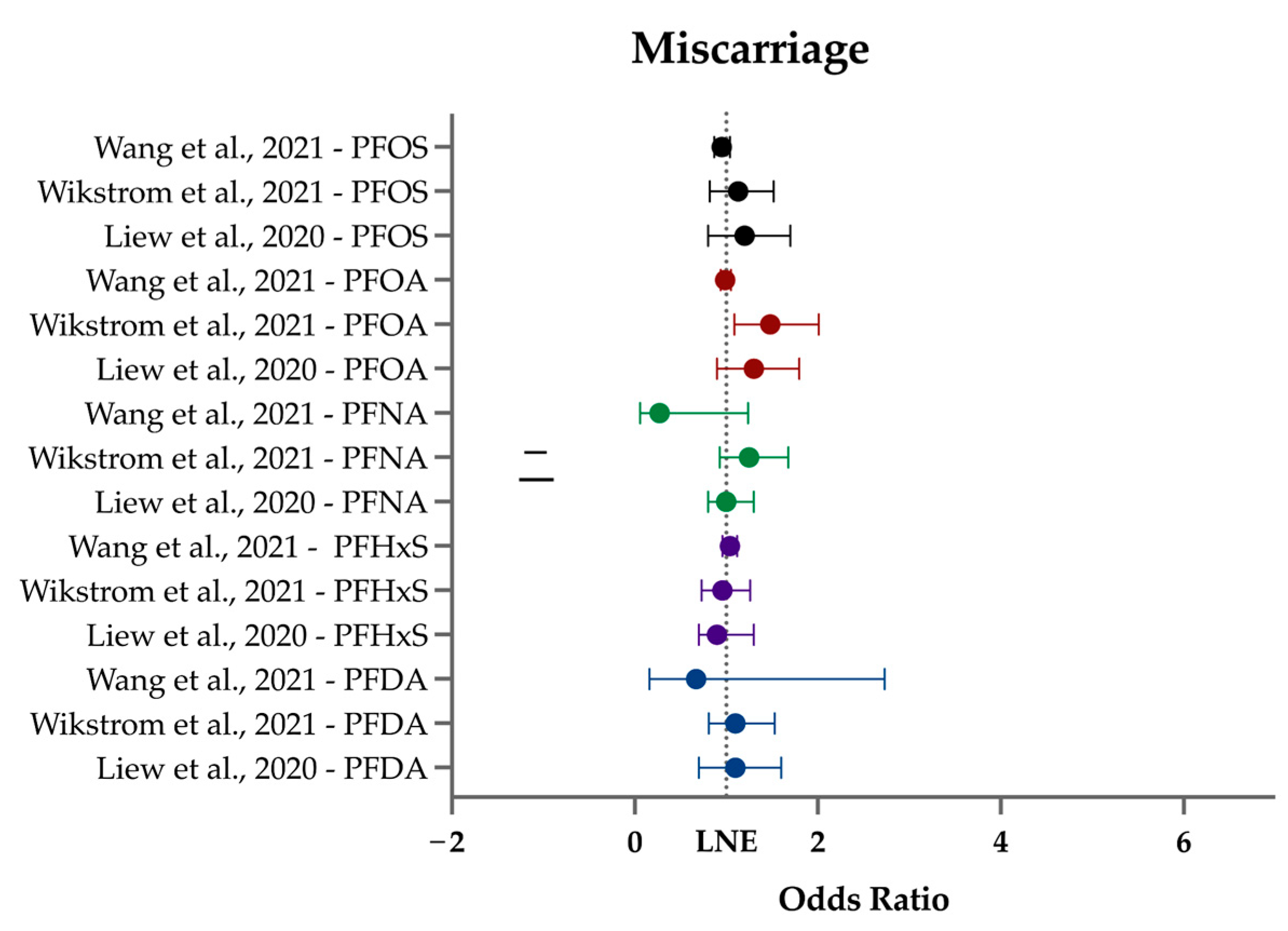
3.4. Miscarriage
3.5. Menopause and Menstruation
3.5.1. Menopause
3.5.2. Menstruation
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Sub-Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|---|
| Ding et al. (2020) [62] | Prospective cohort | 1120 | PFHxS, PFNA, n-PFOA, n-PFOS, Sm-PFOS | PFDA, PFDoDA, Sb-PFOA, PFUnDA | Menopause | Incident natural menopause | Serum | USA |
| Heffernan et al. (2018) [81] | Case–control | Case: 30; Control: 29 | PFHxS, PFNA, PFOA, PFOS | PFBS, PFDA, PFHpA, PFPeA, PFUnDA | Menstruation | Irregular cycle | Serum | UK |
| Singer et al. (2018) [65] | Prospective cohort | 1977 | PFDA, PFHpS, PFHxS, PFNA, PFOA, PFOA, PFUnDA | NA | Menstruation | Cycle regularity and length | Plasma | Norway |
| Wise et al. (2022) [78] | Cohort | 1499 | MeFOSAA, PFDA, PFHxS, PFNA, PFOA, PFOS, PFUnDA | NA | Menstruation | Age at menarche, cycle length and intensity | Plasma | USA |
| Zhang et al. (2018) [75] | Case–control | Cases: 120; Controls: 120 | PFBS, PFDeA, PFDoA, PFHpA, PFHxS, PFNA, PFOA, PFOS, PFUA | PFOSA | Menstruation | Primary ovarian insufficiency | Plasma | China |
| Zhou et al. (2017) [66] | Cohort | 950 | PFBS, PFDeA, PFDoA, PFHpS, PFHxS, PFNA, PFOA, PFOS, PFOSA, PFUA | NA | Menstruation | Cycle regularity, length, and volume | Blood | China |
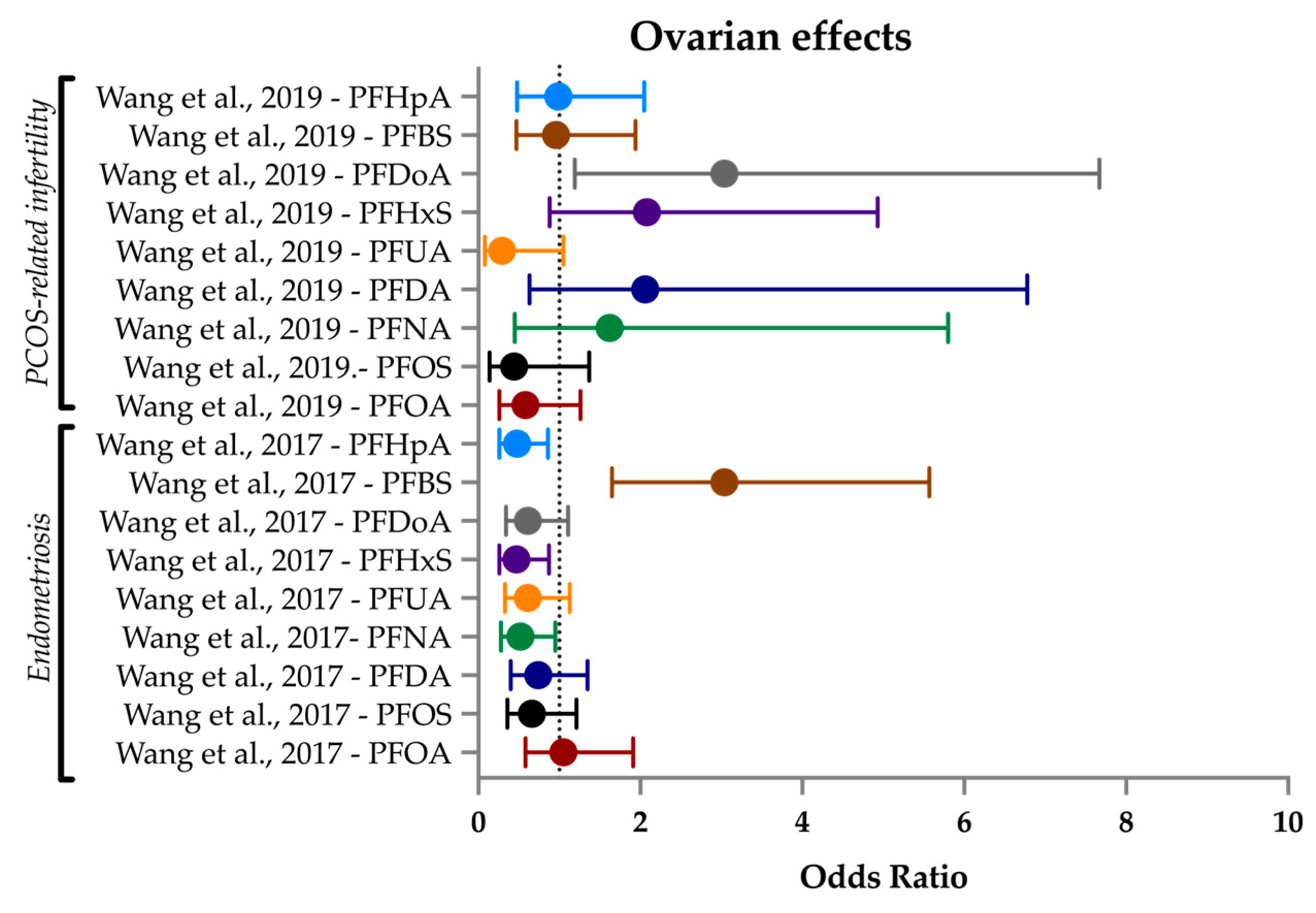
3.6. Ovarian Health
3.6.1. PCOS
3.6.2. Endometriosis
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Sub-Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|---|
| Heffernan et al. (2018) [81] | Case–control | Cases: 30; Controls: 29 | PFHxS, PFNA, PFOA, PFOS | PFBS, PFDA, PFHpA, PFPeA, PFUnDA | Ovarian health | PCOS | Serum, follicular fluid | UK |
| Wang et al. (2017) [72] | Case–control | Cases: 157; Controls: 178 | PFBS, PFDA, PFDoA, PFHpA, PFHxS, PFNA, PFOA, PFOS, PFUA | PFOSA | Ovarian health | Endometriosis-related infertility | Plasma | China |
| Wang et al. (2019) [184] | Case–control | Cases: 180; Controls: 187 | PFBS, PFDA, PFDoA, PFHpA, PFHxS, PFNA, PFOA, PFOS, PFUA | PFOSA | Ovarian health | PCOS-related infertility | Plasma | China |
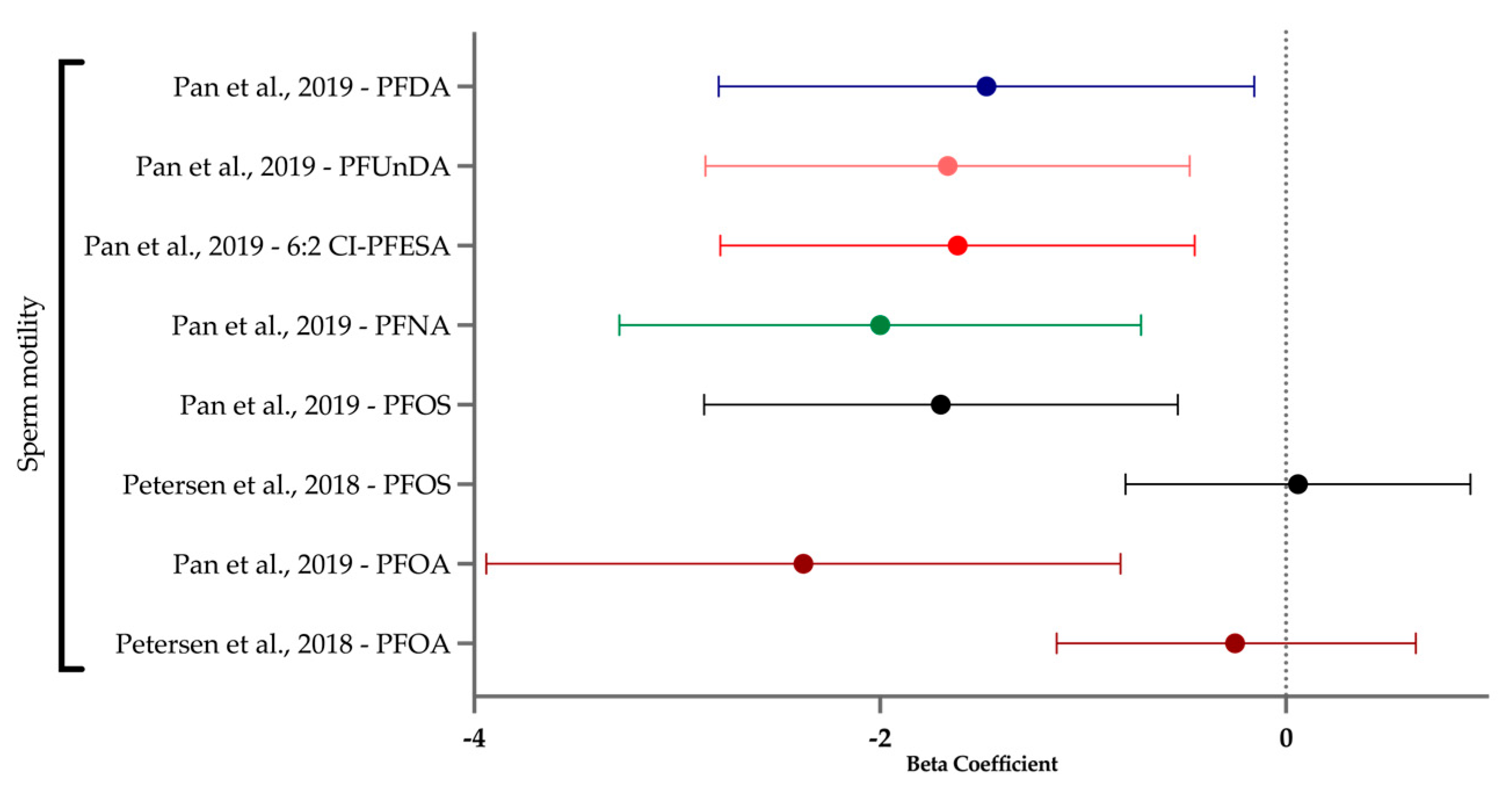
3.7. Sperm Health
3.8. Fetal Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Registration and Protocol
Conflicts of Interest
References
- Roseboom, T.J.; van der Meulen, J.H.P.; Ravelli, A.C.J.; Osmond, C.; Barker, D.J.P.; Bleker, O.P. Effects of Prenatal Exposure to the Dutch Famine on Adult Disease in Later Life: An Overview. Twin Res. 2001, 4, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Laine, J.E.; Ray, P.; Bodnar, W.; Cable, P.H.; Boggess, K.; Offenbacher, S.; Fry, R.C. Placental Cadmium Levels Are Associated with Increased Preeclampsia Risk. PLoS ONE 2015, 10, e0139341. [Google Scholar] [CrossRef] [PubMed]
- Toichuev, R.M.; Zhilova, L.V.; Paizildaev, T.R.; Khametova, M.S.; Rakhmatillaev, A.; Sakibaev, K.S.; Madykova, Z.A.; Toichueva, A.U.; Schlumpf, M.; Weber, R.; et al. Organochlorine Pesticides in Placenta in Kyrgyzstan and the Effect on Pregnancy, Childbirth, and Newborn Health. Environ. Sci. Pollut. Res. 2017, 25, 31885–31894. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, F.; Dubois, M.-F.; Aris, A. Maternal, Placental and Fetal Exposure to Bisphenol a in Women with and without Preeclampsia. Hypertens. Pregnancy 2014, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, D.; Chu, C.; Li, Q.; Zhou, Y.; Hu, L.; Yang, B.-Y.; Dong, G.-H.; Zeng, X.-W.; Chen, D. Transplacental Transfer of Per- and Polyfluoroalkyl Substances (PFASs): Differences between Preterm and Full-Term Deliveries and Associations with Placental Transporter MRNA Expression. Environ. Sci. Technol. 2020, 54, 5062–5070. [Google Scholar] [CrossRef] [PubMed]
- Mamsen, L.S.; Björvang, R.D.; Mucs, D.; Vinnars, M.-T.; Papadogiannakis, N.; Lindh, C.H.; Andersen, C.Y.; Damdimopoulou, P. Concentrations of Perfluoroalkyl Substances (PFASs) in Human Embryonic and Fetal Organs from First, Second, and Third Trimester Pregnancies. Environ. Int. 2019, 124, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Ackerman Grunfeld, D.; Gilbert, D.; Hou, J.; Jones, A.M.; Lee, M.J.; Kibbey, T.C.G.; O’Carroll, D.M. Underestimated Burden of Per- and Polyfluoroalkyl Substances in Global Surface Waters and Groundwaters. Nat. Geosci. 2024, 17, 340–346. [Google Scholar] [CrossRef]
- Kuo, K.-Y.; Chen, Y.; Chuang, Y.; Lin, P.; Lin, Y.-J. Worldwide Serum Concentration-Based Probabilistic Mixture Risk Assessment of Perfluoroalkyl Substances among Pregnant Women, Infants, and Children. Ecotoxicol. Environ. Saf. 2023, 268, 115712. [Google Scholar] [CrossRef]
- Muir, D.; Bossi, R.; Carlsson, P.; Evans, M.; De Silva, A.; Halsall, C.; Rauert, C.; Herzke, D.; Hung, H.; Letcher, R.; et al. Levels and Trends of Poly- and Perfluoroalkyl Substances in the Arctic Environment—An Update. Emerg. Contam. 2019, 5, 240–271. [Google Scholar] [CrossRef]
- Young, C.J.; Furdui, V.I.; Franklin, J.; Koerner, R.M.; Muir, D.C.G.; Mabury, S.A. Perfluorinated Acids in Arctic Snow: New Evidence for Atmospheric Formation. Environ. Sci. Technol. 2007, 41, 3455–3461. [Google Scholar] [CrossRef]
- Miranda, D.d.A.; Leonel, J.; Benskin, J.P.; Johansson, J.; Hatje, V. Perfluoroalkyl Substances in the Western Tropical Atlantic Ocean. Environ. Sci. Technol. 2021, 55, 13749–13758. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding Organofluorine Chemistry. An Introduction to the C–F Bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef]
- Gaines, L.G.T. Historical and Current Usage of Per- and Polyfluoroalkyl Substances (PFAS): A Literature Review. Am. J. Ind. Med. 2022, 66, 353–378. [Google Scholar] [CrossRef] [PubMed]
- Prevedouros, K.; Cousins, I.T.; Buck, R.C.; Korzeniowski, S.H. Sources, Fate and Transport of Perfluorocarboxylates. Environ. Sci. Technol. 2006, 40, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Fenton, S.E.; Ducatman, A.; Boobis, A.; DeWitt, J.C.; Lau, C.; Ng, C.; Smith, J.S.; Roberts, S.M. Per- and Polyfluoroalkyl Substance Toxicity and Human Health Review: Current State of Knowledge and Strategies for Informing Future Research. Environ. Toxicol. Chem. 2020, 40, 606–630. [Google Scholar] [CrossRef] [PubMed]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Tully, J.S.; Needham, L.L. Serum Concentrations of 11 Polyfluoroalkyl Compounds in the U.S. Population: Data from the National Health and Nutrition Examination Survey (NHANES) 1999−2000. Environ. Sci. Technol. 2007, 41, 2237–2242. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey Data: 2017–2018 Data Documentation, Codebook, and Frequencies: Perfluoroalkyl and Polyfluoroalkyl Substances. Available online: https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/PFAS_J.htm#LBXNFOS (accessed on 14 September 2024).
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-Life of Serum Elimination of Perfluorooctanesulfonate, Perfluorohexanesulfonate, and Perfluorooctanoate in Retired Fluorochemical Production Workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Lv, D.; Li, C.; Liu, X.; Liu, W.; Han, W. Human Exposure to F-53B in China and the Evaluation of Its Potential Toxicity: An Overview. Environ. Int. 2022, 161, 107108. [Google Scholar] [CrossRef]
- Schultz, A.A.; Stanton, N.; Shelton, B.; Pomazal, R.; Lange, M.A.; Irving, R.; Meiman, J.; Malecki, K.C. Biomonitoring of Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) from the Survey of the Health of Wisconsin (SHOW) 2014–2016 and Comparison with the National Health and Nutrition Examination Survey (NHANES). J. Expo. Sci. Environ. Epidemiol. 2023, 33, 766–777. [Google Scholar] [CrossRef]
- Shi, Y.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.; Liang, Y.; Cai, Y. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Drinking Water Health Advisory for Perfluorooctanoic Acid (PFOA), Last Revised May 2016. Available online: https://www.epa.gov/sites/default/files/2016-05/documents/pfoa_health_advisory_final_508.pdf (accessed on 14 September 2024).
- Rand, A.A.; Mabury, S.A. Is There a Human Health Risk Associated with Indirect Exposure to Perfluoroalkyl Carboxylates (PFCAs)? Toxicology 2017, 375, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Stein, C.R.; Steenland, K. Serum Perfluorooctanoic Acid and Perfluorooctane Sulfonate Concentrations in Relation to Birth Outcomes in the Mid-Ohio Valley, 2005–2010. Environ. Health Perspect. 2013, 121, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Riise, H.K.R.; Sulo, G.; Tell, G.S.; Igland, J.; Nygård, O.; Iversen, A.; Daltveit, A.K. Association between Gestational Hypertension and Risk of Cardiovascular Disease among 617 589 Norwegian Women. J. Am. Heart Assoc. 2018, 7, e008337. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.F.; Lewandowski, A.J.; Aye, C.; Williamson, W.; Boardman, H.; Huang, R.-C.; Mori, T.A.; Newnham, J.; Beilin, L.J.; Leeson, P. Clinical Cardiovascular Risk during Young Adulthood in Offspring of Hypertensive Pregnancies: Insights from a 20-Year Prospective Follow-up Birth Cohort. BMJ Open 2015, 5, e008136. [Google Scholar] [CrossRef] [PubMed]
- Timpka, S.; Macdonald-Wallis, C.; Hughes, A.D.; Chaturvedi, N.; Franks, P.W.; Lawlor, D.A.; Fraser, A. Hypertensive Disorders of Pregnancy and Offspring Cardiac Structure and Function in Adolescence. J. Am. Heart Assoc. 2016, 5, e003906. [Google Scholar] [CrossRef]
- Timmermann, A.; Avenbuan, O.N.; Romano, M.E.; Braun, J.M.; Tolstrup, J.S.; Vandenberg, L.N.; Fenton, S.E. Per- and Polyfluoroalkyl Substances and Breastfeeding as a Vulnerable Function: A Systematic Review of Epidemiological Studies. Toxics 2023, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Rickard, B.P.; Rizvi, I.; Fenton, S.E. Per- and Poly-Fluoroalkyl Substances (PFAS) and Female Reproductive Outcomes: PFAS Elimination, Endocrine-Mediated Effects, and Disease. Toxicology 2022, 465, 153031. [Google Scholar] [CrossRef]
- Vestergaard, S.; Nielsen, F.; Andersson, A.-M.; Hjollund, N.H.; Grandjean, P.; Andersen, H.R.; Jensen, T.K. Association between Perfluorinated Compounds and Time to Pregnancy in a Prospective Cohort of Danish Couples Attempting to Conceive. Hum. Reprod. 2012, 27, 873–880. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Maternal Levels of Perfluorinated Chemicals and Subfecundity. Hum. Reprod. 2009, 24, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.C.; Liew, Z.; Bech, B.H.; Nohr, E.A.; Fei, C.; Bonefeld-Jorgensen, E.C.; Henriksen, T.B.; Olsen, J. Perfluoroalkyl Acids and Time to Pregnancy Revisited: An Update from the Danish National Birth Cohort. Environ. Health 2015, 14, 59. [Google Scholar] [CrossRef]
- Kahn, L.G.; Harley, K.G.; Siegel, E.L.; Zhu, Y.; Factor-Litvak, P.; Porucznik, C.A.; Klein-Fedyshin, M.; Hipwell, A.E.; Program Collaborators for Environmental Influences on Child Health Outcomes Program. Persistent organic pollutants and couple fecundability: A systematic review. Hum. Reprod. Update 2021, 27, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Hong, X.; Zhao, F.; Wu, J.; Wang, B. The Effects of Perfluoroalkyl and Polyfluoroalkyl Substances on Female Fertility: A Systematic Review and Meta-Analysis. Environ. Res. 2023, 216 Pt 3, 114718. [Google Scholar] [CrossRef] [PubMed]
- Calvert, L.; Green, M.P.; De Iuliis, G.N.; Dun, M.D.; Turner, B.D.; Clarke, B.O.; Eamens, A.L.; Roman, S.D.; Nixon, B. Assessment of the Emerging Threat Posed by Perfluoroalkyl and Polyfluoroalkyl Substances to Male Reproduction in Humans. Front. Endocrinol. 2022, 12, 799043. [Google Scholar] [CrossRef] [PubMed]
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, Y.; Wang, B.; Deng, S.; Zhang, F.; Fu, Z.; Yuan, Y.; Zhang, D. Toxic Effects of Per- and Polyfluoroalkyl Substances on Sperm: Epidemiological and Experimental Evidence. Front. Endocrinol. 2023, 14, 1114463. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Bosagna, C.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance of Male Infertility. Curr. Opin. Genet. Dev. 2014, 26, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Fullston, T.; McPherson, N.O.; Owens, J.A.; Kang, W.X.; Sandeman, L.Y.; Lane, M. Paternal Obesity Induces Metabolic and Sperm Disturbances in Male Offspring That Are Exacerbated by Their Exposure to an “Obesogenic” Diet. Physiol. Rep. 2015, 3, e12336. [Google Scholar] [CrossRef]
- Barouki, R.; Melén, E.; Herceg, Z.; Beckers, J.; Chen, J.; Karagas, M.; Puga, A.; Xia, Y.; Chadwick, L.; Yan, W.; et al. Epigenetics as a Mechanism Linking Developmental Exposures to Long-Term Toxicity. Environ. Int. 2018, 114, 77–86. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent Epigenetic Differences Associated with Prenatal Exposure to Famine in Humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Stener-Victorin, E.; Deng, Q. Epigenetic Inheritance of Polycystic Ovary Syndrome—Challenges and Opportunities for Treatment. Nat. Rev. Endocrinol. 2021, 17, 521–533. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Amalraj Raja, E.; Ruiz Mirazo, E.; Campbell, D.M.; Lee, A.J.; Norman, J.E.; Bhattacharya, S. Inherited Predisposition to Spontaneous Preterm Delivery. Obstet. Gynecol. 2010, 115, 1125–1133. [Google Scholar] [CrossRef]
- Vargesson, N. Thalidomide-Induced Teratogenesis: History and Mechanisms. Birth Defects Res. Part C Embryo Today Rev. 2015, 105, 140–156. [Google Scholar] [CrossRef] [PubMed]
- Mocarelli, P.; Gerthoux, P.M.; Needham, L.L.; Patterson, D.G.; Limonta, G.; Falbo, R.; Signorini, S.; Bertona, M.; Crespi, C.; Sarto, C.; et al. Perinatal Exposure to Low Doses of Dioxin Can Permanently Impair Human Semen Quality. Environ. Health Perspect. 2011, 119, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Mocarelli, P.; Gerthoux, P.M.; Patterson, D.G.; Milani, S.; Limonta, G.; Bertona, M.; Signorini, S.; Tramacere, P.; Colombo, L.; Crespi, C.; et al. Dioxin Exposure, from Infancy through Puberty, Produces Endocrine Disruption and Affects Human Semen Quality. Environ. Health Perspect. 2008, 116, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.C.; Tokranov, A.K.; Liddie, J.; Zhang, X.; Grandjean, P.; Hart, J.E.; Laden, F.; Sun, Q.; Yeung, L.W.Y.; Sunderland, E.M. Tap Water Contributions to Plasma Concentrations of Poly- and Perfluoroalkyl Substances (PFAS) in a Nationwide Prospective Cohort of U.S. Women. Environ. Health Perspect. 2019, 127, 067006. [Google Scholar] [CrossRef] [PubMed]
- Andrews, D.Q.; Naidenko, O.V. Population-Wide Exposure to Per- and Polyfluoroalkyl Substances from Drinking Water in the United States. Environ. Sci. Technol. Lett. 2020, 7, 931–936. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Emerging Contaminants—Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA). EPA 505-F-14-001. Solid Waste and Emergency Responses. Available online: https://semspub.epa.gov/work/HQ/100002767.pdf (accessed on 14 September 2024).
- Müller, C.E.; De Silva, A.O.; Small, J.; Williamson, M.; Wang, X.; Morris, A.; Katz, S.; Gamberg, M.; Muir, D.C.G. Biomagnification of Perfluorinated Compounds in a Remote Terrestrial Food Chain: Lichen–Caribou–Wolf. Environ. Sci. Technol. 2011, 45, 8665–8673. [Google Scholar] [CrossRef]
- Miranda, D.A.; Zachritz, A.M.; Whitehead, H.D.; Cressman, S.R.; Peaslee, G.F.; Lamberti, G.A. Occurrence and Biomagnification of Perfluoroalkyl Substances (PFAS) in Lake Michigan Fishes. Sci. Total Environ. 2023, 895, 164903. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency, Office of Water. Drinking Water Infrastructure Needs Survey and Assessment, 7th Report to Congress. EPA 810R23001; September 2023. Available online: https://www.epa.gov/system/files/documents/2023-09/Seventh%20DWINSA_September2023_Final.pdf (accessed on 13 September 2024).
- The Economic Benefits of Investing in Water Infrastructure How a Failure to Act Would Affect the US Economic Recovery. Available online: https://uswateralliance.org/wp-content/uploads/2023/09/VOW-Economic-Paper_1.pdf (accessed on 14 September 2024).
- United States Environmental Protection Agency. FACT SHEET Bipartisan Infrastructure Law: State Revolving Funds Implementation Memorandum, last revised March 2022. Available online: https://www.epa.gov/system/files/documents/2022-03/bil-srf-memo-fact-sheet-final.pdf (accessed on 14 September 2024).
- Cordner, A.; De La Rosa, V.Y.; Schaider, L.A.; Rudel, R.A.; Richter, L.; Brown, P. Guideline Levels for PFOA and PFOS in Drinking Water: The Role of Scientific Uncertainty, Risk Assessment Decisions, and Social Factors. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 157–171. [Google Scholar] [CrossRef]
- Howard, B.E.; Phillips, J.; Tandon, A.; Maharana, A.; Elmore, R.; Mav, D.; Sedykh, A.; Thayer, K.; Merrick, B.A.; Walker, V.; et al. SWIFT-Active Screener: Accelerated Document Screening through Active Learning and Integrated Recall Estimation. Environ. Int. 2020, 138, 105623. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Inoue, K.; Ritz, B.; Olsen, J.; Liew, Z. Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort. Int. J. Environ. Res. Public Health 2018, 15, 1832. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, S.K.; Rifas-Shiman, S.L.; Fleisch, A.F.; Webster, T.F.; Calafat, A.M.; Ye, X.; Gillman, M.W.; Oken, E. Early-Pregnancy Plasma Concentrations of Perfluoroalkyl Substances and Birth Outcomes in Project Viva: Confounded by Pregnancy Hemodynamics? Am. J. Epidemiol. 2017, 187, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Liew, Z.; Luo, J.; Nohr, E.A.; Bech, B.H.; Bossi, R.; Arah, O.A.; Olsen, J. Maternal Plasma Perfluoroalkyl Substances and Miscarriage: A Nested Case–Control Study in the Danish National Birth Cohort. Environ. Health Perspect. 2020, 128, 047007. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Halling, J.; Jørgensen, N.; Nielsen, F.; Grandjean, P.; Jensen, T.; Weihe, P. Reproductive Function in a Population of Young Faroese Men with Elevated Exposure to Polychlorinated Biphenyls (PCBs) and Perfluorinated Alkylate Substances (PFAS). Int. J. Environ. Res. Public Health 2018, 15, 1880. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Calafat, A.M.; Mukherjee, B.; Batterman, S.; Gold, E.B.; Park, S.K. Associations of Perfluoroalkyl Substances with Incident Natural Menopause: The Study of Women’s Health across the Nation. J. Clin. Endocrinol. Metab. 2020, 105, e3169–e3182. [Google Scholar] [CrossRef]
- Lauritzen, H.B.; Larose, T.L.; Øien, T.; Sandanger, T.M.; Odland, J.Ø.; van de Bor, M.; Jacobsen, G.W. Maternal Serum Levels of Perfluoroalkyl Substances and Organochlorines and Indices of Fetal Growth: A Scandinavian Case–Cohort Study. Pediatr. Res. 2016, 81, 33–42. [Google Scholar] [CrossRef]
- Kalloo, G.; Wellenius, G.A.; McCandless, L.C.; Calafat, A.M.; Sjödin, A.; Romano, M.E.; Karagas, M.R.; Chen, A.; Yolton, K.; Lanphear, B.P.; et al. Exposures to Chemical Mixtures during Pregnancy and Neonatal Outcomes: The HOME Study. Environ. Int. 2020, 134, 105219. [Google Scholar] [CrossRef]
- Singer, A.B.; Whitworth, K.W.; Haug, L.S.; Sabaredzovic, A.; Impinen, A.; Papadopoulou, E.; Longnecker, M.P. Menstrual Cycle Characteristics as Determinants of Plasma Concentrations of Perfluoroalkyl Substances (PFASs) in the Norwegian Mother and Child Cohort (MoBa Study). Environ. Res. 2018, 166, 78–85. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, L.; Tong, C.; Fang, F.; Zhao, S.; Tian, Y.; Tao, Y.; Zhang, J. Plasma Perfluoroalkyl and Polyfluoroalkyl Substances Concentration and Menstrual Cycle Characteristics in Preconception Women. Environ. Health Perspect. 2017, 125, 067012. [Google Scholar] [CrossRef]
- Song, X.; Tang, S.; Zhu, H.; Chen, Z.; Zang, Z.; Zhang, Y.; Niu, X.; Wang, X.; Yin, H.; Zeng, F.; et al. Biomonitoring PFAAs in Blood and Semen Samples: Investigation of a Potential Link between PFAAs Exposure and Semen Mobility in China. Environ. Int. 2018, 113, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Huo, X.; Zhang, L.; Huang, R.; Feng, L.; Wang, W.; Zhang, J. Perfluoroalkyl Substances Exposure in Early Pregnancy and Preterm Birth in Singleton Pregnancies: A Prospective Cohort Study. Environ. Health 2020, 19, 60. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cui, Q.; Wang, J.; Sheng, N.; Jing, J.; Yao, B.; Dai, J. Profiles of Emerging and Legacy Per-/Polyfluoroalkyl Substances in Matched Serum and Semen Samples: New Implications for Human Semen Quality. Environ. Health Perspect. 2019, 127, 127005. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Zhou, Y.; Li, Q.-Q.; Bloom, M.S.; Lin, S.; Yu, Y.-J.; Chen, D.; Yu, H.-Y.; Hu, L.-W.; Yang, B.-Y.; et al. Are Perfluorooctane Sulfonate Alternatives Safer? New Insights from a Birth Cohort Study. Environ. Int. 2020, 135, 105365. [Google Scholar] [CrossRef]
- Wang, B.; Fu, J.; Gao, K.; Liu, Q.; Zhuang, L.; Zhang, G.; Long, M.; Na, J.; Ren, M.; Wang, A.; et al. Early Pregnancy Loss: Do Per- and Polyfluoroalkyl Substances Matter? Environ. Int. 2021, 157, 106837. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, R.; Jin, F.; Lou, H.; Mao, Y.; Zhu, W.; Zhou, W.; Zhang, P.; Zhang, J. Perfluoroalkyl Substances and Endometriosis-Related Infertility in Chinese Women. Environ. Int. 2017, 102, 207–212. [Google Scholar] [CrossRef]
- Costa, O.; Iñiguez, C.; Manzano-Salgado, C.B.; Amiano, P.; Murcia, M.; Casas, M.; Irizar, A.; Basterrechea, M.; Beneito, A.; Schettgen, T.; et al. First-Trimester Maternal Concentrations of Polyfluoroalkyl Substances and Fetal Growth throughout Pregnancy. Environ. Int. 2019, 130, 104830. [Google Scholar] [CrossRef]
- Manzano-Salgado, C.B.; Casas, M.; Lopez-Espinosa, M.-J.; Ballester, F.; Iñiguez, C.; Martinez, D.; Costa, O.; Santa-Marina, L.; Pereda-Pereda, E.; Schettgen, T.; et al. Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes in a Spanish Birth Cohort. Environ. Int. 2017, 108, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, R.; Pan, R.; Xiong, J.; Tian, Y.; Wu, J.; Chen, L. Association of Perfluoroalkyl and Polyfluoroalkyl Substances with Premature Ovarian Insufficiency in Chinese Women. J. Clin. Endocrinol. Metab. 2018, 103, 2543–2551. [Google Scholar] [CrossRef]
- Wikström, S.; Hussein, G.; Lingroth Karlsson, A.; Lindh, C.H.; Bornehag, C.-G. Exposure to Perfluoroalkyl Substances in Early Pregnancy and Risk of Sporadic First Trimester Miscarriage. Sci. Rep. 2021, 11, 3568. [Google Scholar] [CrossRef]
- Ouidir, M.; Buck Louis, G.M.; Kanner, J.; Grantz, K.L.; Zhang, C.; Sundaram, R.; Rahman, M.L.; Lee, S.; Kannan, K.; Tekola-Ayele, F.; et al. Association of Maternal Exposure to Persistent Organic Pollutants in Early Pregnancy with Fetal Growth. JAMA Pediatr. 2020, 174, 149. [Google Scholar] [CrossRef]
- Wise, L.A.; Wesselink, A.K.; Schildroth, S.; Calafat, A.M.; Bethea, T.N.; Geller, R.J.; Coleman, C.M.; Fruh, V.; Henn, B.G.; Botelho, J.C.; et al. Correlates of Plasma Concentrations of Per- and Poly-Fluoroalkyl Substances among Reproductive-Aged Black Women. Environ. Res. 2022, 203, 111860. [Google Scholar] [CrossRef] [PubMed]
- March of Dimes. A Profile of Prematurity of United States. Available online: https://www.marchofdimes.org/peristats/reports/united-states/prematurity-profile (accessed on 5 October 2024).
- Björvang, R.D.; Hallberg, I.; Pikki, A.; Berglund, L.; Pedrelli, M.; Kiviranta, H.; Rantakokko, P.; Ruokojärvi, P.; Lindh, C.H.; Olovsson, M.; et al. Follicular Fluid and Blood Levels of Persistent Organic Pollutants and Reproductive Outcomes among Women Undergoing Assisted Reproductive Technologies. Environ. Res. 2022, 208, 112626. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, A.; Cunningham, T.J.; Drage, D.S.; Aylward, L.L.; Thompson, K.L.; Vijayasarathy, S.; Mueller, J.; Atkin, S.L.; Sathyapalan, T. Perfluorinated Alkyl Acids in the Serum and Follicular Fluid of UK Women with and without Polycystic Ovarian Syndrome Undergoing Fertility Treatment and Associations with Hormonal and Metabolic Parameters. Int. J. Hyg. Environ. Health 2018, 221, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Björvang, R.D.; Hassan, J.; Stefopoulou, M.; Gemzell-Danielsson, K.; Pedrelli, M.; Kiviranta, H.; Rantakokko, P.; Ruokojärvi, P.; Lindh, C.H.; Acharya, G.; et al. Persistent Organic Pollutants and the Size of Ovarian Reserve in Reproductive-Aged Women. Environ. Int. 2021, 155, 106589. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Hom Thepaksorn, E.K.; Izano, M.; Cushing, L.; Wang, Y.; Smith, S.C.; Gao, S.; Park, J.-S.; Padula, A.; DeMicco, E.; et al. Associations between Prenatal Maternal Exposure to Per- and Polyfluoroalkyl Substances (PFAS) and Polybrominated Diphenyl Ethers (PBDEs) and Birth Outcomes among Pregnant Women in San Francisco. Environ. Health 2020, 19, 100. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Wang, B.; Xu, F.; Pang, Y.; Zhang, L.; Zhang, Y.; Jin, L.; Li, Z.; Ren, A. Does Low Maternal Exposure to Per- and Polyfluoroalkyl Substances Elevate the Risk of Spontaneous Preterm Birth? A Nested Case–Control Study in China. Environ. Sci. Technol. 2020, 54, 8259–8268. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are Healthcare Workers’ Intentions to Vaccinate Related to Their Knowledge, Beliefs and Attitudes? A Systematic Review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 10 May 2024).
- The BMJ. Correlation and Regression. Available online: https://www.bmj.com/about-bmj/resources-readers/publications/statistics-square-one/11-correlation-and-regression (accessed on 14 September 2024).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- Rosenthal, J.A. Qualitative Descriptors of Strength of Association and Effect Size. J. Soc. Serv. Res. 1996, 21, 37–59. [Google Scholar] [CrossRef]
- Carson, S.A.; Kallen, A.N. Diagnosis and Management of Infertility: A Review. JAMA 2021, 326, 65–76. [Google Scholar] [CrossRef]
- Bach, C.C.; Vested, A.; Jørgensen, K.T.; Bonde, J.P.E.; Henriksen, T.B.; Toft, G. Perfluoroalkyl and Polyfluoroalkyl Substances and Measures of Human Fertility: A Systematic Review. Crit. Rev. Toxicol. 2016, 46, 735–755. [Google Scholar] [CrossRef] [PubMed]
- Holte, J.; Berglund, L.; Milton, K.; Garello, C.; Gennarelli, G.; Revelli, A.; Bergh, T. Construction of an Evidence-Based Integrated Morphology Cleavage Embryo Score for Implantation Potential of Embryos Scored and Transferred on Day 2 after Oocyte Retrieval. Hum. Reprod. 2006, 22, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Jirge, P. Ovarian Reserve Tests. J. Hum. Reprod. Sci. 2011, 4, 108. [Google Scholar] [CrossRef]
- Olsen, G.W.; Butenhoff, J.L.; Zobel, L.R. Perfluoroalkyl Chemicals and Human Fetal Development: An Epidemiologic Review with Clinical and Toxicological Perspectives. Reprod. Toxicol. 2009, 27, 212–230. [Google Scholar] [CrossRef] [PubMed]
- Appel, M.; Forsthuber, M.; Ramos, R.; Widhalm, R.; Granitzer, S.; Uhl, M.; Hengstschläger, M.; Stamm, T.; Gundacker, C. The Transplacental Transfer Efficiency of Per- and Polyfluoroalkyl Substances (PFAS): A First Meta-Analysis. J. Toxicol. Environ. Health Part B 2021, 25, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Harmon, Q.E.; Upson, K.; Moore, K.R.; Barker-Cummings, C.; Baker, S.; Cooper, T.; Wegienka, G. A Prospective, Ultrasound-Based Study to Evaluate Risk Factors for Uterine Fibroid Incidence and Growth: Methods and Results of Recruitment. J. Women’s Health 2015, 24, 907–915. [Google Scholar] [CrossRef]
- Green, M.P.; Harvey, A.J.; Finger, B.J.; Tarulli, G.A. Endocrine Disrupting Chemicals: Impacts on Human Fertility and Fecundity during the Peri-Conception Period. Environ. Res. 2021, 194, 110694. [Google Scholar] [CrossRef]
- Waitzman, N.J.; Jalali, A.; Grosse, S.D. Preterm Birth Lifetime Costs in the United States in 2016: An Update. Semin. Perinatol. 2021, 45, 151390. [Google Scholar] [CrossRef]
- Beam, A.L.; Fried, I.; Palmer, N.; Agniel, D.; Brat, G.; Fox, K.; Kohane, I.; Sinaiko, A.; Zupancic, J.A.F.; Armstrong, J. Estimates of Healthcare Spending for Preterm and Low-Birthweight Infants in a Commercially Insured Population: 2008–2016. Obstet. Gynecol. Surv. 2020, 75, 717–718. [Google Scholar] [CrossRef]
- Pravia, C.; Benny, M. Long-Term Consequences of Prematurity. Clevel. Clin. J. Med. 2020, 87, 759–767. [Google Scholar] [CrossRef]
- Stock, S.J.; Bauld, L. Maternal Smoking and Preterm Birth: An Unresolved Health Challenge. PLoS Med. 2020, 17, e1003386. [Google Scholar] [CrossRef] [PubMed]
- Albertsen, K. Alcohol Consumption during Pregnancy and the Risk of Preterm Delivery. Am. J. Epidemiol. 2004, 159, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Ikehara, S.; Kimura, T.; Kakigano, A.; Sato, T.; Iso, H.; Saito, H.; Kishi, R.; Yaegashi, N.; Hashimoto, K.; Mori, C.; et al. Association between Maternal Alcohol Consumption during Pregnancy and Risk of Preterm Delivery: The Japan Environment and Children’s Study. BJOG Int. J. Obstet. Gynaecol. 2019, 126, 1448–1454. [Google Scholar] [CrossRef] [PubMed]
- Stone, J.; Sutrave, P.; Gascoigne, E.; Givens, M.B.; Fry, R.C.; Manuck, T.A. Exposure to Toxic Metals and Per- and Polyfluoroalkyl Substances and the Risk of Preeclampsia and Preterm Birth in the United States: A Review. Am. J. Obstet. Gynecol. MFM 2021, 3, 100308. [Google Scholar] [CrossRef]
- Gao, X.; Ni, W.; Zhu, S.; Wu, Y.; Cui, Y.; Ma, J.; Liu, Y.; Qiao, J.; Ye, Y.; Yang, P.; et al. Per- and Polyfluoroalkyl Substances Exposure during Pregnancy and Adverse Pregnancy and Birth Outcomes: A Systematic Review and Meta-Analysis. Environ. Res. 2021, 201, 111632. [Google Scholar] [CrossRef] [PubMed]
- Deji, Z.; Liu, P.; Wang, X.; Zhang, X.; Luo, Y.; Huang, Z. Association between Maternal Exposure to Perfluoroalkyl and Polyfluoroalkyl Substances and Risks of Adverse Pregnancy Outcomes: A Systematic Review and Meta-Analysis. Sci. Total Environ. 2021, 783, 146984. [Google Scholar] [CrossRef]
- Gui, S.-Y.; Chen, Y.-N.; Wu, K.-J.; Liu, W.; Wang, W.-J.; Liang, H.-R.; Jiang, Z.-X.; Li, Z.-L.; Hu, C.-Y. Association between Exposure to Per- and Polyfluoroalkyl Substances and Birth Outcomes: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 855348. [Google Scholar] [CrossRef]
- Yang, B.-Y.; Wu, J.; Niu, X.; He, C.; Bloom, M.S.; Abudoukade, M.; Abulizi, M.; Xu, A.; Li, B.; Li, L.; et al. Low-Level Environmental Per- and Polyfluoroalkyl Substances and Preterm Birth: A Nested Case–Control Study among a Uyghur Population in Northwestern China. Expo. Health 2022, 14, 793–805. [Google Scholar] [CrossRef]
- Bangma, J.; Eaves, L.A.; Oldenburg, K.; Reiner, J.L.; Manuck, T.; Fry, R.C. Identifying Risk Factors for Levels of Per- and Polyfluoroalkyl Substances (PFAS) in the Placenta in a High-Risk Pregnancy Cohort in North Carolina. Environ. Sci. Technol. 2020, 54, 8158–8166. [Google Scholar] [CrossRef]
- Hall, S.M.; Zhang, S.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of Per- and Polyfluoroalkyl Substances (PFAS) in Human Placental Tissues and Associations with Birth Outcomes. Chemosphere 2022, 295, 133873. [Google Scholar] [CrossRef]
- Mokra, K. Endocrine Disruptor Potential of Short- and Long-Chain Perfluoroalkyl Substances (PFASs)—A Synthesis of Current Knowledge with Proposal of Molecular Mechanism. Int. J. Mol. Sci. 2021, 22, 2148. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.S.; Bonefeld-Jørgensen, E.C. Perfluorinated Compounds Affect the Function of Sex Hormone Receptors. Environ. Sci. Pollut. Res. 2013, 20, 8031–8044. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Núñez, Z.; Kinkade, C.W.; Khoury, L.; Brunner, J.; Murphy, H.; Wang, C.; Kannan, K.; Miller, R.K.; O’Connor, T.G.; Barrett, E.S. Prenatal Perfluoroalkyl Substances Exposure and Maternal Sex Steroid Hormones across Pregnancy. Environ. Res. 2023, 220, 115233. [Google Scholar] [CrossRef]
- Xie, X.; Weng, X.; Liu, S.; Chen, J.; Guo, X.; Gao, X.; Fei, Q.; Hao, G.; Jing, C.; Feng, L. Perfluoroalkyl and Polyfluoroalkyl Substance Exposure and Association with Sex Hormone Concentrations: Results from the NHANES 2015–2016. Environ. Sci. Eur. 2021, 33, 69. [Google Scholar] [CrossRef] [PubMed]
- Pavan, A.; Cendron, L.; Di Nisio, A.; Pedrucci, F.; Sabovic, I.; Scarso, A.; Ferlin, A.; Angelini, A.; Foresta, C.; De Toni, L. In Vitro Binding Analysis of Legacy-Linear and New Generation-Cyclic Perfluoro-Alkyl Substances on Sex Hormone Binding Globulin and Albumin, Suggests Low Impact on Serum Hormone Kinetics of Testosterone. Toxicology 2023, 500, 153664. [Google Scholar] [CrossRef] [PubMed]
- National Center for Environmental Health. National Report on Human Exposure to Environmental Chemicals. Updated March 2024. 2023. Available online: https://stacks.cdc.gov/view/cdc/133100 (accessed on 12 September 2024).
- Wang, Q.; Ruan, Y.; Jin, L.; Tao, L.S.; Lai, H.; Li, G.; Yeung, L.W.; Leung, K.M.; Lam, P.K. Legacy and Emerging Per- and Polyfluoroalkyl Substances in a Subtropical Marine Food Web: Suspect Screening, Isomer Profile, and Identification of Analytical Interference. Environ. Sci. Technol. 2023, 57, 8355–8364. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Gravett, M.G.; Iams, J.; Papageorghiou, A.T.; Waller, S.A.; Kramer, M.; Culhane, J.; Barros, F.; Conde-Agudelo, A.; Bhutta, Z.A.; et al. The Preterm Birth Syndrome: Issues to Consider in Creating a Classification System. Am. J. Obstet. Gynecol. 2012, 206, 113–118. [Google Scholar] [CrossRef]
- Kramer, M.S.; Papageorghiou, A.; Culhane, J.; Bhutta, Z.; Goldenberg, R.L.; Gravett, M.; Iams, J.D.; Conde-Agudelo, A.; Waller, S.; Barros, F.; et al. Challenges in Defining and Classifying the Preterm Birth Syndrome. Am. J. Obstet. Gynecol. 2012, 206, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Quenby, S.; Gallos, I.D.; Dhillon-Smith, R.K.; Podesek, M.; Stephenson, M.D.; Fisher, J.; Brosens, J.J.; Brewin, J.; Ramhorst, R.; Lucas, E.S.; et al. Miscarriage Matters: The Epidemiological, Physical, Psychological, and Economic Costs of Early Pregnancy Loss. Lancet 2021, 397, 1658–1667. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Mínguez-Alarcón, L.; Gaskins, A.J.; Missmer, S.A.; Rich-Edwards, J.W.; Manson, J.E.; Pan, A.; Chavarro, J.E. Association of Spontaneous Abortion with All Cause and Cause Specific Premature Mortality: Prospective Cohort Study. BMJ 2021, 372, n530. [Google Scholar] [CrossRef] [PubMed]
- Harty, T.; Trench, M.; Keegan, O.; O’Donoghue, K.; Nuzum, D. The Experiences of Men Following Recurrent Miscarriage in an Irish Tertiary Hospital: A Qualitative Analysis. Health Expect. 2022, 25, 1048–1057. [Google Scholar] [CrossRef] [PubMed]
- Wojnar, D. Miscarriage Experiences of Lesbian Couples. J. Midwifery Women’s Health 2007, 52, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Krieg, S.A.; Shahine, L.K.; Lathi, R.B. Environmental Exposure to Endocrine-Disrupting Chemicals and Miscarriage. Fertil. Steril. 2016, 106, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Darrow, L.A.; Howards, P.P.; Winquist, A.; Steenland, K. PFOA and PFOS Serum Levels and Miscarriage Risk. Epidemiology 2014, 25, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Y.; Scott, K.; Lindh, C.; Jakobsson, K.; Fletcher, T. Half-Lives of PFOA, PFPeS, PFHxS, PFHpS and PFOS after End of Exposure to Contaminated Drinking Water. Environ. Epidemiol. 2019, 3, 237. [Google Scholar] [CrossRef]
- Kolte, A.M.; Bernardi, L.A.; Christiansen, O.B.; Quenby, S.; Farquharson, R.G.; Goddijn, M.; Stephenson, M.D. Terminology for Pregnancy Loss prior to Viability: A Consensus Statement from the ESHRE Early Pregnancy Special Interest Group. Hum. Reprod. 2014, 30, 495–498. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Serum, D.D.T. Age at Menarche, and Abnormal Menstrual Cycle Length. Occup. Environ. Med. 2005, 62, 878–884. [Google Scholar] [CrossRef]
- Watkins, D.J.; Sánchez, B.N.; Téllez-Rojo, M.M.; Lee, J.M.; Mercado-García, A.; Blank-Goldenberg, C.; Peterson, K.E.; Meeker, J.D. Phthalate and Bisphenol a Exposure during in Utero Windows of Susceptibility in Relation to Reproductive Hormones and Pubertal Development in Girls. Environ. Res. 2017, 159, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Gallo, M.V.; Ravenscroft, J.; Carpenter, D.O.; Frye, C.; Akwesasne Task Force on the Environment; Cook, B.; Schell, L.M. Endocrine Disrupting Chemicals and Ovulation: Is There a Relationship? Environ. Res. 2016, 151, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Land, K.L.; Miller, F.G.; Fugate, A.C.; Hannon, P.R. The Effects of Endocrine-Disrupting Chemicals on Ovarian- and Ovulation-Related Fertility Outcomes. Mol. Reprod. Dev. 2022, 89, 608–631. [Google Scholar] [CrossRef]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, E1–E150. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Shearston, J.A.; Kioumourtzoglou, M.A. An Epidemiologic Review of Menstrual Blood Loss as an Excretion Route for Per- and Polyfluoroalkyl Substances. Curr. Environ. Health Rep. 2022, 9, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; MacLeod, M.; Mueller, J.F.; Cousins, I.T. Enhanced Elimination of Perfluorooctane Sulfonic Acid by Menstruating Women: Evidence from Population-Based Pharmacokinetic Modeling. Environ. Sci. Technol. 2014, 48, 8807–8814. [Google Scholar] [CrossRef] [PubMed]
- Ely, D.; Hamilton, B. Trends in Fertility and Mother’s Age at First Birth Among Rural and Metropolitan Counties: United States, 2007–2017. Centers for Disease Control and Prevention: National Center for Health Statistics. 2018. Available online: https://www.cdc.gov/nchs/data/databriefs/db323-h.pdf (accessed on 12 September 2024).
- Lee, Y.J.; Jung, H.W.; Kim, H.Y.; Choi, Y.-J.; Lee, Y.A. Early-Life Exposure to Per- and Poly-Fluorinated Alkyl Substances and Growth, Adiposity, and Puberty in Children: A Systematic Review. Front. Endocrinol. 2021, 12, 683297. [Google Scholar] [CrossRef]
- Anastasiadis, X.; Matsas, A.; Panoskaltsis, T.; Bakas, P.; Papadimitriou, D.T.; Christopoulos, P. Impact of Chemicals on the Age of Menarche: A Literature Review. Children 2023, 10, 1234. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Risk to Human Health Related to the Presence of Perfluorooctane Sulfonic Acid and Perfluorooctanoic Acid in Food. EFSA J. 2018, 16, e05194. [Google Scholar] [CrossRef]
- Ding, N.; Harlow, S.D.; Randolph, J.F.; Loch-Caruso, R.; Park, S.K. Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS) and Their Effects on the Ovary. Hum. Reprod. Update 2020, 26, 724–752. [Google Scholar] [CrossRef]
- Chang, C.-J.; Ryan, P.B.; Smarr, M.M.; Kannan, K.; Panuwet, P.; Dunlop, A.L.; Corwin, E.J.; Barr, D.B. Serum Per- and Polyfluoroalkyl Substance (PFAS) Concentrations and Predictors of Exposure among Pregnant African American Women in the Atlanta Area, Georgia. Environ. Res. 2021, 198, 110445. [Google Scholar] [CrossRef]
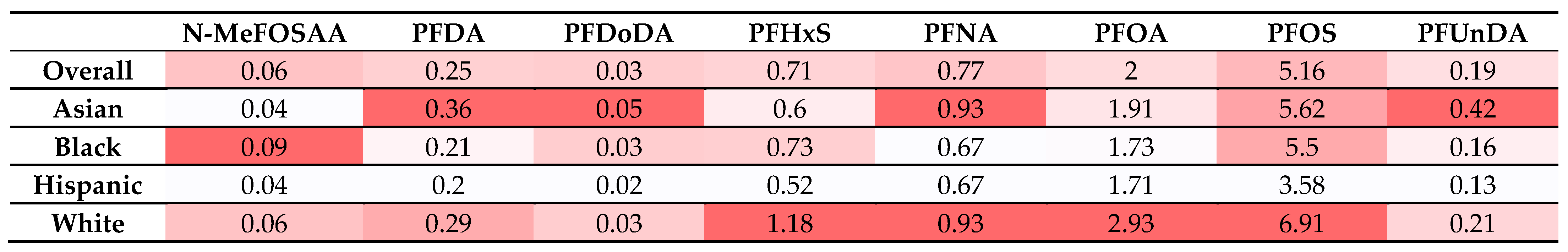
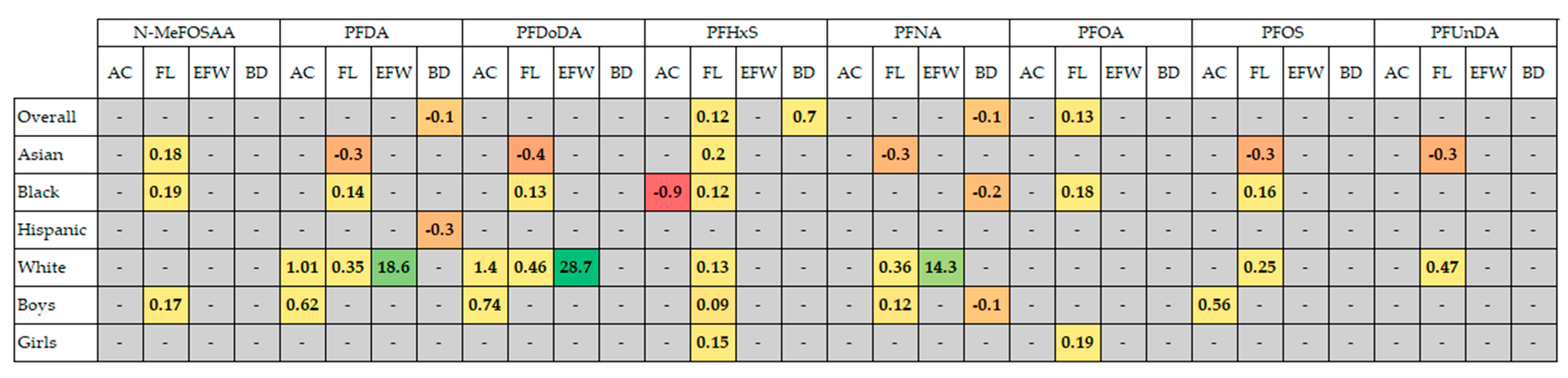
- Park, S.K.; Peng, Q.; Ding, N.; Mukherjee, B.; Harlow, S.D. Determinants of Per- and Polyfluoroalkyl Substances (PFAS) in Midlife Women: Evidence of Racial/Ethnic and Geographic Differences in PFAS Exposure. Environ. Res. 2019, 175, 186–199. [Google Scholar] [CrossRef]
- Taylor, K.W.; Hoffman, K.; Thayer, K.A.; Daniels, J.L. Polyfluoroalkyl Chemicals and Menopause among Women 20–65 Years of Age (NHANES). Environ. Health Perspect. 2014, 122, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Knox, S.S.; Jackson, T.; Javins, B.; Frisbee, S.J.; Shankar, A.; Ducatman, A. Implications of Early Menopause in Women Exposed to Perfluorocarbons. J. Clin. Endocrinol. Metab. 2011, 96, 1747–1753. [Google Scholar] [CrossRef]
- Mathews, T.J.; Hamilton, B.E. Delayed Childbearing: More Women Are Having Their First Child Later in Life. NCHS Data Brief, no 21. Hyattsville, MD: National Center for Health Statistics. 2009. Available online: https://www.cdc.gov/nchs/data/databriefs/db21.pdf (accessed on 10 May 2024).
- Birth Characteristics in England and Wales-Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/livebirths/bulletins/birthcharacteristicsinenglandandwales/2021 (accessed on 12 September 2024).
- De Vos, M.; Devroey, P.; Fauser, B.C. Primary Ovarian Insufficiency. Lancet 2010, 376, 911–921. [Google Scholar] [CrossRef] [PubMed]
- Webber, L.; Davies, M.; Anderson, R.; Bartlett, J.; Braat, D.; Cartwright, B.; Cifkova, R.; de Muinck Keizer-Schrama, S.; Hogervorst, E.; Janse, F.; et al. ESHRE Guideline: Management of Women with Premature Ovarian Insufficiency. Hum. Reprod. 2016, 31, 926–937. [Google Scholar] [CrossRef] [PubMed]
- Vabre, P.; Gatimel, N.; Moreau, J.; Gayrard, V.; Picard-Hagen, N.; Parinaud, J.; Leandri, R.D. Environmental Pollutants, a Possible Etiology for Premature Ovarian Insufficiency: A Narrative Review of Animal and Human Data. Environ. Health 2017, 16, 37. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Hong, X.; Wang, Z.; Zhu, Y. Endometrial Receptivity and Conception Outcome among Women with Light Menstrual Bleeding of Unidentified Etiology. Int. J. Gynecol. Obstet. 2017, 140, 37–41. [Google Scholar] [CrossRef]
- Carroll, J.; Saxena, R.; Welt, C.K. Environmental and Genetic Factors Influence Age at Menarche in Women with Polycystic Ovary Syndrome. J. Pediatr. Endocrinol. Metab. 2012, 25, 459–466. [Google Scholar] [CrossRef]
- Cheng, T.S.; Day, F.R.; Lakshman, R.; Ong, K.K. Association of Puberty Timing with Type 2 Diabetes: A Systematic Review and Meta-Analysis. PLoS Med. 2020, 17, e1003017. [Google Scholar] [CrossRef]
- Yoo, J.-H. Effects of Early Menarche on Physical and Psychosocial Health Problems in Adolescent Girls and Adult Women. Korean J. Pediatr. 2016, 59, 355. [Google Scholar] [CrossRef]
- Schaeffer, K.; Aragão, C. Key Facts About Moms in the U.S. Pew Research Center. Available online: https://www.pewresearch.org/short-reads/2023/05/09/facts-about-u-s-mothers/#:~:text=In%202021%2C%20the%20average%20woman (accessed on 10 May 2024).
- Schell, L.M.; West, C.N. Age at Menarche and Chemical Exposure: Per- and Polyfluoroalkyl Substances (PFAS), Dichloro-Diphenyl-Trichloroethane (DDT), Dichloro-Diphenyl-Dichloroethylene (DDE), and Polychlorinated Biphenyls (PCBs). Ann. Hum. Biol. 2023, 50, 282–292. [Google Scholar] [CrossRef]
- Farahmand, M.; Ramezani Tehrani, F.; Azizi, F. Whether Age of Menarche Is Influenced by Body Mass Index and Lipoproteins Profile? A Retrospective Study. Iran. J. Reprod. Med. 2012, 10, 337–342. [Google Scholar]
- Gong, T.-T.; Wang, Y.-L.; Ma, X.-X. Age at Menarche and Endometrial Cancer Risk: A Dose-Response Meta-Analysis of Prospective Studies. Sci. Rep. 2015, 5, 14051. [Google Scholar] [CrossRef] [PubMed]
- Bullard, R.D.; Mohai, P.; Saha, R.; Wright, B. Toxic Wastes and Race at Twenty: Why Race Still Matters After All of These Years. Environ. Law 2008, 38, 371–411. [Google Scholar]
- Mohai, P.; Saha, R. Which Came First, People or Pollution? A Review of Theory and Evidence from Longitudinal Environmental Justice Studies. Environ. Res. Lett. 2015, 10, 125011. [Google Scholar] [CrossRef]
- Santaliz Casiano, A.; Lee, A.; Teteh, D.; Madak Erdogan, Z.; Treviño, L. Endocrine-Disrupting Chemicals and Breast Cancer: Disparities in Exposure and Importance of Research Inclusivity. Endocrinology 2022, 163, bqac034. [Google Scholar] [CrossRef]
- James-Todd, T.M.; Chiu, Y.-H.; Zota, A.R. Racial/Ethnic Disparities in Environmental Endocrine Disrupting Chemicals and Women’s Reproductive Health Outcomes: Epidemiological Examples across the Life Course. Curr. Epidemiol. Rep. 2016, 3, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Office of Minority Health. Infant Mortality and African Americans. Available online: https://minorityhealth.hhs.gov/infant-mortality-and-african-americans#:~:text=Non%2DHispanic%20blacks%2FAfrican%20Americans (accessed on 5 October 2024).
- Centers for Disease Control and Prevention. Infant Mortality. Available online: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/infantmortality.htm (accessed on 5 October 2024).
- Wallace, M.; Crear-Perry, J.; Richardson, L.; Tarver, M.; Theall, K. Separate and Unequal: Structural Racism and Infant Mortality in the US. Health Place 2017, 45, 140–144. [Google Scholar] [CrossRef]
- Pabayo, R.; Ehntholt, A.; Davis, K.; Liu, S.Y.; Muennig, P.; Cook, D.M. Structural Racism and Odds for Infant Mortality among Infants Born in the United States 2010. J. Racial Ethn. Health Disparities 2019, 6, 1095–1106. [Google Scholar] [CrossRef]
- Chen, F.; Yin, S.; Kelly, B.C.; Liu, W. Isomer-Specific Transplacental Transfer of Perfluoroalkyl Acids: Results from a Survey of Paired Maternal, Cord Sera, and Placentas. Environ. Sci. Technol. 2017, 51, 5756–5763. [Google Scholar] [CrossRef]
- Rush, E.L.; Singer, A.B.; Longnecker, M.P.; Haug, L.S.; Sabaredzovic, A.; Symanski, E.; Whitworth, K.W. Oral Contraceptive Use as a Determinant of Plasma Concentrations of Perfluoroalkyl Substances among Women in the Norwegian Mother and Child Cohort (MoBa) Study. Environ. Int. 2018, 112, 156–164. [Google Scholar] [CrossRef]
- Faubion, S.S.; Kuhle, C.L.; Shuster, L.T.; Rocca, W.A. Long-Term Health Consequences of Premature or Early Menopause and Considerations for Management. Climacteric 2015, 18, 483–491. [Google Scholar] [CrossRef]
- Tsiligiannis, S.; Panay, N.; Stevenson, J.C. Premature Ovarian Insufficiency and Long-Term Health Consequences. Curr. Vasc. Pharmacol. 2019, 17, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Angeles, C.; Castelo-Branco, C. Early Menopause: A Hazard to a Woman’s Health. Indian J. Med. Res. 2016, 143, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Bozdag, G.; Mumusoglu, S.; Zengin, D.; Karabulut, E.; Yildiz, B.O. The Prevalence and Phenotypic Features of Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. 2016, 31, 2841–2855. [Google Scholar] [CrossRef] [PubMed]
- Balen, A.H.; Rutherford, A.J. Managing Anovulatory Infertility and Polycystic Ovary Syndrome. BMJ 2007, 335, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Bulletti, C.; Coccia, M.E.; Battistoni, S.; Borini, A. Endometriosis and Infertility. J. Assist. Reprod. Genet. 2010, 27, 441–447. [Google Scholar] [CrossRef]
- Chambers, W.S.; Hopkins, J.G.; Richards, S.M. A Review of Per- and Polyfluorinated Alkyl Substance Impairment of Reproduction. Front. Toxicol. 2021, 3, 732436. [Google Scholar] [CrossRef] [PubMed]
- Crawford, N.M.; Fenton, S.E.; Strynar, M.; Hines, E.P.; Pritchard, D.A.; Steiner, A.Z. Effects of Perfluorinated Chemicals on Thyroid Function, Markers of Ovarian Reserve, and Natural Fertility. Reprod. Toxicol. 2017, 69, 53–59. [Google Scholar] [CrossRef]
- The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum. Reprod. 2004, 19, 41–47. [Google Scholar] [CrossRef]
- Purwar, A.; Nagpure, S. Insulin Resistance in Polycystic Ovarian Syndrome. Cureus 2022, 14, 511–518. [Google Scholar] [CrossRef]
- Rosenfield, R.L.; Ehrmann, D.A. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocr. Rev. 2016, 37, 467–520. [Google Scholar] [CrossRef]
- Barber, T.M.; Hanson, P.; Weickert, M.O.; Franks, S. Obesity and Polycystic Ovary Syndrome: Implications for Pathogenesis and Novel Management Strategies. Clin. Med. Insights Reprod. Health 2019, 13, 117955811987404. [Google Scholar] [CrossRef] [PubMed]
- Sam, S. Obesity and Polycystic Ovary Syndrome. Obes. Manag. 2007, 3, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Vagi, S.J.; Azziz-Baumgartner, E.; Sjödin, A.; Calafat, A.M.; Dumesic, D.; Gonzalez, L.; Kato, K.; Silva, M.J.; Ye, X.; Azziz, R. Exploring the Potential Association between Brominated Diphenyl Ethers, Polychlorinated Biphenyls, Organochlorine Pesticides, Perfluorinated Compounds, Phthalates, and Bisphenol a in Polycystic Ovary Syndrome: A Case–Control Study. BMC Endocr. Disord. 2014, 14, 86. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Raza, M.; Pollack, A.Z. Perfluoroalkyl Substances and Endometriosis in US Women in NHANES 2003–2006. Reprod. Toxicol. 2016, 65, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Louis, G.M.B.; Peterson, C.M.; Chen, Z.; Hediger, M.L.; Croughan, M.S.; Sundaram, R.; Stanford, J.B.; Fujimoto, V.Y.; Varner, M.W.; Giudice, L.C.; et al. Perfluorochemicals and Endometriosis. Epidemiology 2012, 23, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Kang, Q.; Gao, F.; Zhang, X.; Wang, L.; Liu, J.; Fu, M.; Zhang, S.; Wan, Y.; Shen, H.; Hu, J. Nontargeted Identification of Per- and Polyfluoroalkyl Substances in Human Follicular Fluid and Their Blood-Follicle Transfer. Environ. Int. 2020, 139, 105686. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, W.; Wu, S.; Liang, F.; Li, Y.; Zhang, J.; Cui, L.; Feng, Y.; Wang, Y. Perfluoroalkyl Substances Exposure and Risk of Polycystic Ovarian Syndrome Related Infertility in Chinese Women. Environ. Pollut. 2019, 247, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Christensen, K. Invited Perspective: Challenges in Evaluating the Effect of Per- and Polyfluoroalkyl Substance Mixtures on Polycystic Ovarian Syndrome. Environ. Health Perspect. 2023, 131, 051301. [Google Scholar] [CrossRef]
- Purdue, M.P.; Rhee, J.; Denic-Roberts, H.; McGlynn, K.A.; Byrne, C.; Sampson, J.N.; Botelho, J.C.; Calafat, A.M.; Rusiecki, J. A Nested Case–Control Study of Serum Per- and Polyfluoroalkyl Substances and Testicular Germ Cell Tumors among U.S. Air Force Servicemen. Environ. Health Perspect. 2023, 131, 077007. [Google Scholar] [CrossRef]
- Corsini, C.; Boeri, L.; Candela, L.; Pozzi, E.; Belladelli, F.; Capogrosso, P.; Fallara, G.; Schifano, N.; Cignoli, D.; Ventimiglia, E.; et al. Is There a Relevant Clinical Impact in Differentiating Idiopathic Versus Unexplained Male Infertility? World J. Men’s Health 2023, 41, 354. [Google Scholar] [CrossRef]
- Krzastek, S.C.; Farhi, J.; Gray, M.; Smith, R.P. Impact of Environmental Toxin Exposure on Male Fertility Potential. Transl. Androl. Urol. 2020, 9, 2797–2813. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental Contaminants and Male Infertility: Effects and Mechanisms. Andrologia 2020, 53, e13646. [Google Scholar] [CrossRef] [PubMed]
- Donkin, I.; Barrès, R. Sperm Epigenetics and Influence of Environmental Factors. Mol. Metab. 2018, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-E.; Yang, Y.; Zhao, J.-L.; Liu, Y.-S.; Hu, L.-X.; Li, B.; Li, C.-L.; Ying, G.-G. Legacy and Alternative Per- and Polyfluoroalkyl Substances (PFASs) in the West River and North River, South China: Occurrence, Fate, Spatio-Temporal Variations and Potential Sources. Chemosphere 2021, 283, 131301. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, T.; Wang, P.; Fu, Q.; Lu, Y. Effects of Age, Gender and Region on Serum Concentrations of Perfluorinated Compounds in General Population of Henan, China. Chemosphere 2014, 110, 104–110. [Google Scholar] [CrossRef]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.G.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization Reference Values for Human Semen Characteristics. Hum. Reprod. Update 2009, 16, 231–245. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; World Health Organization: Geneva, Switzerland, 2010. [Google Scholar]
- Dassuncao, C.; Hu, X.C.; Zhang, X.; Bossi, R.; Dam, M.; Mikkelsen, B.; Sunderland, E.M. Temporal Shifts in Poly- and Perfluoroalkyl Substances (PFASs) in North Atlantic Pilot Whales Indicate Large Contribution of Atmospheric Precursors. Environ. Sci. Technol. 2017, 51, 4512–4521. [Google Scholar] [CrossRef] [PubMed]
- Weihe, P.; Debes Joensen, H. Dietary Recommendations Regarding Pilot Whale Meat and Blubber in the Faroe Islands. Int. J. Circumpolar Health 2012, 71, 18594. [Google Scholar] [CrossRef]
- Weihe, P.; Kato, K.; Calafat, A.M.; Nielsen, F.; Wanigatunga, A.A.; Needham, L.L.; Grandjean, P. Serum Concentrations of Polyfluoroalkyl Compounds in Faroese Whale Meat Consumers. Environ. Sci. Technol. 2008, 42, 6291–6295. [Google Scholar] [CrossRef]
- World Health Organization. Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 4th ed.; World Health Organization: Cambridge, UK, 1999. [Google Scholar]
- Menkveld, R.; Stander, F.S.; Kotze, T.J.V.; Kruger, T.F.; Zyl, J.A.V. The Evaluation of Morphological Characteristics of Human Spermatozoa according to Stricter Criteria. Hum. Reprod. 1990, 5, 586–592. [Google Scholar] [CrossRef]
- Barker, D. Infant Mortality, Childhood Nutrition, and Ischaemic Heart Disease in England and Wales. Lancet 1986, 327, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- Dzierlenga, M.W.; Crawford, L.; Longnecker, M.P. Birth Weight and Perfluorooctane Sulfonic Acid: A Random-Effects Meta-Regression Analysis. Environ. Epidemiol. 2020, 4, e095. [Google Scholar] [CrossRef] [PubMed]
- Steenland, K.; Barry, V.; Savitz, D. Serum Perfluorooctanoic Acid and Birthweight. Epidemiology 2018, 29, 765–776. [Google Scholar] [CrossRef] [PubMed]
- Gampel, S.B.; Nomura, Y. Short and Long-Term Effects of Compromised Birth Weight, Head Circumference, and Apgar Scores on Neuropsychological Development. J. Psychol. Abnorm. Child. 2014, 3, 127. [Google Scholar] [CrossRef]
- Hassan, S.; Jahanfar, S.; Inungu, J.; Craig, J.M. Low Birth Weight as a Predictor of Adverse Health Outcomes during Adulthood in Twins: A Systematic Review and Meta-Analysis. Syst. Rev. 2021, 10, 186. [Google Scholar] [CrossRef]
- Nakano, Y. Adult-Onset Diseases in Low Birth Weight Infants: Association with Adipose Tissue Maldevelopment. J. Atheroscler. Thromb. 2020, 27, 397. [Google Scholar] [CrossRef]
- Hadlock, F.P.; Harrist, R.B.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of Fetal Weight with the Use of Head, Body, and Femur Measurements—A Prospective Study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Grewal, J.; Albert, P.S.; Sciscione, A.; Wing, D.A.; Grobman, W.A.; Newman, R.B.; Wapner, R.; D’Alton, M.E.; Skupski, D.; et al. Racial/Ethnic Standards for Fetal Growth: The NICHD Fetal Growth Studies. Am. J. Obstet. Gynecol. 2015, 213, 449.e1–449.e41. [Google Scholar] [CrossRef]
- Buekers, J.; Colles, A.; Cornelis, C.; Morrens, B.; Govarts, E.; Schoeters, G. Socio-Economic Status and Health: Evaluation of Human Biomonitored Chemical Exposure to Per- and Polyfluorinated Substances across Status. Int. J. Environ. Res. Public Health 2018, 15, 2818. [Google Scholar] [CrossRef]
- DeLuca, N.M.; Thomas, K.; Mullikin, A.; Slover, R.; Stanek, L.W.; Pilant, A.N.; Cohen Hubal, E.A. Geographic and Demographic Variability in Serum PFAS Concentrations for Pregnant Women in the United States. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 710–724. [Google Scholar] [CrossRef]
- Kim, M.K.; Lee, S.M.; Bae, S.-H.; Kim, H.J.; Lim, N.G.; Yoon, S.-J.; Lee, J.Y.; Jo, M.-W. Socioeconomic Status Can Affect Pregnancy Outcomes and Complications, Even with a Universal Healthcare System. Int. J. Equity Health 2018, 17, 2. [Google Scholar] [CrossRef]
- Buck Louis, G.M.; Zhai, S.; Smarr, M.M.; Grewal, J.; Zhang, C.; Grantz, K.L.; Hinkle, S.N.; Sundaram, R.; Lee, S.; Honda, M.; et al. Endocrine Disruptors and Neonatal Anthropometry, NICHD Fetal Growth Studies-Singletons. Environ. Int. 2018, 119, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.K.; Eckel, S.P.; Habre, R.; Yang, T.; Faham, D.; Amin, M.; Grubbs, B.H.; Farzan, S.F.; Kannan, K.; Robinson, M.; et al. Detected Prenatal Perfluorooctanoic Acid (PFOA) Exposure Is Associated with Decreased Fetal Head Biometric Parameters in Participants Experiencing Higher Perceived Stress during Pregnancy in the MADRES Cohort. Environ. Adv. 2022, 9, 100286. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Health and Medicine Division; Committee on Population; Board on Health Sciences Policy; Committee on the Use of Race, Ethnicity, and Ancestry as Population Descriptors in Genomics Research. Using Population Descriptors in Genetics and Genomics Research; National Academies Press: Cambridge, MA, USA, 2023. [Google Scholar] [CrossRef]

| Database | Search Terms |
|---|---|
| PubMed | (“PFAS” [Title/Abstract] OR “perfluorinated” [Title/Abstract] OR “polyfluoroalkyl” [Title/Abstract] OR “perfluoroalkyl” [Title/Abstract] AND (reproduct * OR fertile * OR fecund * OR infertile * OR subfertil * OR pregnan * OR menstr * OR menopaus * OR menarche OR endometriosis OR PCOS OR polycystic ovarian syndrome) |
| Embase | PFAS AND (reproduct * OR fertile * OR fecund * OR infertile * OR subfertil * OR pregnan * OR menstr * OR menopaus * OR menarche OR endometriosis OR pcos OR (polycystic AND ovarian AND syndrome)) and (2017:py OR 2018:py OR 2019:py OR 2020:py OR 2021:py OR 2022:py) |
| Web of Science | (AB = (PFAS OR polyfluoroalkyl OR perfluoroalkyl OR perfluorinated) AND ALL = (reproduct * OR fertile * OR fecund * OR infertile * OR subfertil * OR pregnan * OR menstr * OR menopaus * OR menarche OR endometriosis OR PCOS OR polycystic ovarian syndrome)) Refined by: Language: English, Timespan: 2017–2022, Document type: Article |
| HERO | Search For: “PFAS” (match all words) Search For: “reproduct *, fertile *, fecund *, infertile *, subfertil *, pregnan *, menstr *, menopaus *, menarche, endometriosis, PCOS, polycystic ovarian syndrome (match any word) Years: “2017 to 2022” |
| Population | Humans (mothers, men, and women in IVF clinics, young women, fetuses) |
| Exposure | Any PFAS chemical |
| Comparator | Lowest exposed in group compared to more highly exposed in group (i.e., lowest tertile vs. highest tertile), continuous levels, or comparing PFAS levels in groups with or without outcome of interest. |
| Outcome | Reproductive endpoints (fertility, preterm birth, miscarriage, menstruation and menopause, ovarian health, sperm health, and in utero fetal growth) |
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|
| Bjorvang et al. (2021) [82] | Cohort | 50 | PFDA, PFDoA, PFHpA, PFHxA, PFHxS, PFNA, PFOA, PFOS, PFUnA | NA | Ovarian reserve; Growing, Healthy or Atretic Follicle Density; infertility (TTP > 12 months) | Serum | Sweden |
| Bjorvang et al. (2022) [80] | Cohort | 185 | PFDA, PFHpA, PFHxA, PFHxS, PFNA, PFOA, PFOS, PFUnA | PFDoA | Basal antral follicle count, Ovarian sensitivity index, Average embryo score, at least one top quality embryo, Clinical pregnancy from fresh or fresh/frozen transfer, Live birth from fresh or fresh/frozen transfer | Plasma, follicular fluid | Sweden |
| Wang et al. (2021) [71] | Prospective cohort | 305 | PFBA, PFBS, PFDA, PFHxA, PFNA, PFOA, PFOS, PFHxS | PFDoA *, PFHpA, PFPeA *, PFUdA * | Clinical pregnancy failure, hCG test negative 14 d after transfer | Serum | China |
| Wise et al. (2022) [78] | Cross-sectional | 1499 | MeFOSAA, PFDA, PFHxS, PFNA, PFOA, PFOS, PFUnDA | NA | Parity, time since last birth | Plasma | USA |
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Sub-Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|---|
| Bangma et al. (2020) [109] | Cross-sectional cohort | 122 | PFHpS, PFHxS, PFOS | 6:2 FTS, GenX, PFBA, PFBS, PFDA, PFDoA, PFDoS, PFDS, PFHpA, PFHxA, PFHxDA, PFNA, PFNS, PFOA, PFPeA, PFPeS, PFTeDA, PFTrDA, PFUnA | Gestational age at birth, PTB | Infant sex | Placenta | USA |
| Chu et al. (2020) [70] | Cohort | 372 | 6:2 Cl-PFESA, PFBA *, PFDA *, PFDoA*, PFHpA *, PFHpS *, PFHxS *, PFNA *, PFOA, PFOS, PFUdA * | 4:2 FTS, 6:2 FTS, 8:2 Cl-PFESA, 8:2 FTS, FOSA, HFPO-DA, N-EtFOSAA, N-MeFOSAA, PFBS, PFDS, PFHxA, PFNS, PFPeA, PFPeS, PFTeDA, PFTrDA | Gestational age at birth, PTB | Infant sex | Serum | China |
| Eick and Hom Thepaksorn et al. (2020) [83] | Prospective cohort | 506 | Me-PFOSA-AcOH, PFDeA *, PFNA, PFHxS, PFOA, PFOS, PFUdA * | Et-PFOSA-AcOH, PFBS, PFDoA, PFHpA, PFOSA | Gestational age at birth, PTB | Infant sex | Serum | USA |
| Hall et al. (2022) [110] | Prospective cohort | 120 | PFDA, PFNA, PFOA, PFOS | GenX, PFBA, PFBS, PFHpA, PFHxA, PFHxS, PFPeA | Gestational age at birth | Infant sex | Placenta | USA |
| Huo et al. (2020) [68] | Prospective cohort | 2849 | PFBS, PFDA, PFDoA, PFHpA, PFHxS, PFNA, PFOA, PFOS, PFUA | PFOSA | Gestational age at birth, PTB | Spontaneous/non-spontaneous/indicated PTB, spontaneous/indicated late PTB, late PTB (34–36 weeks), length of gestation (weeks); infant sex | Plasma | China |
| Kalloo et al. (2020) [64] | Prospective cohort | 380 | PFHxS, PFNA, PFOA, PFOS | NA | Gestational age at birth | Infant sex | Serum | USA |
| Lauritzen et al. (2017) [63] | Case–cohort | 424 | PFOA, PFOS | NA | Gestational age at birth | Infant sex | Serum | Norway |
| Liu et al. (2020) [84] | Case–control | Cases: 144; Controls: 375 | 6:2 Cl-PFESA, PFDS, PFHpS, PFHxS, PFOA, PFOS | 8:2 Cl-PFESA, PFBA, PFBS, PFDA, PFDoA, PFHpA, PFHxA, PFNA, PFPeA, PFTeDA, PFTrDA, PFUdA * | PTB | Spontaneous PTB | Plasma | China |
| Manzano-Salgado et al. (2017) [74] | Prospective cohort | 1202 | PFHxS, PFNA, PFOA, PFOS | NA | Gestational age at birth, PTB | Infant sex | Plasma | Spain |
| Meng et al. (2018) [58] | Cohort | 3535 (PFOA and PFOS); 2137 (other PFAS) | PFDA, PFHpS, PFHxS, PFNA, PFOA, PFOS | NA | Gestational age at birth, PTB | Infant sex | Plasma | Denmark |
| Sagiv et al.(2017) [59] | Cohort | 1645 | PFHxS, PFNA, PFOA, PFOS | NA | Gestational age at birth, PTB | Infant sex | Plasma | USA |
| Yang et al. (2022) [108] | Case–control | Cases: 384; Controls: 384 | 1 m-PFOS, 11Cl-PF3OUdS, 6 m-PFOS, 9Cl-PF3ONS, branched PFHxS, branched PFOS, linear PFHxS, linear PFOS, PFDA, PFDoA, PFHpA, PFHpS, PFHxA, PFNA, PFOA, PFTeDA, PFTrDA, PFUdA, sum 3 + 4 + 5 m-PFOS, sum m2-PFOS, total PFAS, total PFHxS, total PFOS | 4:2 FTS, 6:2 FTS, 8:2 FTS, FOSA, HFPO-DA, N-EtFOSAA, N-MeFOSAA, PFBA, PFBS, PFDoA, PFDS, PFNS, PFPeA, PFPeS | Gestational age at birth, PTB | Infant sex | Cord serum | China |
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|
| Liew et al. (2020) [60] | Case–control | Cases: 220; Controls: 218 | PFDA, PFHpS, PFHxS, PFNA, PFOA, PFOS, PFOSA | NA | Miscarriage (12–22 w; second trimester) | Plasma | Denmark |
| Wang et al. (2021) [71] | Prospective cohort | 305 (Site 1: 178; Site 2: 127) | PFBA, PFBS, PFDA, PFHxA, PFNA, PFOA, PFOS, PFHxS | PFDoA *, PFHpA, PFPeA *, PFUdA * | Preclinical spontaneous abortion (6 w) | Serum | China |
| Wikström et al. (2021) [76] | Case–control | Cases: 78; Controls: 1449 | PFDA, PFHpA, PFHxS, PFNA, PFOA, PFOS, PFUnDA | NA | Sporadic first trimester miscarriage (≤12 w + 6 d) | Serum | Sweden |
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Sub-Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|---|
| Pan et al. (2019) [69] | Cross-sectional | 664 | 6:2 Cl-PFESA, PFDA, PFNA, PFOA, PFOS, PFUnDA | Semen: PFHpA, PFDoDA, PFTeDA, PFBS, PFHxS, 8:2 Cl-PFESA; Serum: PFHpA, PFTeDA; (PFTriDA >LOQ in most samples regardless of media, but not included in analysis) | DNA instability, semen volume, sperm concentration, sperm morphology, sperm motility, total sperm count | DNA fragmentation index (%), high DNA stainability %, semen volume (mL), sperm concentration (million/mL), morphologically normal sperm (%), progressive motile sperm (%), straight linear sperm velocity (um/s), curvilinear sperm velocity (um/s), sperm count (million) | Serum, semen | China |
| Petersen et al. (2018) [61] | Cross-sectional | 263 | PFOA, PFOS | NA (PFNA, PFHxS, PFDA >LOQ but not included in analysis) | Semen volume, sperm concentration, sperm morphology, sperm motility, total sperm count | Semen volume (mL), sperm concentration (mill/mL), normal morphology (%), motile sperm (%), total sperm count (million) | Serum, semen | Faroe Islands |
| Song et al. (2018) [67] | Cross-sectional | 103 | PFBS, PFBA, PFHpA, PFHS, PFHxA, PFOA, PFOS, PFPeA, PFPrA, total PFAAs | NA | Sperm concentration, sperm motility | Semen concentration, progressive motility of semen | Blood, semen | China |
| Study | Study Type | Study Size (n) | Detected PFAS | PFAS Not Detected in 51% or More Samples | Outcomes | Sub-Outcomes | Media | Country |
|---|---|---|---|---|---|---|---|---|
| Costa et al. (2019) [73] | Cohort | 1230 | PFHxS, PFNA, PFOA, PFOS | NA | Cranium size, appendicular skeleton length, abdominal circumference | Biparietal diameter, femur length, estimated fetal weight, abdominal circumference | Plasma | USA |
| Ouidir et al. (2020) [77] | Cohort | 2284 | N-MeFOSAA, PFDA, PFDoDA, PFHxS, PFNA, PFOA, PFOS, PFUnDA | PFDS, PFHpA PFOSA | Cranium size, appendicular skeleton length, abdominal circumference | Cerebral width, head circumference, inner orbit diameter, occipital–frontal diameter, outer orbit diameter, biparietal diameter, fibula length, humerus length, radial length, tibia length, ulnar length, foot length, femur length, estimated fetal growth, abdominal circumference | Plasma | Spain |
| Endpoint | Conclusions |
|---|---|
| Fertility | Clinical pregnancy and infertility were not affected by PFAS. |
| Preterm birth (PTB)/gestational age at birth (GAB) | In roughly one-third of all PTB/GAB studies, a decrease in gestational age at birth was inversely associated with PFOS levels, though the 1–3-day earlier birth is likely clinically irrelevant for preterm birth, defined as <37 weeks of gestation. |
| Fetal growth | Fetal growth outcomes were inconsistent, though the combination of PFAS and smoking may play an unconfirmed role in reducing growth. |
| Miscarriage | In two of the three miscarriage studies, PFOA was associated with weakly to moderately increased odds of miscarriage, especially in parous women. |
| Menstruation/menopause | Menstrual cycle length and regularity were unaffected, though heavy bleeding was reduced with nearly every PFAS studied. In a single study, increasing tertiles of PFOA and PFOS affected menopause approximately one year earlier than the lowest exposure group, and high total PFAS levels predicted two years earlier. PFOA and PFOS were associated with up to six times higher odds for primary ovarian insufficiency. |
| Ovarian health | Lesser studied PFAS such as PFDoDA and PFBS were implicated in increasing odds of PCOS- and endometriosis-related infertility in women who seek IVF, though this may be due to an unknown lifestyle factor. |
| Sperm health | Sperm motility was decreased with nearly every PFAS when measured in semen but not serum; similarly, most PFASs (except PFOA and PFOS) affected DNA stability in semen and serum. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haimbaugh, A.; Meyer, D.N.; Connell, M.L.; Blount-Pacheco, J.; Tolofari, D.; Gonzalez, G.; Banerjee, D.; Norton, J.; Miller, C.J.; Baker, T.R. Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies. Int. J. Environ. Res. Public Health 2024, 21, 1615. https://doi.org/10.3390/ijerph21121615
Haimbaugh A, Meyer DN, Connell ML, Blount-Pacheco J, Tolofari D, Gonzalez G, Banerjee D, Norton J, Miller CJ, Baker TR. Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies. International Journal of Environmental Research and Public Health. 2024; 21(12):1615. https://doi.org/10.3390/ijerph21121615
Chicago/Turabian StyleHaimbaugh, Alex, Danielle N. Meyer, Mackenzie L. Connell, Jessica Blount-Pacheco, Dienye Tolofari, Gabrielle Gonzalez, Dayita Banerjee, John Norton, Carol J. Miller, and Tracie R. Baker. 2024. "Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies" International Journal of Environmental Research and Public Health 21, no. 12: 1615. https://doi.org/10.3390/ijerph21121615
APA StyleHaimbaugh, A., Meyer, D. N., Connell, M. L., Blount-Pacheco, J., Tolofari, D., Gonzalez, G., Banerjee, D., Norton, J., Miller, C. J., & Baker, T. R. (2024). Environmental Exposure to Per- and Polyfluorylalkyl Substances (PFASs) and Reproductive Outcomes in the General Population: A Systematic Review of Epidemiological Studies. International Journal of Environmental Research and Public Health, 21(12), 1615. https://doi.org/10.3390/ijerph21121615







