Sargassum Inundations and the Risk of Hypertension Disorders Among Pregnant Women Living in the French Caribbean Island of Martinique
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Setting
2.3. Study Population
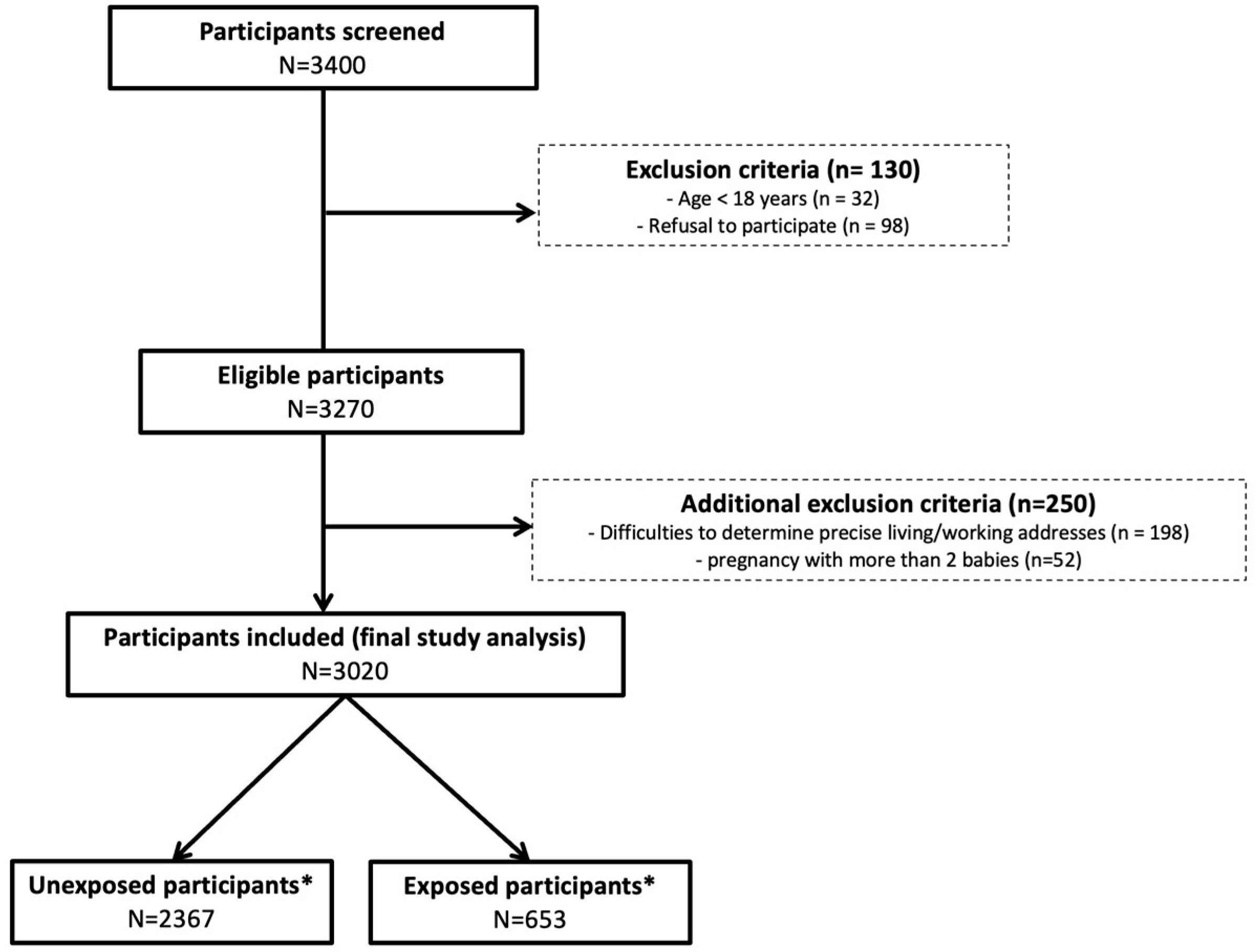
2.4. Study Data
2.5. Definition of Sargassum Exposure (H2S)
2.6. Definition of Hypertensive Disorders of Pregnancy
2.7. Air Pollution
2.8. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmidt, C. Escape from Sargasso Sea: Tremendous Sargassum Blooms Challenge Caribbean and Atlantic Communities. Environ. Health Perspect. 2023, 131, 92001. [Google Scholar] [CrossRef]
- Segaran, T.C.; Azra, M.N.; Handayani, K.S.; Lananan, F.; Xu, J. Seaweed and climate change: A mapping review. Mar. Environ. Res. 2023, 192, 106216. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Neviere, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2019, 392, 2691. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Florentin, J.; Gueye, P.; Lebrun, T.; Blateau, A.; Viguier, J.; Valentino, R.; Brouste, Y.; Kallel, H.; et al. Sargassum seaweed health menace in the Caribbean: Clinical characteristics of a population exposed to hydrogen sulfide during the 2018 massive stranding. Clin. Toxicol. 2021, 59, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Morales-Rodriguez, A.; Zhou, G.; Barrón, D.; Sahuquillo, À.; López-Sánchez, J.F. Survey of arsenic content in edible seaweeds and their health risk assessment. Food Chem. Toxicol. 2024, 187, 114603. [Google Scholar] [CrossRef] [PubMed]
- Legator, M.S.; Singleton, C.R.; Morris, D.L.; Philips, D.L. Health effects from chronic low-level exposure to hydrogen sulfide. Arch. Environ. Health 2001, 56, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Copley, G.B. Chronic low-level hydrogen sulfide exposure and potential effects on human health: A review of the epidemiological evidence. Crit. Rev. Toxicol. 2015, 45, 93–123. [Google Scholar] [CrossRef] [PubMed]
- Batterman, S.; Grant-Alfieri, A.; Seo, S.H. Low level exposure to hydrogen sulfide: A review of emissions, community exposure, health effects, and exposure guidelines. Crit. Rev. Toxicol. 2023, 53, 244–295. [Google Scholar] [CrossRef] [PubMed]
- Van den Hooven, E.H.; de Kluizenaar, Y.; Pierik, F.H.; Hofman, A.; van Ratingen, S.W.; Zandveld, P.Y.; Mackenbach, J.P.; Steegers, E.A.; Miedema, H.M.; Jaddoe, V.W. Air pollution, blood pressure, and the risk of hypertensive complications during pregnancy: The generation R study. Hypertension 2011, 57, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.C.; Talbott, E.O.; Roberts, J.M.; Catov, J.M.; Bilonick, R.A.; Stone, R.A.; Sharma, R.K.; Ritz, B. Ambient air pollution exposure and blood pressure changes during pregnancy. Environ. Res. 2012, 117, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Ha, S.; Roth, J.; Kearney, G.; Talbott, E.O.; Xu, X. Ambient Air Pollution and Hypertensive Disorders of Pregnancy: A Systematic Review and Meta-analysis. Atmos. Environ. 2014, 97, 336–345. [Google Scholar] [CrossRef]
- Pedersen, M.; Stayner, L.; Slama, R.; Sørensen, M.; Figueras, F.; Nieuwenhuijsen, M.J.; Raaschou-Nielsen, O.; Dadvand, P. Ambient air pollution and pregnancy-induced hypertensive disorders: A systematic review and meta-analysis. Hypertension 2014, 64, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Nobles, C.J.; Williams, A.; Ouidir, M.; Sherman, S.; Mendola, P. Differential Effect of Ambient Air Pollution Exposure on Risk of Gestational Hypertension and Preeclampsia. Hypertension 2019, 74, 384–390. [Google Scholar] [CrossRef]
- Bai, W.; Li, Y.; Niu, Y.; Ding, Y.; Yu, X.; Zhu, B.; Duan, R.; Duan, H.; Kou, C.; Li, Y.; et al. Association between ambient air pollution and pregnancy complications: A systematic review and meta-analysis of cohort studies. Environ. Res. 2020, 185, 109471. [Google Scholar] [CrossRef] [PubMed]
- Decrue, F.; Townsend, R.; Miller, M.R.; Newby, D.; Reynolds, R.M. Ambient air pollution and maternal cardiovascular health in pregnancy. Heart 2023, 109, 1586–1593. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, P.; Zhang, L.; Shi, H.; Li, J.; Meng, X.; Xiao, X.; Dai, H.; Zhang, Y. Ozone Exposure During Pregnancy and Risk of Gestational Hypertension or Preeclampsia in China. JAMA Netw. Open 2023, 6, e236347. [Google Scholar] [CrossRef] [PubMed]
- Ausma, T.; De Kok, L.J. Atmospheric H2S: Impact on Plant Functioning. Front. Plant Sci. 2019, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shackelford, R.E.; Shen, X.; Dominic, P.; Kevil, C.G. Sulfide regulation of cardiovascular function in health and disease. Nat. Rev. Cardiol. 2023, 20, 109–125. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, Z.; Luo, S.; Guo, W.; Zhu, Y.Z. The Cardioprotective Effects of Hydrogen Sulfide in Heart Diseases: From Molecular Mechanisms to Therapeutic Potential. Oxid. Med. Cell Longev. 2015, 2015, 925167. [Google Scholar] [CrossRef] [PubMed]
- Lv, B.; Chen, S.; Tang, C.; Jin, H.; Du, J.; Huang, Y. Hydrogen sulfide and vascular regulation—An update. J. Adv. Res. 2020, 27, 85–97. [Google Scholar] [CrossRef]
- Song, Y.; Xu, Z.; Zhong, Q.; Zhang, R.; Sun, X.; Chen, G. Sulfur signaling pathway in cardiovascular disease. Front. Pharmacol. 2023, 14, 1303465. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Jing, G.; Zhu, S. Regulation of Mitochondrial Respiration by Hydrogen Sulfide. Antioxidants 2023, 12, 1644. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.J.; Liu, Y.H.; Khin, E.S.; Bian, J.S. Vasoconstrictive effect of hydrogen sulfide involves downregulation of cAMP in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2008, 295, C1261–C1270. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ping, N.N.; Cao, L.; Mi, Y.-N.; Cao, Y.-X. H2S induces vasoconstriction of rat cerebral arteries via cAMP/adenylyl cyclase pathway. Toxicol. Appl. Pharmacol. 2015, 289, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.R.; Alexander, S.P.; Ralevic, V.; Roberts, R.E. Effects of hydrogen sulphide in smooth muscle. Pharmacol. Ther. 2016, 158, 101–113. [Google Scholar] [CrossRef]
- Orlov, S.N.; Gusakova, S.V.; Smaglii, L.V.; Koltsova, S.V.; Sidorenko, S.V. Vasoconstriction triggered by hydrogen sulfide: Evidence for Na+,K+,2Cl-cotransport and L-type Ca2+ channel-mediated pathway. Biochem. Biophys. Rep. 2017, 12, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, R.; Provitera, L.; Cavallaro, G.; Lattuada, D.; Ercoli, G.; Mosca, F.; Villamor, E. Vasomotor effects of hydrogen sulfide in human umbilical vessels. J. Physiol. Pharmacol. 2017, 68, 737–747. [Google Scholar] [PubMed]
- Liu, T.; Zhang, M.; Hanson, S.; Juarez, R.; Wilson, S.; Schroeder, H.; Li, Q.; Zhu, L.; Zhang, G.; Blood, A.B. H2S Increases Blood Pressure via Activation of L-Type Calcium Channels with Mediation by HS• Generated from Reactions with Oxyhemoglobin. Adv. Sci. 2024, 11, e2305866. [Google Scholar] [CrossRef] [PubMed]
- Cindrova-Davies, T.; Herrera, E.A.; Niu, Y.; Kingdom, J.; Giussani, D.A.; Burton, G.J. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: Hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 2013, 182, 1448–1458. [Google Scholar] [CrossRef]
- Wang, K.; Ahmad, S.; Cai, M.; Rennie, J.; Fujisawa, T.; Crispi, F.; Baily, J.; Miller, M.R.; Cudmore, M.; Hadoke, P.W.; et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation 2013, 127, 2514–2522. [Google Scholar] [CrossRef]
- Possomato-Vieira, J.S.; Palei, A.C.; Pinto-Souza, C.C.; Cavalli, R.; Dias-Junior, C.A.; Sandrim, V. Circulating levels of hydrogen sulphide negatively correlate to nitrite levels in gestational hypertensive and preeclamptic pregnant women. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Banydeen, R.; Lacavalerie, M.R.; Florentin, J.; Boullanger, C.; Medhaoui, H.; Resiere, D.; Neviere, R. Central sleep apnea and exposure to ambient hydrogen sulfide emissions from massive strandings of decomposing sargassum in the Caribbean. Sci. Total Environ. 2024, 912, 168886. [Google Scholar] [CrossRef] [PubMed]
- Merle, H.; Resière, D.; Mesnard, C.; Pierre, M.; Jean-Charles, A.; Béral, L.; Nevière, R. Case Report: Two Cases of Keratoconjunctivitis Tied to Sargassum Algae Emanations. Am. J. Trop. Med. Hyg. 2021, 104, 403–405. [Google Scholar] [CrossRef]
- De Lanlay, D.B.; Monthieux, A.; Banydeen, R.; Jean-Laurent, M.; Resiere, D.; Drame, M.; Neviere, R. Risk of preeclampsia among women living in coastal areas impacted by sargassum strandings on the French Caribbean Island of Martinique. Environ. Toxicol. Pharmacol. 2022, 94, 103894. [Google Scholar] [CrossRef] [PubMed]
- Hypertension in Pregnancy: Diagnosis and Management. Available online: https://www.nice.org.uk/guidance/ng133/resources/hypertension-in-pregnancy-diagnosis-and-management-pdf-66141717671365 (accessed on 1 October 2024).
- Gheibi, S.; Jeddi, S.; Kashfi, K.; Ghasemi, A. Regulation of vascular tone homeostasis by NO and H2S: Implications in hypertension. Biochem. Pharmacol. 2018, 149, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Ping, N.N.; Li, S.; Mi, Y.N.; Cao, L.; Cao, Y. H Hydrogen sulphide induces vasoconstriction of rat coronary artery via activation of Ca2+ influx. Acta Physiol. 2015, 214, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Blackstone, E.; Morrison, M.; Roth, M.B. H2S Induces a Suspended Animation-Like State in Mice. Science 2005, 308, 518. [Google Scholar] [CrossRef] [PubMed]
- Volpato, G.P.; Searles, R.; Yu, B.; Scherrer-Crosbie, M.; Bloch, K.D.; Ichinose, F.; Zapol, W.M. Inhaled hydrogen sulfide: A rapidly reversible inhibitor of cardiac and metabolic function in the mouse. Anesthesiology 2008, 108, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Bustaffa, E.; Cori, L.; Manzella, A.; Nuvolone, D.; Minichilli, F.; Bianchi, F.; Gorini, F. The health of communities living in proximity of geothermal plants generating heat and electricity: A review. Sci. Total Environ. 2020, 706, 135998. [Google Scholar] [CrossRef] [PubMed]
- Bustaffa, E.; Minichilli, F.; Nuvolone, D.; Voller, F.; Cipriani, F.; Bianchi, F. Mortality of populations residing in geothermal areas of Tuscany during the period 2003–2012. Ann. Ist. Super Sanita 2017, 53, 108–117. [Google Scholar]
- Minichilli, F.; Nuvolone, D.; Bustaffa, E.; Cipriani, F.; Vigotti, M.A.; Bianchi, F. State of health of populations residing in geothermal areas of Tuscany. Epidemiol. Prev. 2012, 36, 1–104. [Google Scholar] [PubMed]
- Nuvolone, D.; Petri, D.; Biggeri, A.; Barbone, F.; Voller, F. Health effects associated with short-term exposure to hydrogen sulfide from geothermal power plants: A case-crossover study in the geothermal areas in Tuscany. Int. Arch. Occup. Environ. Health 2020, 3, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Bustaffa, E.; Chatzianagnostou, K.; Bianchi, F.; Vassalle, C. Hydrogen sulfide and cardiovascular disease: Doubts, clues, and interpretation difficulties from studies in geothermal areas. Sci. Total Environ. 2020, 743, 140818. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Hypertensive Disorders of Pregnancy | |||
|---|---|---|---|---|
| All N = 3020 | Yes N = 351 | No N = 2669 | p-Value | |
| Age, years | 29.8 ± 6.2 | 31.2 ± 7.0 | 29.6 ± 6.1 | <0.001 * |
| Age > 30 years | 1431 (47.4%) | 197 (56.1%) | 1234 (46.2%) | <0.001 * |
| BMI, kg/m2 | 26.3 ± 6.8 | 30.1 ± 8.0 | 25.8 ± 6.4 | <0.001 * |
| BMI > 30 kg/m2 n = 2897 | 1325 (43.9%) | 224 (63.8%) | 1101 (41.3%) | <0.001 * |
| Primiparity | 1430 (47.4%) | 188 (53.6%) | 1247 (46.7%) | 0.001 * |
| Primipaternity n = 2050 | 1675 (82%) | 215 (88%) | 1460 (81%) | 0.004 * |
| Twin pregnancy n = 3018 | 71 (2.4%) | 10 (2.8%) | 61 (2.3%) | 0.308 |
| Active tobacco use n = 3014 | 196 (6.5) | 24 (6.9%) | 172 (6.5%) | 0.417 |
| Medical history | ||||
| Endometriosis | 60 (2.0%) | 7 (2.0%) | 53 (2.0%) | 0.557 |
| Thyroid diseases | 55 (1.8%) | 4 (1.1%) | 51 (1.9%) | 0.216 |
| Chronic hypertension n = 3016 | 87 (2.9%) | 54 (15.5%) | 33 (1.2%) | <0.001 * |
| Diabetes | 90 (3.0%) | 26 (7.4%) | 64 (2.4%) | <0.001 * |
| Sickle cell disease | 10 (0.3%) | 4 (1.1%) | 6 (0.2%) | 0.203 |
| Polycystic ovary syndrome | 49 (1.6%) | 8 (2.3%) | 41 (1.5%) | 0.365 |
| Personal pre-eclampsia | 56 (1.9%) | 25 (7.1%) | 31 (1.2%) | <0.001 * |
| Pregnancy outcome | ||||
| Term pregnancy, weeks | 38.7 ± 3.0 | 37.6 ± 3.9 | 38.9 ± 2.8 | <0.001 * |
| Weight gain, kg | 11.0 ± 6.0 | 12.0 ± 7.4 | 10.9 ± 5.8 | 0.010 * |
| Newborn weight, kg | 3.04 ± 0.67 | 2.77 ± 0.89 | 3.07 ± 0.62 | <0.001 * |
| Apgar score 1th min | 8.9 ± 2.5 | 8.0 ± 3.2 | 9.0 ± 2.3 | <0.001 * |
| Apgar score 5th min | 9.5 ± 1.7 | 8.9 ± 2.5 | 9.6 ± 1.6 | <0.001 * |
| Umbilical cord blood lactate | 4.4 ± 3.4 | 5.2 ± 4.8 | 4.3 ± 3.0 | 0.061 |
| Pregnancy complication | ||||
| Gestational diabetes | 274 (9.1%) | 70 (20.0%) | 204 (7.7%) | <0.001 * |
| Threat of premature labor | 690 (22.8%) | 111 (31.6%) | 579 (21.7) | <0.001 * |
| Pre-eclampsia | 194 (6.4%) | 194 (55.3%) | 0 (0%) | <0.001 * |
| Eclampsia | 4 (0.1%) | 4 (1.1%) | 0 (0.0) | <0.001 * |
| HELLP syndrome | 26 (0.9%) | 26 (7.4%) | 0 (0) | <0.001 * |
| Obstetrical hemorrhage | 127 (4.2%) | 28 (8.0%) | 99 (3.7%) | <0.001 * |
| Neonatal death | 66 (2.2%) | 19 (5.4%) | 47 (1.8%) | <0.001 * |
| Biochemistry markers | ||||
| Urea, mmol/L | 3.0 ± 1.3 | 3.1 ± 1.1 | 3.0 ± 1.3 | 0,979 |
| Creatinine, µmol/L | 58.4 ± 13.5 | 58.1 ± 11.4 | 58.5 ± 13.8 | 0.833 |
| Total bilirubin, µmol/L | 9.7 ± 12.3 | 8.8 ± 6.6 | 10.1 ± 14.3 | 0.171 |
| Aspartate aminotransferase, IU/L | 43 ± 105 | 53 ± 111 | 38 ± 101 | 0.046 * |
| Alanine aminotransferase, IU/L | 30 ± 77 | 39 ± 85 | 25 ± 71 | 0.008 * |
| Alkaline phosphatase, IU/L | 181 ± 159 | 201 ± 205 | 171 ± 130 | 0.009 * |
| C reactive protein, mg/L | 29 ± 47 | 38 ± 55 | 28 ± 46 | 0.003 * |
| Blood count and coagulation tests | ||||
| Red blood cells, million/mm3 | 4.0 ± 0.5 | 4.0 ± 0.6 | 4.0 ± 0.5 | 0.514 |
| Hemoglobin, g/dL | 11.0 ± 1.4 | 11.0 ± 1.5 | 11.0 ± 1.4 | 0.521 |
| Hematocrit, % | 33.9 ± 3.9 | 33.7 ± 4.5 | 33.9 ± 3.9 | 0.251 |
| White blood cells, mm−3 | 11.9 ± 4.3 | 12.9 ± 4.9 | 11.7 ± 4.2 | <0.001 * |
| Neutrophils, % | 73.8 ± 10.0 | 76.5 ± 9.2 | 73.3 ± 10.0 | <0.001 * |
| Lymphocytes, % | 18.1 ± 8.4 | 16.4 ± 7.7 | 18.4 ± 8.5 | <0.001 * |
| Monocytes, % | 7.7 ± 2.3 | 7.3 ± 2.4 | 7.8 ± 2.3 | 0.001 * |
| Eosinophils, % | 1.7 ± 1.7 | 2.1 ± 1.6 | 1.6 ± 1.7 | <0.001 * |
| Basophils, % | 0.37± 0.21 | 0.43 ± 0.23 | 0.36 ± 0.20 | <0.001 * |
| Platelets, ×10 µL−1 | 232 ± 70 | 221 ± 73 | 234 ± 69 | 0.001 * |
| Fibrinogen, g/L | 4.7 ± 1.0 | 4.6 ± 1.1 | 4.7 ± 1.0 | 0.031 * |
| PT, % | 104 ± 14 | 103 ± 15 | 104 ± 14 | 0.111 |
| APTT, s. | 29 ± 9 | 29 ± 3 | 28 ± 10 | 0.375 |
| Environmental exposure | ||||
| H2S concentration (ppm) | 0.036 ± 0.236 | 0.073 ± 0.375 | 0.032 ± 0.211 | 0.002 * |
| NH3 concentration (ppm) | 0.052 ± 0.184 | 0.063 ± 0.207 | 0.037 ± 0.146 | 0.188 |
| Active sargassum strandings | 653 (21.6%) | 87 (24.8%) | 566 (21.2) | 0.073 |
| Characteristics | Sargassum Stranding Exposure | |||
|---|---|---|---|---|
| All N = 3020 | Yes (N = 653) | No N = 2367 | p-Value | |
| Age, years | 29.8 ± 6.2 | 30.3 ± 6.6 | 29.7 ± 6.1 | 0.021 * |
| Age > 30 years | 1431 (47.4%) | 322 (49.3%) | 1109 (46.9%) | 0.142 |
| BMI, kg/m2 | 26.3 ± 6.8 | 26.2 ± 7.5 | 26.2 ± 6.6 | 0.241 |
| BMI > 30 kg/m2 n = 2897 | 1325 (43.9%) | 320 (49%) | 1110 (47%) | 0.386 |
| Primiparity | 1430 (47.4%) | 188 (54%) | 1247 (47%) | 0.607 |
| Primipaternity n = 2050 | 1675 (82%) | 371 (82%) | 1304 (82%) | 0.394 |
| Twin pregnancy n = 3018 | 71 (2.4%) | 12 (1.8%) | 59 (2.5%) | 0.204 |
| Active tobacco use n = 3014 | 196 (6.5) | 39 (6.0%) | 157 (6.6%) | 0.309 |
| Medical history | ||||
| Endometriosis | 60 (2.0%) | 18 (2.8%) | 42 (1.8%) | 0.079 |
| Thyroid diseases | 55 (1.8%) | 13 (2.0%) | 42 (1.8%) | 0.409 |
| Chronic hypertension n = 3016 | 87 (2.9%) | 23 (3.5%) | 64 (2.7%) | 0.164 |
| Diabetes | 90 (3.0%) | 24 (3.7%) | 66 (2.8%) | 0.147 |
| Sickle cell disease | 10 (0.3%) | 2 (0.3%) | 8 (0.3%) | 0.628 |
| Polycystic ovary syndrome | 49 (1.6%) | 8 (1.2%) | 41 (1.7%) | 0.237 |
| Personal pre-eclampsia | 56 (1.9%) | 16 (2.5%) | 40 (1.7%) | 0.134 |
| Pregnancy outcome | ||||
| Term pregnancy, weeks | 38.7 ± 3.0 | 38.6 ± 3.0 | 38.8 ± 3.0 | 0.364 |
| Weight gain, kg | 11.0 ± 6.0 | 11.3 ± 5.7 | 11.0 ± 6.1 | 0.322 |
| Newborn weight, kg | 3.04 ± 0.67 | 3.01 ± 0.67 | 3.04 ± 0.66 | 0.237 |
| Apgar score 1th min | 8.9 ± 2.5 | 8.8 ± 2.5 | 8.9 ± 2.5 | 0.235 |
| Apgar score 5th min | 9.5 ± 1.7 | 9.5 ± 1.7 | 9.5 ± 1.7 | 0.339 |
| Umbilical cord blood lactate | 4.4 ± 3.4 | 4.5 ± 3.0 | 4.4 ± 3.5 | 0.800 |
| Pregnancy complication | ||||
| Gestational diabetes | 274 (9.1%) | 53 (8.1%) | 221 (9.4%) | 0.186 |
| Threat of premature labor | 690 (22.8%) | 150 (23.0%) | 540 (22.8) | 0.485 |
| Hypertensive disorders | 353 (11.7%) | 89 (13.6%) | 264 (11.2%) | 0.049 * |
| Pre-eclampsia | 194 (6.4%) | 49 (7.5%) | 145 (6.1%) | 0.120 |
| Eclampsia | 4 (0.1%) | 0 (0%) | 4 (0.2%) | 0.377 |
| HELLP syndrome | 26 (0.9%) | 11 (1.7%) | 15 (0.6%) | 0.014 * |
| Obstetrical hemorrhage | 127 (4.2%) | 28 (4.3%) | 99 (4.2%) | 0.489 |
| Neonatal death | 66 (2.2%) | 13 (2.0%) | 53 (2.2%) | 0.908 |
| Biochemistry markers | ||||
| Urea, mmol/L | 3.0 ± 1.3 | 3.1 ± 1.1 | 3.0 ± 1.3 | 0.828 |
| Creatinine, µmol/L | 58.4 ± 13.5 | 58.1 ± 11.4 | 58.5 ± 13.8 | 0.762 |
| Total bilirubin, µmol/L | 9.7 ± 12.3 | 8.8 ± 6.6 | 10.1 ± 14.3 | 0.434 |
| Aspartate aminotransferase, IU/L | 43 ± 105 | 53 ± 111 | 38 ± 101 | 0.144 |
| Alanine aminotransferase, IU/L | 30 ± 77 | 39 ± 85 | 25 ± 71 | 0.180 |
| Alkaline phosphatase, IU/L | 181 ± 159 | 201 ± 205 | 171 ± 130 | 0.314 |
| C reactive protein, mg/L | 29 ± 47 | 29 ± 44 | 30 ± 48 | 0.734 |
| Blood count and coagulation tests | ||||
| Red blood cells, million/mm3 | 4.0 ± 0.5 | 4.0 ± 0.6 | 4.0 ± 0.5 | 0.466 |
| Hemoglobin, g/dL | 11.0 ± 1.4 | 10.9 ± 1.4 | 11.0 ± 1.4 | 0.628 |
| Hematocrit, % | 33.9 ± 3.9 | 33.8 ± 4.0 | 33.9 ± 3.9 | 0.655 |
| White blood cells, mm−3 | 11.9 ± 4.3 | 11.7 ± 4.0 | 11.9 ± 4.4 | 0.319 |
| Neutrophils, % | 73.8 ± 10.0 | 73.7 ± 9.3 | 73.8 ± 10.0 | 0.866 |
| Lymphocytes, % | 18.1 ± 8.4 | 16.4 ± 7.7 | 18.4 ± 8.5 | 0.920 |
| Monocytes, % | 7.7 ± 2.3 | 7.7 ± 2.2 | 7.7 ± 2.3 | 0.475 |
| Eosinophils, % | 1.7 ± 1.7 | 1.9 ± 1.8 | 1.7 ± 1.6 | 0.023 * |
| Basophils, % | 0.37± 0.21 | 0.38 ± 0.21 | 0.37 ± 0.21 | 0.366 |
| Platelets, ×10 µL−1 | 232 ± 70 | 234 ± 76 | 231 ± 68 | 0.362 |
| Fibrinogen, g/L | 4.7 ± 1.0 | 4.6 ± 1.1 | 4.7 ± 1.0 | 0.946 |
| PT, % | 104 ± 14 | 103 ± 15 | 104 ± 14 | 0.282 |
| APTT, s. | 29 ± 9 | 29 ± 7 | 29 ± 10 | 0.916 |
| Environmental exposure | ||||
| H2S concentration (ppm) | 0.036 ± 0.236 | 0.169 ± 0.486 | 0 ± 0 | <0.001 * |
| NH3 concentration (ppm) | 0.052 ± 0.184 | 0.073 ± 0.242 | 0 ± 0 | <0.001 * |
| Mean NO2 concentration, µg·m−3 | 7.2 ± 0.9 | 7.2 ± 0.9 | 7.3 ± 0.9 | 0.123 |
| Mean SO2 concentration, µg·m−3 | 7.7 ± 3.8 | 7.5 ± 3.9 | 7.8 ± 3.7 | 0.496 |
| Mean O3 concentration, µg·m−3 | 45.0 ± 8.4 | 44.7 ± 8.4 | 45.2 ± 8.5 | 0.634 |
| Mean PM10 concentration, µg·m−3 | 22.5 ± 3.2 | 22.6 ± 3.3 | 22.4 ± 3.1 | 0.598 |
| Mean PM2.5 concentration, µg·m−3 | 12.8 ± 3.1 | 13.2 ± 2.9 | 12.3 ± 4.1 | 0.673 |
| Characteristics | Sargassum Stranding Exposure | |||
|---|---|---|---|---|
| All N = 351 | Yes N = 87 | No N = 264 | p-Value | |
| Age, years | 31.2 ± 7.0 | 31.6 ± 7.3 | 31.1 ± 6.9 | 0.523 |
| Age > 30 years | 197 (56.1%) | 50 (56.2%) | 147 (55.7%) | 0.518 |
| BMI, kg/m2 | 30.1 ± 8.0 | 29.6 ± 7.7 | 30.3 ± 8.1 | 0.481 |
| BMI > 30 kg/m2 | 224 (63.8%) | 53 (63.1%) | 171 (66.3%) | 0.342 |
| Primiparity | 188 (53.6%) | 48 (55%) | 140 (53%) | 0.129 |
| Primipaternity n = 245 | 215 (88%) | 57 (89%) | 158 (87%) | 0.451 |
| Twin pregnancy | 10 (2.8%) | 1 (1.1%) | 9 (3.4%) | 0.245 |
| Active tobacco use n = 349 | 24 (6.9%) | 8 (9.3%) | 16 (6.1%) | 0.214 |
| Medical history | ||||
| Endometriosis | 7 (2.0%) | 4 (4.6%) | 3 (1.1%) | 0.067 |
| Thyroid diseases | 4 (1.1%) | 1 (1.1%) | 3 (1.1%) | 0.682 |
| Chronic hypertension | 54 (15.4%) | 18 (20.7%) | 36 (13.6%) | 0.082 |
| Diabetes | 26 (7.4%) | 5 (5.7%) | 21 (8.0%) | 0.339 |
| Sickle cell disease | 4 (1.1%) | 0 (0%) | 4 (1.5%) | 0.318 |
| Polycystic ovary syndrome | 8 (2.3%) | 1 (1.1%) | 7 (2.7%) | 0.370 |
| Personal pre-eclampsia | 25 (7.1%) | 10 (11.5%) | 15 (5.7%) | 0.061 |
| Pregnancy outcome | ||||
| Term pregnancy, weeks | 37.6 ± 3.9 | 37.6 ± 3.9 | 38.9 ± 2.8 | 0.006 * |
| Weight gain, kg | 12.0 ± 7.4 | 11.7 ± 7.2 | 12.0 ± 7.5 | 0.714 |
| Newborn weight, kg | 2.77 ± 0.89 | 2.56 ± 0.96 | 2.84 ± 0.85 | 0.011 * |
| Apgar score 1th min | 8.0 ± 3.2 | 7.5 ± 3.5 | 8.1 ± 3.2 | 0.143 |
| Apgar score 5th min | 8.9 ± 2.5 | 8.5 ± 3.0 | 9.1 ± 2.4 | 0.076 |
| Umbilical cord blood lactate | 5.2 ± 4.8 | 4.2 ± 3.0 | 5.6 ± 5.3 | 0.399 |
| Pregnancy complication | ||||
| Gestational diabetes | 70 (20.0%) | 17 (19.8%) | 53 (20.1%) | 0.543 |
| Threat of premature labor | 111 (31.6%) | 31 (35.6%) | 80 (30.3%) | 0.213 |
| Pre-eclampsia | 194 (55.3%) | 49 (56.3%) | 145 (54.9%) | 0.460 |
| Eclampsia | 4 (1.1%) | 0 (0%) | 4 (1.5) | 0.318 |
| HELLP syndrome | 26 (7.4%) | 11 (12.6%) | 15 (5.7%) | 0.032 * |
| Obstetrical hemorrhage | 28 (8.0%) | 8 (9.3%) | 20 (7.6%) | 0.377 |
| Neonatal death | 19 (5.4%) | 13 (2.0%) | 6 (2.2%) | 0.198 |
| Biochemistry markers | ||||
| Urea, mmol/L | 3.1 ± 1.1 | 2.8 ± 1.1 | 3.2 ± 1.1 | 0,248 |
| Creatinine, µmol/L | 58.1 ± 11.4 | 56.1 ± 14.9 | 59.1 ± 10.2 | 0.419 |
| Total bilirubin, µmol/L | 8.8 ± 6.6 | 8.7 ± 7.3 | 9.0 ± 6.4 | 0.171 |
| Aspartate aminotransferase, IU/L | 53 ± 111 | 71 ± 181 | 46 ± 67 | 0.082 |
| Alanine aminotransferase, IU/L | 39 ± 85 | 51 ± 120 | 35 ± 67 | 0.149 |
| Alkaline phosphatase, IU/L | 201 ± 205 | 238 ± 365 | 188 ± 80 | 0.062 |
| C reactive protein, mg/L | 38 ± 55 | 40 ± 58 | 36 ± 53 | 0.632 |
| Blood count and coagulation tests | ||||
| Red blood cells, million/mm3 | 4.0 ± 0.6 | 3.9 ± 0.6 | 4.0 ± 0.6 | 0.100 |
| Hemoglobin, g/dL | 11.0 ± 1.5 | 10.8 ± 1.5 | 11.0 ± 1.5 | 0.415 |
| Hematocrit, % | 33.7 ± 4.5 | 33.7 ± 4.5 | 33.9 ± 3.9 | 0.251 |
| White blood cells, mm−3 | 12.9 ± 4.9 | 12.8 ± 4.3 | 12.9 ± 5.1 | 0.841 |
| Neutrophils, % | 76.5 ± 9.2 | 76.2 ± 9.1 | 76.6 ± 9.3 | 0.709 |
| Lymphocytes, % | 16.4 ± 7.7 | 17.0 ± 7.8 | 16.2 ± 7.6 | 0.412 |
| Monocytes, % | 7.3 ± 2.4 | 7.5 ± 2.5 | 7.3 ± 2.4 | 0.377 |
| Eosinophils, % | 2.1 ± 1.6 | 2.2 ± 1.7 | 2.0 ± 1.6 | 0.472 |
| Basophils, % | 0.43± 0.23 | 0.46 ± 0.25 | 0.42 ± 0.22 | 0.201 |
| Platelets, ×10 µL−1 | 221 ± 73 | 224 ± 91 | 218 ± 67 | 0.556 |
| Fibrinogen, g/L | 4.6 ± 1.1 | 4.6 ± 1.2 | 4.5 ± 1.1 | 0.547 |
| PT, % | 103 ± 15 | 102 ± 15 | 103 ± 14 | 0.546 |
| APTT, s. | 29 ± 3 | 29 ± 3 | 29 ± 3 | 0.754 |
| Environmental exposure | ||||
| H2S concentration (ppm) | 0.073 ± 0.375 | 0.297 ± 0.713 | 0 ± 0 | <0.001 * |
| NH3 concentration (ppm) | 0.047 ± 0.181 | 0.0797 ± 0.236 | 0 ± 0 | <0.001 * |
| Mean NO2 concentration, µg·m−3 | 7.2 ± 0.9 | 7.2 ± 0.9 | 7.3 ± 0.9 | 0.123 |
| Mean SO2 concentration, µg·m−3 | 7.7 ± 3.8 | 7.5 ± 3.9 | 7.8 ± 3.7 | 0.496 |
| Mean O3 concentration, µg·m−3 | 45.0 ± 8.4 | 44.7 ± 8.4 | 45.2 ± 8.5 | 0.634 |
| Mean PM10 concentration, µg·m−3 | 22.5 ± 3.2 | 22.6 ± 3.3 | 22.4 ± 3.1 | 0.598 |
| Mean PM2.5 concentration, µg·m−3 | 13.4 ± 2.3 | 13.0 ± 3.1 | 13.8 ± 2.4 | 0.676 |
| Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Age | 1.045 (0.997–1.09) | 0.159 * | 1.05 (1.02–1.07) | 0.001 |
| BMI | 1.07 (1.03–1.10) | <0.001 * | 1.08 (1.05–1.10) | <0.001 |
| Nulliparity | 0.91 (0.75–1.10) | 0.339 | ||
| Active tobacco use | 1.29 (0.67–2.47) | 0.448 | ||
| Medical history | ||||
| Endometriosis | 0.72 (0.24–2.18) | 0.562 | ||
| Diabetes | 2.09 (0.87–5.03) | 0.099 * | ||
| Sickle cell disease | 18.64 (1.95–178.30) | 0.011 * | 18.11 (2.15–152.57) | 0.008 |
| Polycystic ovary syndrome | 2.06 (0.83–5.14) | 0.120 * | ||
| Pregnancy | ||||
| Primipaternity | 2.37 (1.30–4.33) | 0.005 * | 2.52 (1.50–4.24) | 0.001 |
| Twin pregnancy | 0.83 (0.28–2.42) | 0.729 | ||
| Weight gain | 1.06 (1.03–1.08) | <0.001 * | 1.05 (1.03–1.08) | <0.001 |
| Gestational diabetes | 1.82 (1.08–3.07) | 0.024 * | 2.04 (1.26–3.30) | 0.004 |
| Environmental exposure | ||||
| Sargassum stranding (H2S exposure) | 1.37 (0.90–2.10) | 0.146 * | 1.59 (1.09–2.34) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banydeen, R.; Rejaudry Lacavalerie, M.; Savoyen, L.; Monthieux, A.; Jean-Laurent, M.; Florentin, J.; Radouani, F.; Mehdaoui, H.; Resiere, D.; Neviere, R. Sargassum Inundations and the Risk of Hypertension Disorders Among Pregnant Women Living in the French Caribbean Island of Martinique. Int. J. Environ. Res. Public Health 2024, 21, 1612. https://doi.org/10.3390/ijerph21121612
Banydeen R, Rejaudry Lacavalerie M, Savoyen L, Monthieux A, Jean-Laurent M, Florentin J, Radouani F, Mehdaoui H, Resiere D, Neviere R. Sargassum Inundations and the Risk of Hypertension Disorders Among Pregnant Women Living in the French Caribbean Island of Martinique. International Journal of Environmental Research and Public Health. 2024; 21(12):1612. https://doi.org/10.3390/ijerph21121612
Chicago/Turabian StyleBanydeen, Rishika, Mickael Rejaudry Lacavalerie, Loic Savoyen, Alice Monthieux, Mehdi Jean-Laurent, Jonathan Florentin, Fatima Radouani, Hossein Mehdaoui, Dabor Resiere, and Remi Neviere. 2024. "Sargassum Inundations and the Risk of Hypertension Disorders Among Pregnant Women Living in the French Caribbean Island of Martinique" International Journal of Environmental Research and Public Health 21, no. 12: 1612. https://doi.org/10.3390/ijerph21121612
APA StyleBanydeen, R., Rejaudry Lacavalerie, M., Savoyen, L., Monthieux, A., Jean-Laurent, M., Florentin, J., Radouani, F., Mehdaoui, H., Resiere, D., & Neviere, R. (2024). Sargassum Inundations and the Risk of Hypertension Disorders Among Pregnant Women Living in the French Caribbean Island of Martinique. International Journal of Environmental Research and Public Health, 21(12), 1612. https://doi.org/10.3390/ijerph21121612






