Co-Creation in the Development of Digital Therapeutics: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Narrative
3.1. Digital Therapeutics
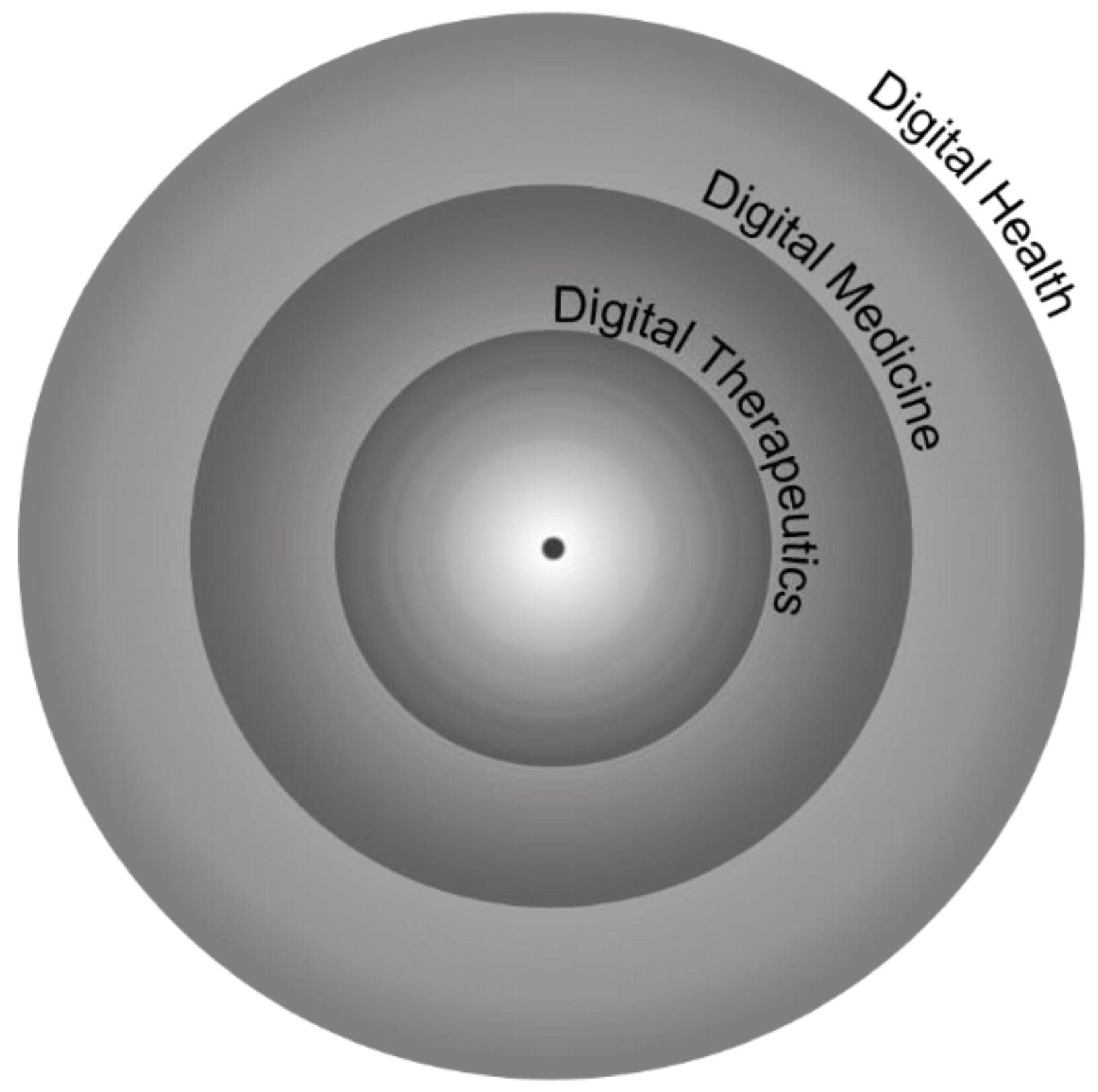
3.1.1. Digital Health vs. Digital Medicine vs. DTx
3.1.2. DTx vs. mHealth
3.1.3. Evaluation, Regulation, and Approval
3.1.4. Major DTx Products and Companies
3.1.5. DTx Advantages and Disadvantages
3.1.6. User Perceptions and Experiences
3.1.7. Healthcare Professional’s Perceptions and Experiences
3.2. Co-Creation: Co-Design
3.2.1. Co-Design Activities
3.2.2. Benefits and Challenges Associated with the Use of Co-Creation in DTx Development
3.2.3. Examples of How Co-Creation Has Been Used in the Development of DTx
4. Conclusions
5. Future Directions and Recommendations
Author Contributions
Funding
Conflicts of Interest
References
- Adler, R.F.; Baez, K.; Morales, P.; Sotelo, J.; Victorson, D.; Magasi, S. Evaluating the Usability of an mHealth App for Empowering Cancer Survivors With Disabilities: Heuristic Evaluation and Usability Testing. JMIR Hum. Factors 2024, 11, e51522. [Google Scholar] [CrossRef] [PubMed]
- Dang, A.; Arora, D.; Rane, P. Role of digital therapeutics and the changing future of healthcare. J. Fam. Med. Prim. Care 2020, 9, 2207–2213. [Google Scholar] [CrossRef] [PubMed]
- Henni, S.H.; Maurud, S.; Fuglerud, K.S.; Moen, A. The experiences, needs and barriers of people with impairments related to usability and accessibility of digital health solutions, levels of involvement in the design process and strategies for participatory and universal design: A scoping review. BMC Public Health 2022, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Petracca, F.; Ciani, O.; Cucciniello, M.; Tarricone, R. Harnessing Digital Health Technologies During and After the COVID-19 Pandemic: Context Matters. J. Med. Internet Res. 2020, 22, e21815. [Google Scholar] [CrossRef] [PubMed]
- Warraich, H.J.; Califf, R.M.; Krumholz, H.M. The digital transformation of medicine can revitalize the patient-clinician relationship. NPJ Digit. Med. 2018, 1, 49. [Google Scholar] [CrossRef] [PubMed]
- Farnood, A.; Johnston, B.; Mair, F.S. A mixed methods systematic review of the effects of patient online self-diagnosing in the ‘smart-phone society’ on the healthcare professional-patient relationship and medical authority. BMC Med. Inform. Decis. Mak. 2020, 20, 253. [Google Scholar] [CrossRef]
- DTA. Product Library. 2024. Available online: https://dtxalliance.org/understanding-dtx/product-library/ (accessed on 6 August 2024).
- Lofton, J.C. AMCP Partnership Forum: Digital Therapeutics-What Are They and Where Do They Fit in Pharmacy and Medical Benefits? J. Manag. Care Spec. Pharm. 2020, 26, 674–681. [Google Scholar]
- Bally, E.L.S.; Cheng, D.; van Grieken, A.; Ferri Sanz, M.; Zanutto, O.; Carroll, A.; Darley, A.; Roozenbeek, B.; Dippel, D.W.J.; Raat, H. Patients’ Perspectives Regarding Digital Health Technology to Support Self-management and Improve Integrated Stroke Care: Qualitative Interview Study. J. Med. Internet Res. 2023, 25, e42556. [Google Scholar] [CrossRef]
- Berry, N.; Machin, M.; Ainsworth, J.; Berry, K.; Edge, D.; Haddock, G.; Lewis, S.; Morris, R.; Bucci, S. Developing a Theory-Informed Smartphone App for Early Psychosis: Learning Points From a Multidisciplinary Collaboration. Front. Psychiatry 2020, 11, 602861. [Google Scholar] [CrossRef]
- Bevan Jones, R.; Stallard, P.; Agha, S.S.; Rice, S.; Werner-Seidler, A.; Stasiak, K.; Kahn, J.; Simpson, S.A.; Alvarez-Jimenez, M.; Rice, F.; et al. Practitioner review: Co-design of digital mental health technologies with children and young people. J. Child Psychol. Psychiatry Allied Discip. 2020, 61, 928–940. [Google Scholar] [CrossRef]
- Lee, N.J.; Ahn, S.; Lee, M. Mixed-method investigation of health consumers’ perception and experience of participation in patient safety activities. BMJ Open 2020, 10, e035831. [Google Scholar] [CrossRef] [PubMed]
- Adler, R.F.; Morales, P.; Sotelo, J.; Magasi, S. Developing an mHealth App for Empowering Cancer Survivors with Disabilities: Co-design Study. JMIR Form. Res. 2022, 6, e37706. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Olivencia, S.; Carrillo-Leal, J.; Rao, A.; Sasangohar, F. User-Centered Design of a Diabetes Self-Management Tool for Underserved Populations. J. Diabetes Sci. Technol. 2024, 18, 22–29. [Google Scholar] [CrossRef]
- Barbaric, A.; Munteanu, C.; Ross, H.; Cafazzo, J.A. Design of a Patient Voice App Experience for Heart Failure Management: Usability Study. JMIR Form. Res. 2022, 6, e41628. [Google Scholar] [CrossRef] [PubMed]
- Gilson, A.; Gassman, M.; Dodds, D.; Lombardo, R.; Ford Ii, J.H.; Potteiger, M. Refining a Digital Therapeutic Platform for Home Care Agencies in Dementia Care to Elicit Stakeholder Feedback: Focus Group Study With Stakeholders. JMIR Aging 2022, 5, e32516. [Google Scholar] [CrossRef] [PubMed]
- MSF Sweden Innovation Unit. Digital Therapeutics (DTx) for Diabetes. 2023. Available online: https://msf-siu.org/development-stage-cases/digital-therapeutics-for-diabetes (accessed on 6 August 2024).
- DTA. What is a DTx? 2024. Available online: https://dtxalliance.org/understanding-dtx/ (accessed on 4 July 2024).
- Denecke, K.; May, R.; Gabarron, E.; Lopez-Campos, G.H. Assessing the Potential Risks of Digital Therapeutics (DTX): The DTX Risk Assessment Canvas. J. Pers. Med. 2023, 13, 1523. [Google Scholar] [CrossRef] [PubMed]
- DTA. Is This Product a DTx? 2022. Available online: https://dtxalliance.org/understanding-dtx/what-is-a-dtx/#difference (accessed on 9 July 2024).
- Hong, J.S.; Wasden, C.; Han, D.H. Introduction of digital therapeutics. Comput. Methods Programs Biomed. 2021, 209, 106319. [Google Scholar] [CrossRef]
- Brönneke, J.B.; Herr, A.; Reif, S.; Stern, A.D. Dynamic HTA for digital health solutions: Opportunities and challenges for patient-centered evaluation. Int. J. Technol. Assess. Health Care 2023, 39, e72. [Google Scholar] [CrossRef]
- FDA. Software as a Medical Device (SaMD). 2018. Available online: https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd (accessed on 4 July 2024).
- Huh, K.Y.; Oh, J.; Lee, S.; Yu, K.S. Clinical Evaluation of Digital Therapeutics: Present and Future. Healthc. Inform. Res. 2022, 28, 188–197. [Google Scholar] [CrossRef]
- Palanica, A.; Docktor, M.J.; Lieberman, M.; Fossat, Y. The Need for Artificial Intelligence in Digital Therapeutics. Digit. Biomark. 2020, 4, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Cornet, V.P.; Daley, C.; Bolchini, D.; Toscos, T.; Mirro, M.J.; Holden, R.J. Patient-centered Design Grounded in User and Clinical Realities: Towards Valid Digital Health. Proc. Int. Symp. Hum. Factors Ergon. Health Care 2019, 8, 100–104. [Google Scholar] [CrossRef]
- Abdulhussein, F.S.; Pinkney, S.; Görges, M.; van Rooij, T.; Amed, S. Designing a Collaborative Patient-Centered Digital Health Platform for Pediatric Diabetes Care in British Columbia: Formative Needs Assessment by Caregivers of Children and Youths Living With Type 1 Diabetes and Health Care Providers. JMIR Pediatr. Parent. 2023, 6, e46432. [Google Scholar] [CrossRef] [PubMed]
- Ahlqvist, J.; Kalliola, M. How Can Digital Therapeutics Help Europe? Sitra: Singapore, 2021. [Google Scholar]
- European Union. Digital Therapeutics (Dtx). 2024. Available online: https://www.edps.europa.eu/press-publications/publications/techsonar/digital-therapeutics-dtx (accessed on 11 October 2024).
- Bochicchio, M.A.; Vaira, L.; Mortara, A.; Maria, R.D. Which Usability Assessment for Digital Therapeutics and Patient Support Programs? In Proceedings of the 2021 IEEE International Conference on Digital Health (ICDH), Chicago, IL, USA, 5–10 September 2021; pp. 276–282. [Google Scholar]
- Patel, N.A.; Butte, A.J. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digit. Med. 2020, 3, 159. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Zhang, W.; Evans, R.; Cao, G.; Rui, T.; Shen, L. Inequities in Health Care Services Caused by the Adoption of Digital Health Technologies: Scoping Review. J. Med. Internet Res. 2022, 24, e34144. [Google Scholar] [CrossRef]
- Gan, D.Z.Q.; McGillivray, L.; Larsen, M.E.; Torok, M. Promoting engagement with self-guided digital therapeutics for mental health: Insights from a cross-sectional survey of end-users. J. Clin. Psychol. 2023, 79, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, K.M.; Lee, S.; Park, S.; Hong, M.; Shin, J.; Lee, E. Understanding patient perspectives on digital therapeutics and its platform for insomnia: Insights from focused group interviews. BMC Health Serv. Res. 2024, 24, 859. [Google Scholar] [CrossRef]
- Carrera, A.; Lettieri, E.; Lietti, G.; Martignoni, S.; Sgarbossa, C.; Cafazzo, J. Therapies go digital. What drives physicians’ acceptance? PLoS ONE 2024, 19, e0303302. [Google Scholar] [CrossRef]
- Laurisz, N.; Ćwiklicki, M.; Żabiński, M.; Canestrino, R.; Magliocca, P. Co-Creation in Health 4.0 as a New Solution for a New Era. Healthcare 2023, 11, 363. [Google Scholar] [CrossRef]
- Carrera, A.; Manetti, S.; Lettieri, E. Rewiring care delivery through Digital Therapeutics (DTx): A machine learning-enhanced assessment and development (M-LEAD) framework. BMC Health Serv. Res. 2024, 24, 237. [Google Scholar] [CrossRef]
- Barros, J.P.; Brandão, P. End-Stage Renal Disease Self-management: Mobile app development. In Proceedings of the 2021 IEEE Symposium on Computers and Communications (ISCC), Athens, Greece, 5–8 September 2021; pp. 1–4. [Google Scholar]
- Denecke, K.; Von Kaenel, F.; Miletic, M.; Fernández-Llatas, C.; Ibañez-Sánchez, G.; Valero-Ramón, Z.; Martînez-Millana, A.; Segura, M.; Rivera Romero, O. How to Design Successful Participatory Design Workshops for Digital Health Solutions? In Caring is Sharing—Exploiting the Value in Data for Health and Innovation; Studies in Health Technology and Informatics; IOS Press: Amsterdam, The Netherlands, 2023; pp. 641–645. [Google Scholar]
- Slattery, P.; Saeri, A.K.; Bragge, P. Research co-design in health: A rapid overview of reviews. Health Res. Policy Syst. 2020, 18, 17. [Google Scholar] [CrossRef]
- SDT. User Scenarios. 2024. Available online: https://servicedesigntools.org/tools/user-scenarios (accessed on 25 July 2024).
- SDT. Personas. 2024. Available online: https://servicedesigntools.org/tools/personas (accessed on 25 July 2024).
- Muratovski, G. Research for Designers; Steele, M., Ed.; SAGE: Washington, DC, USA, 2016; pp. 56–117. [Google Scholar]
- Moran, K. Usability Testing 101. 2019. Available online: https://www.nngroup.com/articles/usability-testing-101/ (accessed on 18 June 2024).
- Borycki, E.M.; Kushniruk, A.W. Health technology, quality and safety in a learning health system. Healthc. Manag. Forum 2022, 36, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.; Arora, S.; Shah, N.; King, D.; Darzi, A. A regulatory perspective on the influence of health information technology on organisational quality and safety in England. Health Inform. J. 2020, 26, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Ebnali, M.; Kennedy-Metz, L.R.; Conboy, H.M.; Clarke, L.A.; Osterweil, L.J.; Avrunin, G.; Miccile, C.; Arshanskiy, M.; Phillips, A.; Zenati, M.A.; et al. A Coding Framework for Usability Evaluation of Digital Health Technologies. In Human-Computer Interaction. Theoretical Approaches and Design Methods; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Jeong, H.; Yoo, J.H.; Goh, M. Virtual Agents in Internet-Based Cognitive Behavioral Therapy: Enhancing Engagement and Alleviating Depression. In Proceedings of the 2023 IEEE International Conference on Agents (ICA), Kyoto, Japan, 4–6 December 2023. [Google Scholar]
- Grannell, A.; Hallson, H.; Gunlaugsson, B.; Jonsson, H. Exercise therapy as a digital therapeutic for chronic disease management: Consideration for clinical product development. Front. Digit. Health 2023, 5, 1250979. [Google Scholar] [CrossRef] [PubMed]
- W3C. Web Content Accessibility Guidelines (WCAG) 2.1. 2023. Available online: https://www.w3.org/TR/WCAG21/ (accessed on 18 June 2024).
- Noorbergen, T.J.; Adam, M.T.P.; Teubner, T.; Collins, C.E. Using Co-design in Mobile Health System Development: A Qualitative Study with Experts in Co-design and Mobile Health System Development. JMIR Mhealth Uhealth 2021, 9, e27896. [Google Scholar] [CrossRef]
- Papoutsi, C.; Wherton, J.; Shaw, S.; Morrison, C.; Greenhalgh, T. Putting the social back into sociotechnical: Case studies of co-design in digital health. J. Am. Med. Inform. Assoc. 2021, 28, 284–293. [Google Scholar] [CrossRef]
- MSF Sweden Innovation Unit. Prioritizing People with Lived Experience as Partners to Co-Create a Diabetes Digital Therapeutic (DTx) in Lebanon. 2023. Available online: https://msf-siu.org/blog/co-creating-a-diabetes-digital-therapeutic-in-lebanon (accessed on 30 July 2024).

| Product Name | Classification | Company | Therapeutic Area | Approval Status | Website |
|---|---|---|---|---|---|
| Dario® Platform | Non- Prescription DTx | Dario | Diabetes (type 1 and type 2) Hypertension | FDA-cleared Class II device; CE mark received by European Notified Body | https://www.dariohealth.com (accessed on 5 June 2024) |
| Insulia | Prescription DTx | Voluntis | Type 2 diabetes | FDA-510(k) EU-CE Mark | https://insulia.com/ (accessed on 5 June 2024) |
| Daylight® | Non- Prescription DTx | Big Health | Generalised anxiety disorder | EU-CE Mark FDA enforcement discretion | https://www.bighealth.com/daylight/ (accessed on 5 June 2024) |
| EndeavorOTC® | Non- Prescription DTx | Akili | Attention-deficit/ hyperactivity disorder (ADHD) | FDA-authorised Class II Medical Device | https://www.akiliinteractive.com/ (accessed on 5 June 2024)) |
| Freespira® | Prescription DTx | Palo Alto Health Sciences | Post-traumatic stress disorder (PTSD), panic disorder, panic/anxiety attacks | FDA-cleared Class II Medical Device | https://freespira.com/ (accessed on 5 June 2024) |
| GameChange® | Non- Prescription DTx | RealizedCare | Agoraphobic avoidance and distress | Class I CE mark | https://www.realizedcare.com/ (accessed on 5 June 2024) |
| HelloBetter® Chronic Pain | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Chronic Pain | Class I CE mark (MDR) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| HelloBetter® Diabetes | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Diabetes | Class I CE mark (MDD) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| HelloBetter® Panic | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Panic disorder with or without agoraphobia | Class I CE mark (MDR) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| HelloBetter® Sleep | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Insomnia | Class I CE mark (MDR) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| HelloBetter® Stress and Burnout | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Stress and burnout | Class I CE mark (MDR) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| HelloBetter® Vaginismus Plus | Non- Prescription DTx (Eligible in ‘DiGA’) | HelloBetter | Vaginismus, dyspareunia, and genito-pelvic pain/penetration disorder | Class I CE mark (MDR) | https://hellobetter.de/en/ (accessed on 5 June 2024) |
| JOGO-Gx® | Non- Prescription DTx (Reimbursed by Medicare) | Jogo Health | Migraine and chronic lower back pain | FDA 510K exempted (reviewed by the FDA and registered) | https://www.jogohealth.com/ (accessed on 5 June 2024) |
| Leva® | Non- Prescription DTx | Leva | Urinary and faecal incontinence | FDA-cleared Class II Medical Device | https://www.levatherapy.com/ (accessed on 5 June 2024) |
| Propeller® | Non- Prescription DTx | ResMed | Asthma and chronic obstructive pulmonary disease (COPD) | FDA-cleared Class II Medical Device and EU Class I Medical Device | https://propellerhealth.com/ (accessed on 5 June 2024) |
| Sleepio® | Non- Prescription DTx | Big Health | Insomnia | FDA enforcement discretion; Class I CE mark | https://www.bighealth.com/sleepio/ (accessed on 5 June 2024) |
| Welldoc® App | Non- Prescription DTx | Welldoc | Type 1 and 2 diabetes, pre-diabetes, hypertension, heart failure, weight and obesity management | FDA-cleared Class II Medical Device; Class IIa CE mark; Health Canada-licenced Class II Medical Device | https://www.welldoc.com/ (accessed on 5 June 2024) |
| reSET® | Prescription DTx | Pear Therapeutics | Substance-use disorder (SUD) | FDA-cleared Class II Medical Device | https://peartherapeutics.com/products/reset-reset-o/ (accessed on 5 June 2024) |
| Natural Cycles | - | Natural Cycles | Birth control | FDA-de novo | https://www.naturalcycles.com (accessed on 5 June 2024) |
| Oleena | Prescription DTx | Voluntis | All cancer | FDA-510(k) | https://oleena.com/ (accessed on 5 June 2024) |
| CureApp-SC | Non- Prescription DTx | CuraApp Inc. | Smoking cessation | MHLW (Japan) | https://sc.cureapp.com/d/ (accessed on 5 June 2024) |
| PainChek® | Non-Prescription DT | PainChek | Pain in people living with dementia | Class I CE Mark | https://www.painchek.com/ (accessed on 5 June 2024) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mimoso, I.; Figueiredo, T.; Midão, L.; Carrilho, J.; Henriques, D.V.; Alves, S.; Duarte, N.; Bessa, M.J.; Facal, D.; Felpete, A.; et al. Co-Creation in the Development of Digital Therapeutics: A Narrative Review. Int. J. Environ. Res. Public Health 2024, 21, 1589. https://doi.org/10.3390/ijerph21121589
Mimoso I, Figueiredo T, Midão L, Carrilho J, Henriques DV, Alves S, Duarte N, Bessa MJ, Facal D, Felpete A, et al. Co-Creation in the Development of Digital Therapeutics: A Narrative Review. International Journal of Environmental Research and Public Health. 2024; 21(12):1589. https://doi.org/10.3390/ijerph21121589
Chicago/Turabian StyleMimoso, Inês, Teodora Figueiredo, Luís Midão, Joana Carrilho, Diogo Videira Henriques, Sara Alves, Natália Duarte, Maria João Bessa, David Facal, Alba Felpete, and et al. 2024. "Co-Creation in the Development of Digital Therapeutics: A Narrative Review" International Journal of Environmental Research and Public Health 21, no. 12: 1589. https://doi.org/10.3390/ijerph21121589
APA StyleMimoso, I., Figueiredo, T., Midão, L., Carrilho, J., Henriques, D. V., Alves, S., Duarte, N., Bessa, M. J., Facal, D., Felpete, A., Fidalgo, J. M., & Costa, E. (2024). Co-Creation in the Development of Digital Therapeutics: A Narrative Review. International Journal of Environmental Research and Public Health, 21(12), 1589. https://doi.org/10.3390/ijerph21121589








