Restricted Daily Exposure of Environmental Enrichment: Bridging the Practical Gap from Animal Studies to Human Application
Abstract
1. Introduction
2. Domains of Environmental Enrichment
3. From Rodent Cages to Human Environments: The Environmental Enrichment Discrepancies
3.1. The Disparity between the Environments of Laboratory Rodents and Humans
3.2. Challenges in Standardizing Environmental Enrichment Across Preclinical and Clinical Settings
3.3. Unresolved Biological Mechanisms and Translational Challenges of Environmental Enrichment
3.4. Limited Understanding of Which Specific Domains of Enrichment—Such as Physical, Social, Sensory, or Cognitive Stimulation—Are Crucial for Enhancing Brain Plasticity
3.5. The Optimal “Dose” of Enrichment Remains Uncertain, as Most Laboratory Studies Use Continuous Periods of Enrichment, Which Is Seen as Impractical for Human Application
4. Overview of Short-Term or Restricted Daily Environmental Enrichment from Rodent Studies
4.1. Disease Models and Research Endpoints of Restricted Environmental Enrichment in Rodent Studies
4.2. Settings and Duration of Restricted Environmental Enrichment in Rodent Studies
4.2.1. Cognitive and Neural Benefits of 2-h Daily REE
4.2.2. Neurodevelopmental Effects and Recovery with 2-h REE
4.2.3. Enhanced Memory and Synaptic Function with 3-h Daily REE
4.2.4. Additional Findings: Age-Related Synaptic Decline, Mental Health, and TBI Recovery
4.2.5. Time Efficiency in REE Protocols for Practical Research Settings
5. Real-World Applications of Restricted Daily Environmental Enrichment in Human Settings
6. The Future of REE: Making Environmental Enrichment Work at Home
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newberry, R.C. Environmental enrichment: Increasing the biological relevance of captive environments. Appl. Anim. Behav. Sci. 1995, 44, 229–243. [Google Scholar] [CrossRef]
- Figuracion, K.C.F.; Lewis, F.M. Environmental enrichment: A concept analysis. Nurs. Forum 2021, 56, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Crofton, E.J.; Zhang, Y.; Green, T.A. Inoculation stress hypothesis of environmental enrichment. Neurosci. Biobehav. Rev. 2015, 49, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Hebb, D.O. The effects of early experience on problem-solving at maturity. Am. Psychol. 1947, 2, 737–745. [Google Scholar]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; John Wiley & Sons Inc.: New York, NY, USA, 1949. [Google Scholar]
- Rosenzweig, M.R.; Krech, D.; Bennett, E.L.; Diamond, M.C. Effects of environmental complexity and training on brain chemistry and anatomy: A replication and extension. J. Comp. Physiol. Psychol. 1962, 55, 429–437. [Google Scholar] [CrossRef]
- Diamond, M.C.; Krech, D.; Rosenzweig, M.R. The effects of an enriched environment on the histology of the rat cerebral cortex. J. Comp. Neurol. 1964, 123, 111–119. [Google Scholar] [CrossRef]
- Cummins, R.; Livesey, P.; Evans, J. A developmental theory of environmental enrichment. Science 1977, 197, 692–694. [Google Scholar] [CrossRef]
- Shepherdson, D.J.; Mellen, J.D.; Hutchins, M. Second Nature: Environmental Enrichment for Captive Animals; Smithsonian Institution Press: Washington, DC, USA, 1998; p. 350. [Google Scholar]
- Shepherdson, D.J. Environmental enrichment: Past, present and future. Int. Zoo Yearb. 2003, 38, 118–124. [Google Scholar] [CrossRef]
- Kim-McCormack, N.N.E.; Smith, C.L.; Behie, A.M. Is interactive technology a relevant and effective enrichment for captive great apes? Appl. Anim. Behav. Sci. 2016, 185, 1–8. [Google Scholar] [CrossRef]
- Piggin, J. What Is Physical Activity? A Holistic Definition for Teachers, Researchers and Policy Makers. Front. Sports Act. Living 2020, 2, 72. [Google Scholar] [CrossRef]
- Pang, T.Y.C.; Hannan, A.J. Enhancement of cognitive function in models of brain disease through environmental enrichment and physical activity. Neuropharmacology 2013, 64, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Rebar, A.L.; Stanton, R.; Geard, D.; Short, C.; Duncan, M.J.; Vandelanotte, C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol. Rev. 2015, 9, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Oatess, T.L.; Harrison, F.E.; Himmel, L.E.; Jones, C.P. Effects of Acrylic Tunnel Enrichment on Anxiety-Like Behavior, Neurogenesis, and Physiology of C57BL/6J Mice. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2021, 60, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Eime, R.M.; Young, J.A.; Harvey, J.T.; Charity, M.J.; Payne, W.R. A Systematic Review of the Psychological and Social Benefits of Participation in Sport for Children and adolescents: Informing Development of a Conceptual Model of Health through Sport. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Salmon, P. Effects of physical exercise on anxiety, depression, and sensitivity to stress. Clin. Psychol. Rev. 2001, 21, 33–61. [Google Scholar] [CrossRef]
- Vuu, S.; Barr, C.J.; Killington, M.; Jill, G.; Van Den Berg, M.E. Physical exercise for people with mild traumatic brain injury: A systematic review of randomized controlled trials. Neurorehabilitation 2022, 51, 185–200. [Google Scholar] [CrossRef]
- Knöchel, C.; Oertel-Knöchel, V.; O’Dwyer, L.; Prvulovic, D.; Alves, G.; Kollmann, B.; Hampel, H. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog. Neurobiol. 2012, 96, 46–68. [Google Scholar] [CrossRef]
- Gelfo, F. Does Experience Enhance Cognitive Flexibility? An Overview of the Evidence Provided by the Environmental Enrichment Studies. Front. Behav. Neurosci. 2019, 13, 150. [Google Scholar] [CrossRef]
- Watson, S.L.; Shively, C.A.; Voytko, M.L. Can puzzle feeders be used as cognitive screening instruments? Differential performance of young and aged female monkeys on a puzzle feeder task. Am. J. Primatol. 1999, 49, 195–202. [Google Scholar] [CrossRef]
- Stern, C.; Munn, Z. Cognitive leisure activities and their role in preventing dementia. Int. J. Evid. -Based Healthc. 2010, 8, 2–17. [Google Scholar] [CrossRef]
- Froeliger, B.E.; Garland, E.L.; Modlin, L.A.; McClernon, F.J. Neurocognitive correlates of the effects of yoga meditation practice on emotion and cognition: A pilot study. Front. Integr. Neurosci. 2012, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- House, G.M.; Sobotik, E.B.; Nelson, J.R.; Archer, G.S. Effects of Ultraviolet Light Supplementation on Pekin Duck Production, Behavior, and Welfare. Animals 2020, 10, 833. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias, M.; Aruanno, B.; Cianferoni, T.; Rossini, M.; Komarova, S.; Molteni, F. Neuro Rehabilitation System Through Virtual Reality, Music and Fragrance Therapy. In Biosystems & Biorobotics; Springer: Cham, Switzerland, 2018; pp. 848–852. [Google Scholar] [CrossRef]
- Birte-Antina, W.; Ilona, C.; Antje, H.; Thomas, H. Olfactory training with older people. Int. J. Geriatr. Psychiatry 2017, 33, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Yamada, K.; Sugita, M.; Ichitani, Y. Social enrichment improves social recognition memory in male rats. Anim. Cogn. 2018, 21, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.I.; Leventhal, B.L.; Nielsen, B.N.; Hinshaw, S.P. Reducing mental-illness stigma via high school clubs: A matched-pair, cluster-randomized trial. Stigma Health 2020, 5, 230–239. [Google Scholar] [CrossRef]
- Meyer, M.S.; Agner, J.; Botero, A.; Cha, T. Mapping community: A scoping review of clubhouse members’ social networks and their impact on recovery in mental illness. Psychiatr. Rehabil. J. 2023, 46, 250–264. [Google Scholar] [CrossRef]
- McDonald, M.W.; Hayward, K.S.; Rosbergen, I.C.M.; Jeffers, M.S.; Corbett, D. Is Environmental Enrichment Ready for Clinical Application in Human Post-stroke Rehabilitation? Front. Behav. Neurosci. 2018, 12, 135. [Google Scholar] [CrossRef]
- Clemenson, G.D.; Deng, W.; Gage, F.H. Environmental enrichment and neurogenesis: From mice to humans. Curr. Opin. Behav. Sci. 2015, 4, 56–62. [Google Scholar] [CrossRef]
- Queen, N.J.; Hassan, Q.N.; Cao, L. Improvements to Healthspan Through Environmental Enrichment and Lifestyle Interventions: Where Are We Now? Front. Neurosci. 2020, 14, 605. [Google Scholar] [CrossRef]
- Torres-Reveron, A.; Dow-Edwards, D. Scoping review on environmental enrichment: Are critical periods and sex differences adequately studied? Pharmacol. Biochem. Behav. 2022, 218, 173420. [Google Scholar] [CrossRef]
- Simpson, J.; Kelly, J.P. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behav. Brain Res. 2011, 222, 246–264. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Carvajal, M.; Sequeira-Cordero, A.; Brenes, J.C. Neurobehavioral Effects of Restricted and Unpredictable Environmental Enrichment in Rats. Front. Pharmacol. 2020, 11, 674. [Google Scholar] [CrossRef] [PubMed]
- Rosenzweig, M.R.; Love, W.; Bennett, E.L. Effects of a Few Hours a Day of Enriched Experience on Brain Chemistry and Brain Weights. Physiol. Behav. 1968, 3, 819–825. [Google Scholar] [CrossRef]
- Widman, D.R.; Abrahamsen, G.C.; Rosellini, R.A. Environmental enrichment: The influences of restricted daily exposure and subsequent exposure to uncontrollable stress. Physiol. Behav. 1992, 51, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Scarola, S.J.; Bardi, M. Environmental enrichment modulates inflammation during development in long-evans rats (Rattus norvegicus). Dev. Psychobiol. 2021, 63, 183–191. [Google Scholar] [CrossRef]
- Pereira, L.O.; Arteni, N.S.; Petersen, R.C.; da Rocha, A.P.; Achaval, M.; Netto, C.A. Effects of daily environmental enrichment on memory deficits and brain injury following neonatal hypoxia-ischemia in the rat. Neurobiol. Learn. Mem. 2007, 87, 101–108. [Google Scholar] [CrossRef]
- Widman, D.R.; Rosellini, R.A. Restricted daily exposure to environmental enrichment increases the diversity of exploration. Physiol. Behav. 1990, 47, 57–62. [Google Scholar] [CrossRef]
- Fukushiro, D.F.; Josino, F.S.; Saito, L.P.; Costa, J.M.; Zanlorenci, L.H.; Berro, L.F.; Fernandes-Santos, L.; Morgado, F.; Mári-Kawamoto, E.; Frussa-Filho, R. Differential effects of intermittent and continuous exposure to novel environmental stimuli on the development of amphetamine-induced behavioral sensitization in mice: Implications for addiction. Drug Alcohol Depend. 2012, 124, 135–141. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Pastoriza, J.M.; Severance, J.E.; Foster, T.C.; Ormerod, B.K. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol. Aging 2013, 34, 263–274. [Google Scholar] [CrossRef]
- Ji, M.H.; Wang, X.M.; Sun, X.R.; Zhang, H.; Ju, L.S.; Qiu, L.L.; Yang, J.J.; Jia, M.; Wu, J.; Yang, J. Environmental Enrichment Ameliorates Neonatal Sevoflurane Exposure-Induced Cognitive and Synaptic Plasticity Impairments. J. Mol. Neurosci. MN 2015, 57, 358–365. [Google Scholar] [CrossRef]
- Ji, M.H.; Wang, Z.Y.; Sun, X.R.; Tang, H.; Zhang, H.; Jia, M.; Qiu, L.L.; Zhang, G.F.; Peng, Y.G.; Yang, J.J. Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Mol. Neurobiol. 2017, 54, 3759–3770. [Google Scholar] [CrossRef] [PubMed]
- Rampon, C.; Tang, Y.P.; Goodhouse, J.; Shimizu, E.; Kyin, M.; Tsien, J.Z. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 2000, 3, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Fernandez, S.M. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol. Aging 2003, 24, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.J.; Fernandez, S.M.; Frick, K.M. Different types of environmental enrichment have discrepant effects on spatial memory and synaptophysin levels in female mice. Neurobiol. Learn. Mem. 2005, 83, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.C.; McRae, P.A.; Levy, L.J.; Frick, K.M. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol. Learn. Mem. 2006, 85, 139–152. [Google Scholar] [CrossRef]
- Sampedro-Piquero, P.; Begega, A.; Zancada-Menendez, C.; Cuesta, M.; Arias, J.L. Age-dependent effects of environmental enrichment on brain networks and spatial memory in Wistar rats. Neuroscience 2013, 248, 43–53. [Google Scholar] [CrossRef]
- Radabaugh, H.L.; LaPorte, M.J.; Greene, A.M.; Bondi, C.O.; Lajud, N.; Kline, A.E. Refining environmental enrichment to advance rehabilitation based research after experimental traumatic brain injury. Exp. Neurol. 2017, 294, 12–18. [Google Scholar] [CrossRef]
- Stein, L.R.; O’Dell, K.A.; Funatsu, M.; Zorumski, C.F.; Izumi, Y. Short-term environmental enrichment enhances synaptic plasticity in hippocampal slices from aged rats. Neuroscience 2016, 329, 294–305. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Vega-Rivera, N.M.; Meneses-San Juan, D.; Ortiz-López, L.; Estrada-Camarena, E.M.; Flores-Ramos, M. Short Daily Exposure to Environmental Enrichment, Fluoxetine, or Their Combination Reverses Deterioration of the Coat and Anhedonia Behaviors with Differential Effects on Hippocampal Neurogenesis in Chronically Stressed Mice. Int. J. Mol. Sci. 2021, 22, 10976. [Google Scholar] [CrossRef]
- Konkle, A.T.; Kentner, A.C.; Baker, S.L.; Stewart, A.; Bielajew, C. Environmental-enrichment-related variations in behavioral, biochemical, and physiologic responses of Sprague-Dawley and Long Evans rats. J. Am. Assoc. Lab. Anim. Sci. JAALAS 2010, 49, 427–436. [Google Scholar]
- Skillings, E.A.; Wood, N.I.; Morton, A.J. Beneficial effects of environmental enrichment and food entrainment in the R6/2 mouse model of Huntington’s disease. Brain Behav. 2014, 4, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Luo, Z.; Shan, W.; Zuo, Z. Role of Sox2 in Learning, Memory, and Postoperative Cognitive Dysfunction in Mice. Cells 2021, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Brito, D.V.C.; Esteves, F.; Rajado, A.T.; Silva, N.; ALFA score Consortium; Araújo, I.; Bragança, J.; Castelo-Branco, P.; Nóbrega, C. Assessing cognitive decline in the aging brain: Lessons from rodent and human studies. Npj Aging 2023, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Dewabhrata, W.; Ahsan, A.; Bella, A.; Amalia, N.; Kusuma, D.; Pertiwi, Y.B.A. Mental Health, Environmental, and Socioeconomic Geographic Factors of Severe Drug Addiction: Analysis of Rehabilitation Center Data in Indonesia. Subst. Abus. Res. Treat. 2023, 17. [Google Scholar] [CrossRef]
- González, M.M.C. Dim Light at Night and Constant Darkness: Two Frequently Used Lighting Conditions That Jeopardize the Health and Well-being of Laboratory Rodents. Front. Neurol. 2018, 9, 609. [Google Scholar] [CrossRef]
- Gaynor, A.M.; Gazes, Y.; Haynes, C.R.; Babukutty, R.S.; Habeck, C.; Stern, Y.; Gu, Y. Childhood engagement in cognitively stimulating activities moderates relationships between brain structure and cognitive function in adulthood. Neurobiol. Aging 2024, 138, 36–44. [Google Scholar] [CrossRef]
- Schoentgen, B.; Gagliardi, G.; Défontaines, B. Environmental and Cognitive Enrichment in Childhood as Protective Factors in the Adult and Aging Brain. Front. Psychol. 2020, 11, 1814. [Google Scholar] [CrossRef]
- Weaver, A.N.; Jaeggi, S.M. Activity Engagement and Cognitive Performance Amongst Older Adults. Front. Psychol. 2021, 12, 620867. [Google Scholar] [CrossRef]
- Nik Ramli, N.N.; Asokan, A.; Mayakrishnan, D.; Annamalai, H. Exploring Stroke Rehabilitation in Malaysia: Are Robots Better than Humans for Stroke Recuperation? Malays. J. Med. Sci. MJMS 2021, 28, 14–23. [Google Scholar] [CrossRef]
- Lee, M.; Choi, H.; Shin, J.; Suh, H.S. The Effects of Adding Art Therapy to Ongoing Antidepressant Treatment in Moderate-to-Severe Major Depressive Disorder: A Randomized Controlled Study. Int. J. Environ. Res. Public Health 2022, 20, 91. [Google Scholar] [CrossRef]
- Millard, E.; Cardona, J.; Fernandes, J.; Priebe, S.; Carr, C. I know what I like, and I like what I know: Patient preferences and expectations when choosing an arts therapies group. Arts Psychother. 2021, 75, 101829. [Google Scholar] [CrossRef] [PubMed]
- Han, P.P.; Han, Y.; Shen, X.Y.; Gao, Z.K.; Bi, X. Enriched environment-induced neuroplasticity in ischemic stroke and its underlying mechanisms. Front. Cell. Neurosci. 2023, 17, 1210361. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.; Ahmed, F.M. Effect of the Nursing Practice of Creative Art Therapy on Psychiatric Symptoms among Schizophrenic Patients. Am. J. Nurs. Res. 2019, 7, 14–23. [Google Scholar] [CrossRef]
- Hamad, A.; Jia, B. How Virtual Reality Technology Has Changed Our Lives: An Overview of the Current and Potential Applications and Limitations. Int. J. Environ. Res. Public Health 2022, 19, 11278. [Google Scholar] [CrossRef] [PubMed]
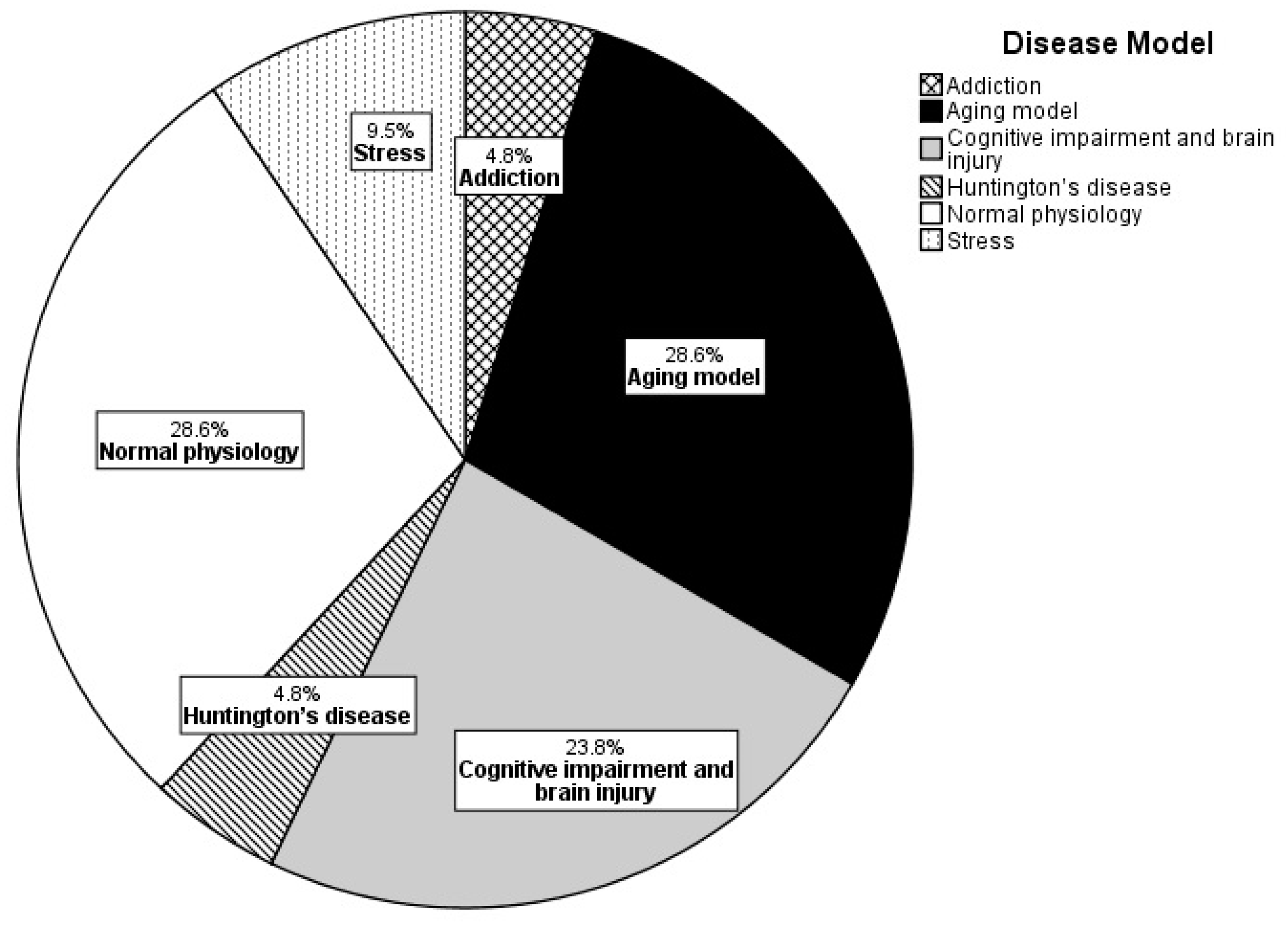

| Reference | Disease Model | Rodent Species | Research Endpoint | Duration of REE | Enrichment Setting |
|---|---|---|---|---|---|
| [38] | Aging model | Long-Evans male rats | Stress behavior, Neurobiology of inflammation | 30 min (3 days/week, total of 5 weeks) |
|
| [39] | Prenatal hypoxia-ischemia | Male Wistar rat | Reference memory, Working memory, Histology | 1 h daily (6 days/week, total of 9 weeks) |
|
| [36] | Normal model | Male Berkeley S3 strain rat | Neuroanatomy, Neurobiology of synaptic transmission | 2 h, 2.5 h, 4.5 h (total of 55 days) |
|
| [40] | Normal model | Male Sprague Dawley rats | Exploratory Behavior | 2 h (total of 1 month) |
|
| [37] | Uncontrollable stress model | Male Sprague Dawley rats | Learning behavior | 2 h (total of 40 days) | Same setting as Widman et al. [40] |
| [41] | Addiction model | Adult male Swiss mice | Addiction behavior | 2 h (total of 13 days) |
|
| [42] | Aging model | Young and aged male Fischer 344 rats | Spatial learning and memory, Neurogenesis | 2–3 h (total of 10 weeks) |
|
| [43] | Sevoflurane-induced cognitive impairment model | Male C57BL/6 mice | Learning behavior, Neuroplasticity | 2 h (total of 34 days) |
|
| [44] | Sevoflurane-induced cognitive impairment model | C57BL/6 male pups | Learning behavior, Memory behavior, Neurobiology of synaptic transmission | 2 h (total of 82 days) |
|
| [35] | Normal model | Male Wistar rats | Exploratory Behavior, Neuroplasticity | 2 to 48 h unpredictably (total of 1 month) |
|
| [45] | Genetically modified model | CA1 specific NMDA receptor 1 subunit knockout (CA1-KO) mice | Learning behavior, Neuroplasticity | 3 h (total of 2 months) |
|
| [46] | Aging model | Young and aged female C57BL/6 mice | Learning behavior, Neurobiology of synaptic transmission | 3 h (total of 23 days) |
|
| [47] | Normal model | Young female C57BL/6 mice | Spatial learning and memory, Neurobiology of synaptic transmission | 3 h (total of 6 weeks) | Cognitive: The 56.5 × 41.5 × 22 cm cage contained various objects, (toys, PVC pipes, hollow metal cylinders, Legos, and a toy rope). Running wheels were excluded, and four to five objects were rearranged daily. Exercise: Each cage had three running wheels (11.5 cm diameter), repositioned daily. Acrobatic: Ten bridges connected six wooden platforms (10 × 10 cm, 34.5 cm high) in two rows. The bridges were made from chains, rubber bands, metal rods, wires, and ropes, with changes made weekly. |
| [48] | Aging model | Young and aged male C57BL/6 mice | Spatial learning and memory, Neurobiology of synaptic transmission | 3 h (total of 10 weeks) |
|
| [49] | Aging model | Young and aged male Wistar rats | Spatial learning and memory, Neurobiology of aging | 3 h (total of 2 months) |
|
| [50] | Traumatic brain injury model | Adult male Sprague- Dawley rats | Neurological function, Histology | 3 h (twice) or 6 h (total of 21 days) |
|
| [51] | Aging model | Male Sprague–Dawley rats | Neuroplasticity | 3 h (total of 3 weeks) |
|
| [52] | Chronic mild stress model | Balb/C female mice | Stress behavior, Neurogenesis | 3 h (total of 4 weeks) |
|
| [53] | Normal model | Male Long Evans and Sprague– Dawley rats | Stress behavior, Neurobiology of stress | 8 h (5 days/week, total of 6 weeks) |
|
| [54] | Huntington’s disease model | R6/2 mice | Cognitive functions, Neuroanatomy | 14 h (total of 8, 12, 16, 22 weeks) |
|
| [55] | Postoperative cognitive dysfunction (POCD) model | Male C57BL/6 | Cognitive functions, Neurobiology of circadian rhythm | 15 h (total of 2 weeks) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nik Ramli, N.N.; Kamarul Sahrin, N.A.; Nasarudin, S.N.A.Z.; Hashim, M.H.; Abdul Mutalib, M.; Mohamad Alwi, M.N.; Abd Rashed, A.; Ramasamy, R. Restricted Daily Exposure of Environmental Enrichment: Bridging the Practical Gap from Animal Studies to Human Application. Int. J. Environ. Res. Public Health 2024, 21, 1584. https://doi.org/10.3390/ijerph21121584
Nik Ramli NN, Kamarul Sahrin NA, Nasarudin SNAZ, Hashim MH, Abdul Mutalib M, Mohamad Alwi MN, Abd Rashed A, Ramasamy R. Restricted Daily Exposure of Environmental Enrichment: Bridging the Practical Gap from Animal Studies to Human Application. International Journal of Environmental Research and Public Health. 2024; 21(12):1584. https://doi.org/10.3390/ijerph21121584
Chicago/Turabian StyleNik Ramli, Nik Nasihah, Nurin Amalia Kamarul Sahrin, Siti Nur Atiqah Zulaikah Nasarudin, Mohamad Hisham Hashim, Maisarah Abdul Mutalib, Muhammad Najib Mohamad Alwi, Aswir Abd Rashed, and Rajesh Ramasamy. 2024. "Restricted Daily Exposure of Environmental Enrichment: Bridging the Practical Gap from Animal Studies to Human Application" International Journal of Environmental Research and Public Health 21, no. 12: 1584. https://doi.org/10.3390/ijerph21121584
APA StyleNik Ramli, N. N., Kamarul Sahrin, N. A., Nasarudin, S. N. A. Z., Hashim, M. H., Abdul Mutalib, M., Mohamad Alwi, M. N., Abd Rashed, A., & Ramasamy, R. (2024). Restricted Daily Exposure of Environmental Enrichment: Bridging the Practical Gap from Animal Studies to Human Application. International Journal of Environmental Research and Public Health, 21(12), 1584. https://doi.org/10.3390/ijerph21121584








