Abstract
There is a lack of groundwater quality monitoring, especially in developing countries like South Africa. This study aimed to evaluate borehole water quality. Groundwater was analysed for pH, dissolved oxygen (DO), temperature, electrical conductivity (EC), total dissolved solids (TDSs), turbidity, chemical oxygen demand (COD), nitrogen (N), sulphate (SO42−), fluoride (F−), chloride (Cl−), calcium (Ca2+), magnesium (Mg2+), potassium (K+), and sodium (Na+) using a multi-parameter device, spectrophotometer, turbidity meter, and inductively coupled plasma optical emission spectrophotometer. Total coliforms and Escherichia coli were quantified using the Colilert system. The water quality index (WQI) was calculated using the arithmetic weighting method. The parameters ranged as follows: pH (6.71–7.94), DO (2.19–7.79 mg/L), EC (379.67–1317.33 µS/cm), TDSs (190–659 mg/L), temperature (16.75–22.31 °C), turbidity (0.17–3.21 NTU), COD (9–50 mg/L), F− (0.17–2.09 mg/L), Cl− (36.1–184.55 mg/L), N (0.64–28.56 mg/L), SO42− (27.18–112.13 mg/L), K+ (1.71–21.77 mg/L), Ca2+ (29.59–134.59 mg/L), Mg2+ (16.72–110.78 mg/L), and Na+ (38.52–170.63 mg/L). One borehole was polluted with E. coli (9 MPN/100 mL) and 25% were contaminated with coliforms beyond 10 MPN/100 mL. The WQI ranged from 50.430 to 190.220. The results underscore the importance of regular monitoring of groundwater.
1. Introduction
There is a constantly increasing water stress globally due to the rapid population and industrial growth, the increase in urbanisation, low rainfall, climate change, and water pollution. Recently, there has been heavy reliance not only on surface water but also on groundwater for drinking, farming, and industrial purposes [1]. In fact, over 35% of global total water withdrawal for human use is obtained from groundwater. Groundwater contributes 36%, 42%, and 27% of water for domestic, irrigation, and industrial use, respectively [2]. Limpopo is one of the provinces in South Africa that heavily relies on groundwater as a source of domestic water supply. In Limpopo province, groundwater accounts for approximately 70% of the rural domestic water supply [3]. The reliance on groundwater has increased manifold over the past years due to the cost-effective and advanced drilling technologies that ease accessibility [4].
Groundwater is generally considered less susceptible to contamination and free from impurities compared to surface water bodies. However, concerns have been raised regarding the depletion and degradation of groundwater quality due to various chemical and microbial pollutants. The deterioration and degradation of groundwater are often due to various factors, including infiltration of excess nutrients into the ground, poor industrial and mining practices, sub-surface geochemical processes, rainfall, and stormwater runoff [5]. Moreover, inadequate provision of proper sanitation infrastructure, such as toilets (pit latrines) and sewage systems, especially in rural areas in developing worlds, poses a high risk of faecal contamination of nearby groundwater sources [6,7]. Furthermore, the maintenance and management of existing sanitation infrastructure are often subpar, leading to leakages, overflows, or seepage of untreated sewage into groundwater, thereby introducing harmful pathogens and other pollutants, compromising its quality and biosafety. The main water quality problems linked to poor groundwater quality include health issues related to gastrointestinal effects, dental fluorosis, and skin lesions, among others [8]. Therefore, this necessitates the implementation of appropriate management strategies to safeguard groundwater quality and to ensure the prevention of groundwater pollution and potential adverse health risks.
The risk mitigation strategies for poor groundwater quality, including regular monitoring and assessment of groundwater quality, can assist not only in the conservation of groundwater but also in the identification of potential risks and the implementation of timely interventions. Therefore, monitoring compliance for both physicochemical and microbiological parameters of groundwater can avert outbreaks of water-related diseases and complications. Globally, the World Health Organisation (WHO) and in South Africa, the South African National Standard (SANS), are the institutes that set groundwater quality guidelines to safeguard humans [9,10]. However, the lack of groundwater quality surveillance and enforcement of water legislature due to limited resources, especially in peri-urban and rural regions of Limpopo, South Africa, hinders active interventions to safeguard humans against contaminated groundwater [11].
Assessing the quality of groundwater involves a multifaceted approach aimed at safeguarding public health and ensuring compliance with regulatory standards. Water quality rating (Qn) and water quality index (WQI) are tools used to assess and communicate the overall quality of water based on multiple parameters. Qn is a qualitative classification system that assigns a rating or score to water samples based on predefined criteria for various parameters such as pH, turbidity, dissolved oxygen, metals, and nutrients. These ratings are typically categorised into different classes (e.g., excellent, good, fair, poor) to indicate the suitability of water for specific uses such as drinking, irrigation, or recreational purposes. On the other hand, WQI is a quantitative indicator that integrates multiple water quality parameters into a single numerical value, providing a comprehensive assessment of overall water quality. WQI combines individual parameter measurements using weighted averaging or mathematical models to derive an index score that reflects the overall condition of water [12]. It does, therefore, simplify the communication of water quality data to laymen as well as policymakers [13]. Several researchers in various fields, such as groundwater quality assessment, have effectively used WQI to analyse the quality of water [14,15,16].
Mankweng is a township in the Capricorn District Municipality in the Limpopo province of South Africa, with a population density of approximately 2800/km2. It consists of the University of Limpopo, Mankweng Hospital, clinics, shopping centres, filling stations, large human settlements, and agricultural farms, which rely heavily on water use. However, since Mankweng is a semiarid township, the treated water supply from the municipality is inadequate; thus, some households, especially in new settlements, are not connected to the municipal line. The unconnected households tend to rely on groundwater from boreholes as an alternative source. Due to various activities and poor sanitation in those areas, the groundwater may be contaminated by different physicochemical and biological pollutants that might percolate into the groundwater. However, according to our knowledge, there are currently no published data on the status of groundwater and possible health risks associated with its consumption in Mankweng. This lack of studies might be due to the general misconception that groundwater is free from pollutants such as chemicals and pathogens, or the high costs involved.
Our study focused on the evaluation of the quality of groundwater in Mankweng township in Limpopo, South Africa, from physicochemical as well as bacteriological perspectives. We also arithmetically assessed the quality of the groundwater using the WQI.
2. Materials and Methods
2.1. Description of the Study Area
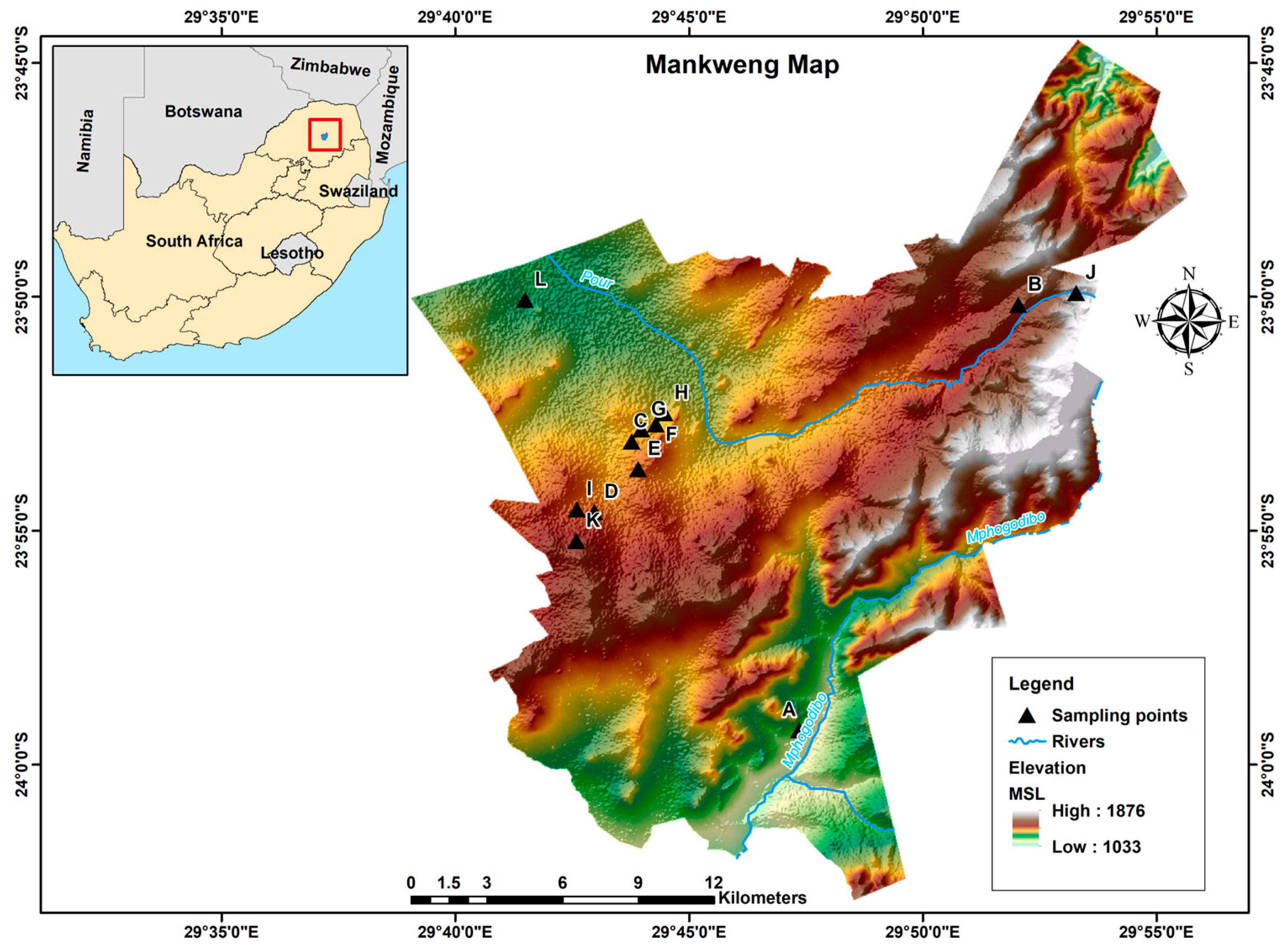
The study was conducted in Mankweng, previously known as Turfloop, which is a township found within Capricorn District Municipality in the Limpopo province of South Africa. The area is located at two corner coordinates: 23°53′10″ S, 29°43′05″ E and 29°42′19.2″ E, 23°50′58.7″ S, about 30 km east of Polokwane city. Mankweng is home to the University of Limpopo and covers a total area of 600 km2, with a population size of approximately 3,000,000 inhabitants. The area is characterised by hills (rocky outcrops, known as ‘koppies’) amidst mostly flat regions with elevations ranging from 1033 to 1876 m, as illustrated in Figure 1. The predominant geological formations in the area consist of gneiss, granite, and lava. These rock types play a significant role in shaping the landscape and influencing soil properties and groundwater availability in the region.
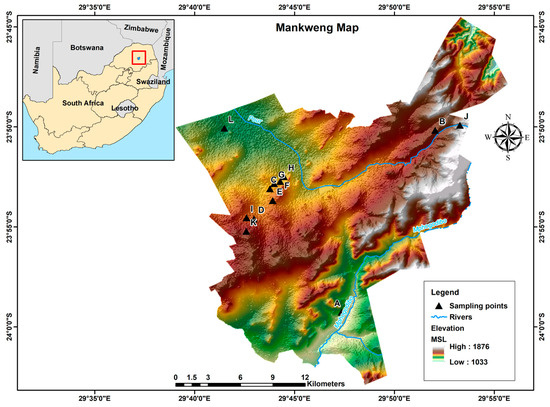
Figure 1.
Map of South Africa showing the location and topography of Mankweng township. A–L: sampling point.
2.2. Mankweng Climate
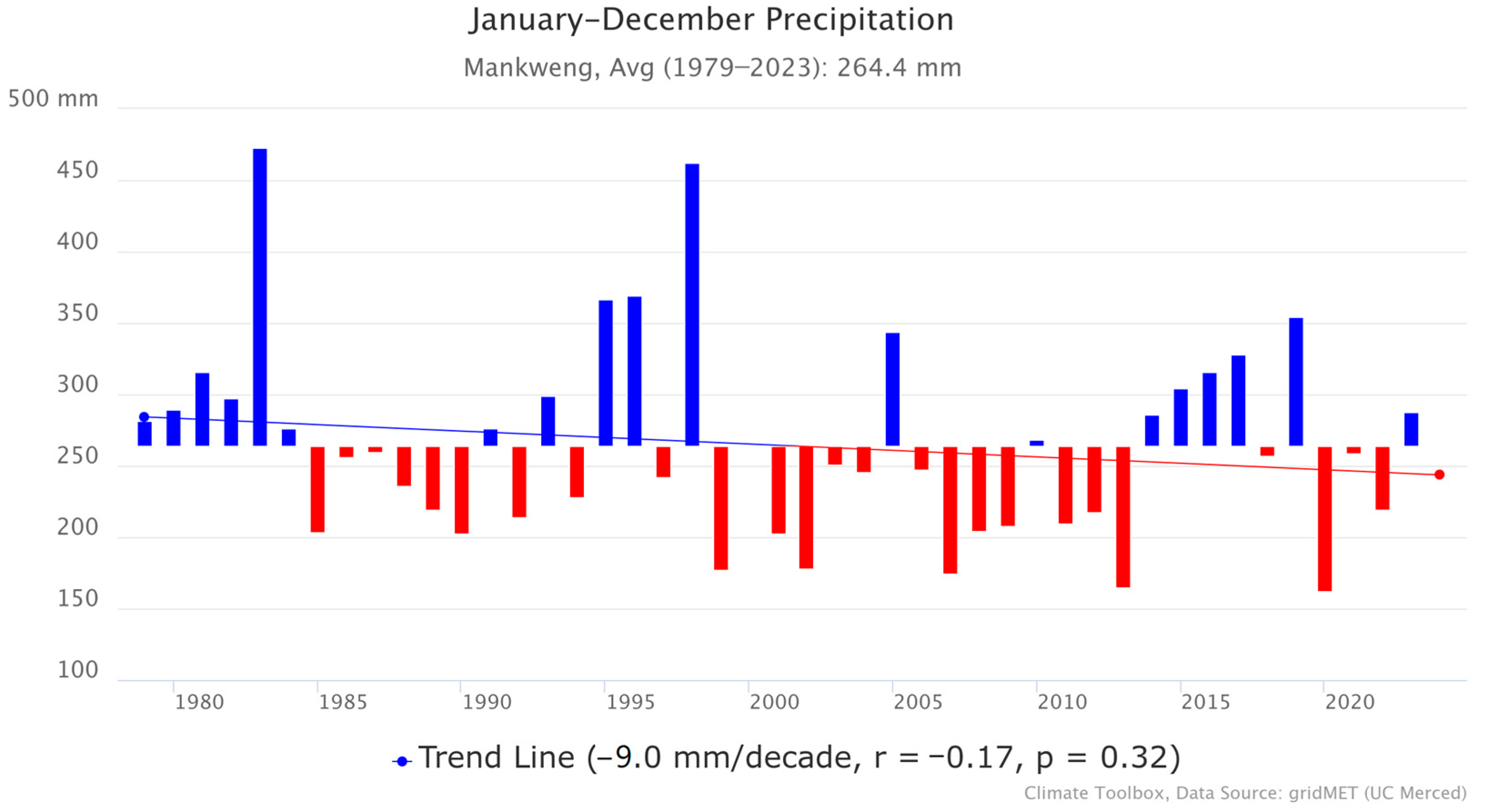
Mankweng township has a semi-arid climate. Based on precipitation data from the Mankweng Meteorological Station, illustrated in Figure 2, the monthly average precipitation for the period 1979–2023 ranged between 200 and 600 mm per annum [17]. The area receives its highest rainfall in summer (134 mm), from December to February, and the lowest average rainfall (0 mm) in Winter, in June and July. In addition, there has been a slight downward trend in precipitation (Figure 2), which shows a decline of −9.0 mm per decade. Nonetheless, the p-value (0.32) shows that the trend was not statistically significant, and a weak correlation coefficient (r = −0.17) was also observed between time and precipitation. Despite the small downward trend, the rainfall in Mankweng remains highly variable year-to-year.
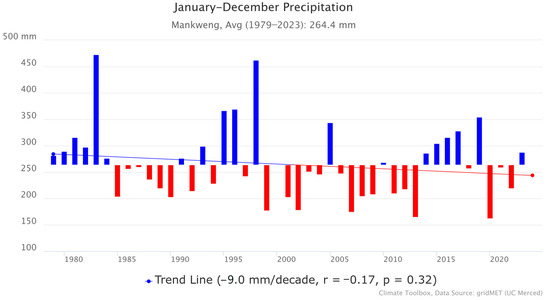
Figure 2.
Precipitation in Mankweng.
The annual mean temperature is 18.0 °C, with average maximum midday temperatures ranging from 19.2 °C in June and July to 26.6 °C in January. The average coldest midnight temperatures are in July, when the mercury drops to 3.1 °C.
2.3. Collection of Groundwater Samples
Groundwater samples were collected in the month of June 2024, from twelve (n = 12) residential boreholes that were randomly selected. The groundwater samples were collected in accordance with standard sampling procedures of the American Public Health Association (APHA) [18]. Prior to sampling, sterile Scott bottles were rinsed twice with groundwater from the sampling points. The collected samples were preserved on ice in a cooler box to maintain their integrity and to minimise degradation during transportation to the analytic laboratory at the University of Limpopo. Each sample was analysed for physicochemical and bacterial parameters.
2.4. Physicochemical Analysis of the Ground Water
Physicochemical parameters, such as temperature, dissolved oxygen (DO) pH, and electrical conductivity (EC), were measured on-site during sample collection using a HANNA HI98494 multi-parameter device (Hanna Instruments, Inc., Smithfield, RI, USA), following the standard protocol. The turbidity, chemical oxygen demand (COD), anions, and cations were determined within 12 h at the laboratory. Turbidity was measured using a turbidity meter (HACH, HQ 40d, Johannesburg, South Africa). COD was determined in the laboratory following standard methods as described by APHA [19]. Spectrophotometric measurements were used to determine COD using a COD broad-range kit (Hanna Instruments, HI839800, Woonsocket, RI, USA) in accordance with the manufacturer’s procedure. Fluoride (F−), chloride (Cl−), nitrate (N), sulphate (SO42−), calcium (Ca2+), magnesium (Mg2+), potassium (K+), and sodium (Na+) were analysed using Perkin Elmer 8000 Optima 8000 inductively coupled plasma optical emission spectrophotometer (ICP-OES) (Perkin Elmer, Washington, USA) [20]. Total dissolved solids (TDSs) were arithmetically calculated based on in situ measurements of EC using Equation (1) [21].
2.5. Bacterial Analysis
Bacterial isolation was conducted within 6 h of sample collection. Viable total coliform (TC) and Escherichia coli were quantified using the IDEXX technique. Colilert media was added to 100 mL of the water sample and mixed until completely dissolved. The solutions were then poured into an IDEXX Quanti-Tray/2000 and sealed using the Quanti-Tray sealer. Subsequently, the samples were incubated at 35 °C for 24 h. After incubation, trays that exhibited a yellow colour (indicative of o-nitrophenol production) due to the hydrolysis of the substrate o-nitrophenyl-β-D-galactopyranoside (ONPG) by β-galactosidase were considered as TC. All trays confirmed as TC were examined under a fluorescent UV lamp at 365 nm. The wells that fluoresced as a result of 4-methylumbelliferone production caused by the hydrolysis of 4-methylumbelliferone-glucuronide (MUG) by β-glucuronidase, were considered to illustrate the presence of E. coli. The counts for both TC and E. coli were determined using the most probable number (MPN) table [22].
2.6. Determination of WQI
The arithmetic weighting method, according to Brown et al. [23] and Brown et al. [24], was used to determine the water quality index and the status of the groundwater using the descriptive data of the selected parameters. WQI was analysed in accordance with the WHO standards for drinking water [9]. WQI was computed using Equation (2).
where Qn is the water quality rating of the nth water quality parameter and Wn is the unit weight of the nth water quality parameter.
WQI = ΣQn × Wn/ΣWn,
The water quality rating (Qn) is assessed using Equation (3):
where Vn is the observed value of the parameter, Vi is the ideal value of that water parameter, [Vi = 0, except for pH (Vi = 7) and DO (Vi = 14.6 mg/L)], and Vs is the standard permissible value for the nth water quality parameter.
Qn = 100 × [(Vn − Vi)/(Vs − Vi)],
The unit weight (Wn) was calculated using the formula below:
where K = 1/ΣXs is the proportionality constant and Vs is the water quality standard for the parameter. K is computed using Equation (5).
Wn = K/Vs
K = [1/Σ 1/Vs = 1, 2, …n].
The water quality status (WQS) based on WQI is displayed in Table 1 [24].

Table 1.
WQI range and status of the water samples.
2.7. Data Analysis
The experiments were conducted in triplicates and results are expressed as mean plus standard error. Analysis of variance (ANOVA) and Tukey’s honestly significant difference test were used to determine the mean separation with significant difference between the treatments indicated at p ≤ 0.05 using Graph Pad prism™ version 8.4.2. Pearson correlation was measured using OriginPro 2024b software to analyse the relationship between the tested parameters. A value between 0 and 1 implied a positive correlation while values between 0 and −1 signified negative correlations between two variables at a significant level of p < 0.05. Zero value denotes no correlation between two parameters. A strong correlation was reflected when r > 0.7, whereas r between 0.5 and 0.7 indicated a positive moderate correlation. Principal component analysis (PCA) was carried out on the selected water quality parameters using OriginPro 2024b software. Principal components with an eigenvalue > 1 were extracted. The principal components were classified as strong when the loading values were greater or equal to 0.75, moderate when the loading values were between 0.75 and 0.50, and weak when the loading values were less than 0.50 [25]. OriginPro 2024b software was also used for hierarchical cluster analysis (HCA) to analyse the generated data. Euclidean distance was used to determine the similarities between the selected parameters, and Ward’s technique was employed as the joining rule.
3. Results
3.1. Physicochemical Analysis of the Ground Water
The descriptive groundwater parameters are displayed in Table 2. The pH of the groundwater was slightly acid to alkaline, ranging from 6.71 to 7.95, with a mean average value of 7.43. The distribution range of DO was 2.19–7.80 mg/L, averaging 4.57 mg/L. Fifty percent (n = 6/12) of the boreholes had DO concentrations lower than 4 mg/L. The EC content ranged between 380 and 1317 μS/cm, with an average of 766.5 μS/cm. One hundred percent (n = 12/12) of the boreholes had EC contents greater than the WHO [9] limit standard of 300 µS/cm. TDSs varied between 190 and 659 mg/L, with an average of 383 mg/L. All boreholes complied with the standard of less than 600 mg/L set by the WHO [20]. The ranges for temperature and turbidity were 16.76–22.31 °C (averaging 19.13 °C) and 0.17–3.22 NTU (averaging 0.59 NTU), respectively. Only Site H, with 3.22 NTU, exceeded the WHO [19] set standard of 1 NTU. The average mean concentration of COD was 31.17 mg/L. The highest concentration of 50 mg/L was found at Site H, whereas the lowest (9 mg/L) was reported at Site B. This lowest COD concentration was the only one that complied with the WHO standard guideline of equal to or less than 10 mg/L. The compositions of F− and Cl− in the groundwater were relatively high, with averages of 0.76 and 98.86 mg/L. F− concentration values varied between 0 and 3.5 mg/L. F− contents of 1.87 and 2.09 mg/L at sites C and F, respectively, exceeded the WHO [9] and SANS [10] guidelines of 1.5 mg/L for drinking. However, Cl− concentrations at all sites complied with the WHO [9] (≤250 mg/L) and SANS [10] (≤300 mg/L) guidelines. The contents of N and SO42− averaged 10.61 and 60.73 mg/L, respectively. N concentrations of 28.56, 11.37, 15.74, and 21.44 at sites B, C, D, and E did not comply with the limit standards of the WHO [9] (10 mg/L) and SANS [20] (11 mg/L). The detected divalent cations Ca2+ and Mg2+ were on average 57.49 and 47.26 mg/L, respectively. Ca2+ concentrations at Site B (134.59 mg/L) and Site L (85.91 mg/L) exceeded the limit set by WHO [9]. Thirty-three percent (n = 4/12) of the boreholes had Mg2+ concentrations greater than the WHO [9] set standard of 50 mg/L. The monovalent cations K+ and Na+ were relatively less, averaging 6.99 and 98.57 mg/L, respectively. However, the K+ concentration at Site L (21.97 mg/L) was higher than the limit of 12 mg/L set by WHO [9]. One hundred percent (n = 12/12) of the boreholes demonstrated compliance with the WHO [9] limit standard of 200 mg/L for Na+ concentrations.

Table 2.
Descriptive physicochemical parameters of the groundwater in Mankweng.
3.2. Bacterial Analysis of the Groundwater
Bacterial analysis of the groundwater samples was conducted to detect the presence of total coliforms and E. coli. Based on the results, only Site L was polluted with E. coli, with a maximum count of 9 MPN/100 mL. Twenty-five percent (n = 3/12) of the groundwater sources were not contaminated with coliforms; however, samples from Site A and Site B had high levels of coliform pollution, which were both greater than 201 MPN/100 mL (Table 3).

Table 3.
Total coliforms and E. coli concentrations (MPN/100 mL) in the groundwater.
3.3. Pearson Correlation Coefficient
Computation of Pearson correlation coefficients was conducted to assess the interrelationships between the parameters and the results are illustrated in Table 4. Significant and positively strong correlations (p < 0.05) were observed between E. coli–K+ (r = 0.8226), E. coli–Mg2+ (r = 0.81457), Na+–F− (r = 0.81151), Mg2+–K+ (r = 0.77712), and pH–F− (r = 0.76883). COD-N had a strong significant negative correlation coefficient of 0.84531. Significantly positive moderate correlations were shown between Ca2+–Cl− (r = 0.69887), K+–Cl− (r = 069874), Ca2+–Mg2+ (r = 0.66298), Ca2+–N (r = 0.64954), EC–N (r = 0.64402), TDS–N (r = 0.64355), Ca2+– SO42− (r = 0.62266), COD–DO (r = 0.61059), pH–F− (r = 0.60956), TC–Ca2+ (r = 0.60148), EC–Cl− (r = 0.57743), and TDS (r = 0.5771). Moderate negatively correlated coefficient values of -0.63831 and 0.63792 were observed between EC–COD and TDS–COD.

Table 4.
Pearson correlation coefficients of the selected parameters.
3.4. PCA of the Tested Parameters
Four principal components (PCs) had eigenvalues greater than one and were extracted. The four components explained 82.69% of the total variance extracted; PC 1 had the highest eigenvalue of 5.5867 and explained 32.86% of the variance. PC 2 had the second highest eigenvalue (4.05801) and explained 23.87% of the variance. PCs 3 and 4 had eigenvalues of 2.44654 and 1.96674 and explained 14.39% and 4 11.57% of the variance, respectively (Table 5).

Table 5.
PCA of the evaluated selected parameters.
3.5. Bioplot of the Two Main PCs
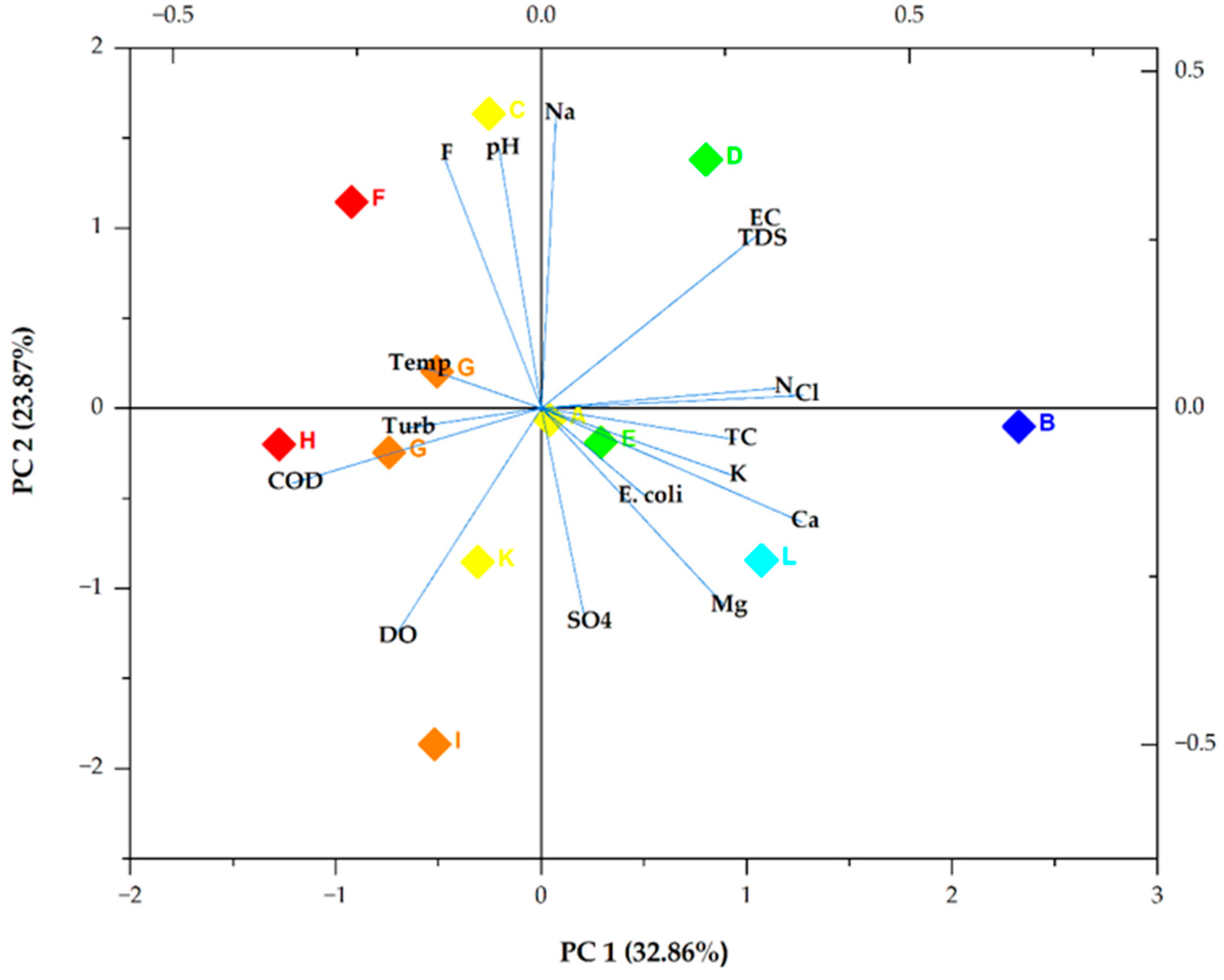
Figure 3 demonstrates a biplot of PC 1 and PC 2. PC 1 exhibited a low positive loading for Cl− (0.35606243), Ca2+ (0.35353), and N (0.32414). It also revealed the highest negative loading for COD (−0.33884). PC 2 demonstrated weak positive loadings of 0.43733, 0.38542, and 0.37657 for Na+, pH, and F−, respectively. It also illustrated a weak negative loading for DO (−0.34007), which was followed by SO42− with a loading value of −0.31866.
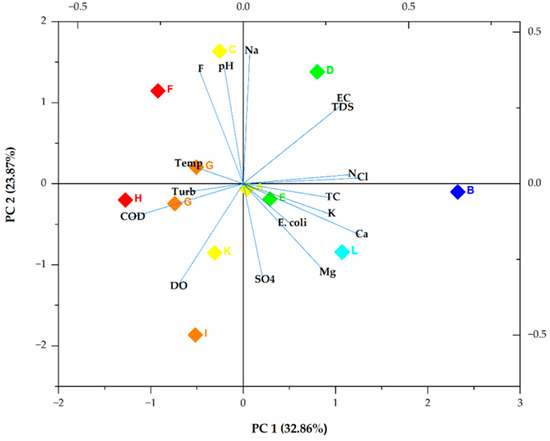
Figure 3.
Bioplot of the two main PCs. Turb, Temp, and SO4 denote turbidity, temperature, and SO42−, respectively. The box colours show the different sampling points.
3.6. Hierarchical Cluster Analysis of the Dataset
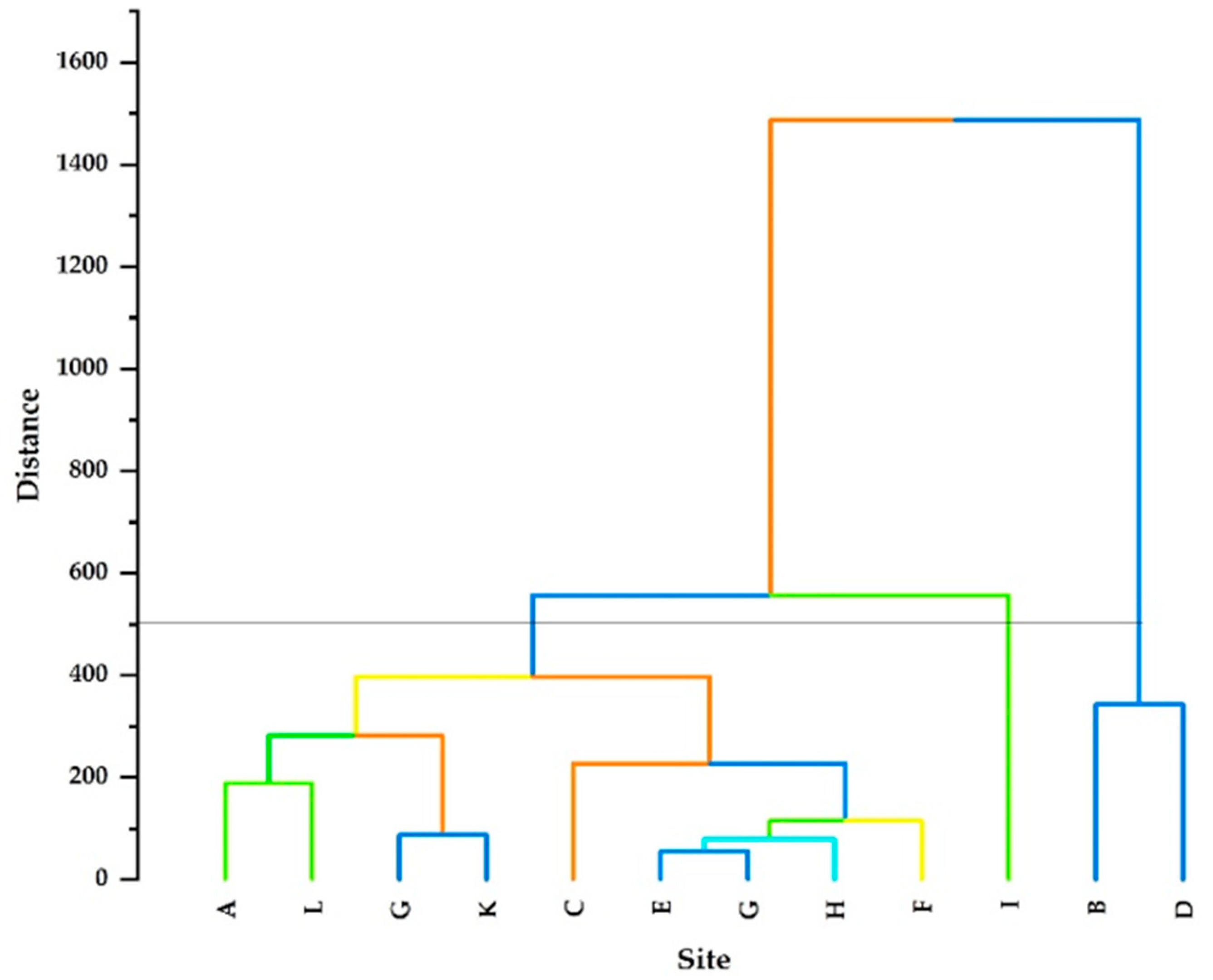
A dendrogram of the observed parameters was generated using Euclidean distance and the results are displayed in Figure 4. Based on Euclidean distance, three major clustering groups (Cluster 1, Cluster 2, and Cluster 3) were observed. Cluster 1 is characterised by a lower Euclidean distance than Clusters 2 and 3, corresponding to two sites (Site B and Site D). Cluster 2, which had a high Euclidean distance, is correlated with only Site I. Cluster 3 is composed of 9 sites, namely, sites A, C, D, E, F, G, H, J, K, and L. Cluster 3 further revealed different subclusters with significance Euclidean distances. The subcluster E–G was the closest, with the smallest Euclidean distance, followed by H. Furthermore, G–K were also among the closest, with small Euclidean distances.
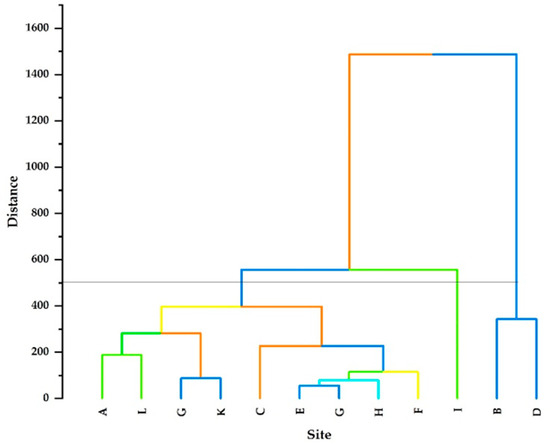
Figure 4.
Dendrogram of HCA for the 12 groundwater sampling sites. The coloured lines illustrate the different main and sub clusters whereas the parallel line at 500 is a cutoff line.
3.7. Water Quality Index of the Groundwater
The WQIs of the groundwater were calculated and their values are shown in Table 6. The WQI values of groundwater from different sampling sites ranged between 50.430 and 190.220. The largest WQI value was obtained at Site H whereas the smallest value was from Site A. The average WQI value was 72.311. Seventy-five percent (n = 9) of the groundwater sites can be classified as poor, 17% (n = 2) as very poor, and 8% (n = 1) as unfit for consumption. Generally, the overall water quality based on the obtained average WQI value (72.311) was concluded to be poor.

Table 6.
WQI of groundwater from different sites in Mankweng.
4. Discussion
The consumption of high-quality water has been strongly associated with improved health outcomes globally. However, only the water supplied by municipalities is often measured against the national standards for drinking water to assess its fitness for consumption or recreational use. There are scant attempts to assess the quality of groundwater such as from boreholes, especially in peri-urban and rural regions of developing countries such as South Africa [26].
The pH of water serves as an indicator of biological systems and the nature of chemical reactions in waterbodies [27]. The mean pH values of the groundwater samples from all points fell within the permissible limits recommended by the WHO [9] and the SANS [10] guidelines for drinking water, indicative of the fitness of the groundwater for consumption. DO determines the level of water contamination by organic pollutants, the degradation of organic substances, as well as the self-purification capability of waterbodies [24]. Low levels of DO (<4 mg/L) may result in obnoxious odour as a result of anaerobic microbial reactions. The low concentrations of DO observed in this study might be due to the high concentrations of dissolved organic substances such as N and SO42−, as they have a tendency to reduce oxygen in groundwater.
EC is the ability of groundwater to carry an electric current due to the presence of various ions. Dissolved solids such as Ca2+, Mg2+, Na+, Cl−, SO42−, etc., contribute to the EC of groundwater. The EC findings in this study did not comply with the WHO standard as they exceeded the limit value of 300 µS/cm, implying that the water had high concentrations of dissolved ions. This was confirmed by the high concentrations of Ca2+ and Mg2+ in some groundwater samples in this study, which did not comply with WHO standards [9]. The high EC may result in a loathsome taste, rendering the water unpalatable. High TDS values suggest that the water is highly mineralised. This means that the water contains a high amount of minerals such as Ca2+, Mg2+, Na+, Cl−, SO42−, etc. High concentrations of TDSs in water result in loathsome taste and obnoxious odour [28]. Fortunately, the groundwater samples in this study complied with the WHO limit guidelines, indicative of the high margin of biosafety upon drinking.
Temperature is an important water quality factor as it affects the availability of DO, solubility of chemicals, and microbial diversity and population. The temperature range in this study was within the acceptable WHO [9] limit of drinking water, suggesting that it can support oxygen and chemical solubilities and microbial growth. Moreover, the E. coli and TC observed in some sites in this study might be psychrotrophs (growing best at temperatures between 4 and 25 °C) and mesophiles, growing at an optimal temperature range of 20–45 °C, as the groundwater temperature ranged between 16.76 and 22.31 °C.
Turbidity is often utilised to monitor source water quality. Most of the sampled sites recorded low turbidity values, indicative of good aesthetic properties of the water, in compliance with WHO [9] and SANS [10] guidelines. Only the turbidity value of groundwater from Site H exceeded the drinking water standard of 1 NTU set by WHO [9]. High turbidity levels could be due to the presence of suspended solids such as clay and organic matter, consequently making it visually less appealing to consumers. This calls for urgent interventions such as the implementation of coagulation–flocculation and/or filtration processes to reduce turbidity to acceptable levels [29].
Chemical oxygen demand is a water indicator that measures all organics, including non-biodegradable and biodegradable matter; thus, COD generally provides a broad picture of water quality. In this study, only Site B demonstrated COD concentrations less than 10 mg/L recommended by WHO guidelines, indicative of safe drinking water. However, the rest of the sampled groundwater illustrated COD levels greater than 10 mg/L, implying that the groundwater is polluted and may pose health threats upon consumption.
Two borehole water samples did not comply with the WHO [9] and SANS [10] standards for F− concentrations, thus, posing a health threat to consumers. The potential health risks associated with the consumption of groundwater with high concentrations of F− include dental damage and pronounced skeletal fluorosis [30]. Thus, people at the two polluted sites are at high risk of these diseases. The high levels of geogenic F− exceeding the permissible level of 1.5 mg/L set by the WHO [9] and SANS [10] have previously been reported in the Limpopo province of South Africa [30,31]. Cl− occurs naturally in groundwater; however, the high concentrations may indicate water pollution. Cl− concentrations were not a health threat as they were well below the permissible WHO [9] set limits at all sites, indicative of a high level of biosafety upon consumption. Nevertheless, Cl− levels at sites B, D, and L were very high (>170 mg/L), reaching closer to WHO [9] and SANS [10] limits. This implies that the boreholes need more purification considerations in this regard in future. High Cl− might be from diverse sources, such as weathering, leaching of rocks, and domestic and municipal effluents. Chlorides are vital in balancing the level of electrolytes in the body; however, high concentrations can lead to hyperchloremia and the formation of kidney stones [32].
Thirty-three percent (n = 4/12) of the boreholes had nitrate ion concentrations exceeding the WHO [9] threshold concentration for drinking water. The frequent droughts in Mankweng have made flush toilets unusable, pressurising the residents to use pit latrines. Moreover, due to small land allocations for residential use, most of the septic tanks and pit latrines are constructed close (<15 m) to the boreholes. Therefore, the high N concentrations observed in this study might have been due to the underdeveloped sanitation and the percolation of animal and human excreta from animal kraals, septic tanks, and latrines nearby [5]. High concentrations of N cause diseases such as methemoglobinemia and cyanosis, especially in infants [33,34]. The high N levels in some boreholes in this study correlated with the findings obtained in Limpopo, South Africa, by Mutileni et al. [35], who reported N contents greater than the WHO [9] set limit. Concentrations of SO42− were not of concern as they aligned with the permissible WHO set limits at all sites, indicative of biosafety upon consumption. Therefore, since nitrate pollution is strongly correlated with poor sanitation infrastructure in Mankweng, construction of boreholes further from animal kraals, septic tanks, and latrines (15–30 m away) can be one of the cost-effective mitigation strategies for reducing pollution and its associated health risks [36].
SO42− occurs naturally in groundwater; however, high concentrations can occur due to leaching of natural deposits of sodium sulphate or magnesium sulphate. High concentrations are reported to cause objectionable tastes and laxative side effects [35]. In this study, the levels of SO42− complied with the WHO [9] and SANS [10] guideline limits at all sites, implying that the water had a high level of biosafety.
Mg2+, Ca2+, Na+, and K+ are vital to human metabolism and osmoregulation [37,38]. However, high concentrations of these elements in groundwater tend to increase its salinity and hardness, consequently making it less appealing for consumption. Thus, all sites with elevated Mg2+, Ca2+, and K+ beyond WHO [9] and SANS [10] limits in this study might have non-palatable groundwater [39]. In terms of Na+ levels, the findings in this study are contrary to those obtained by Barbieri et al. [40] in the Limpopo National Park, Gaza province, Mozambique, where the groundwater was unsuitable for drinking purposes due to the high Na+ concentration.
The WHO [9] and SANS [10] drinking water guidelines recommend zero E. coli in drinking water. WHO also recommends zero TC, while SANS recommend less than 10 [10]. Therefore, the high TC and E. coli contamination of the borehole at Site L beyond the limits set by WHO [9] and SANS [10] might be because Site L is an animal farm consisting of cows, goats, sheep, and chickens. Moreover, Site L receives partially treated wastewater for irrigation and animal drinking from the Mankweng Wastewater Treatment Plant. However, currently, the plant is not fully functional as it does not include a chlorination process to reduce microbial load in wastewater. Therefore, the microbiologically polluted wastewater might penetrate the soil into groundwater, consequently causing microbial transmission from animal waste or sewage, thereby polluting the groundwater [41]. The high concentration of TC beyond the WHO [9] and SANS [10] limit standards at Site A and Site B was attributed to faecal matter from pit latrines and septic tanks, which are constructed within 15 m of the boreholes. The high N content, because of leaching into the groundwater, is strongly associated with a high count of coliforms [41,42]. This phenomenon was also observed at Site B in this study. Apparently, the presence of TC and E. coli beyond set limits poses a health threat to water consumers. Apart from E. coli, TC is composed of pathogens such as Salmonella spp., Klebsiella spp., and Enterobacter spp., which can cause severe gastrointestinal infections [43]. It is therefore important for Mankweng residents to construct boreholes far from septic tanks and latrines to avoid groundwater pollution. Furthermore, to reduce microbial health risks, residents are advised to use treatment techniques such as boiling and filtration prior to groundwater consumption [44]. Moreover, the efficiency of the Mankweng Wastewater Treatment Plant in reducing microbial load and other pollutants to comply with effluent standards prior to supplying wastewater to sites such as Site L ought to be improved. The findings by Edokpayi et al. [11] supported ours, as they reported the occurrence of microbial pollution of groundwater in rural areas of Limpopo province, South Africa. The rest of the borehole water samples in this study had acceptable total coliform and E. coli counts in accordance with WHO [9] and SANS [10] standards, rendering them suitable and safe for drinking.
Pearson correlation was performed to understand the nature of the relationships between the selected parameters. The significantly strong positive correlation between E. coli and Mg2+ and K+ implied that these elements can be predictors of the prevalence of E. coli in groundwater. Moreover, this meant that the higher the concentration of these elements, the higher the concentration of E. coli. Na+ and F− had a significantly strong positive correlation, meaning that the higher the Na+ concentration, the higher the F− content and vice versa. The significant strong negative correlation displayed by COD–N implied that any increase in one of these parameters would automatically lead to a decrease in the other, and vice versa. The significant moderate positive correlation between TC and Ca2+ also meant that Ca2+ content could be utilised as an indicator of the prevalence of TC. Ca2+ correlated moderately with SO42−, N, and Cl−, implying that calcium ions in the groundwater samples might be coming from sulphate, nitrogenous or chloride salts. Moreover, Ca2+ in the water samples existed in the form of calcium chloride. The significant positive relationship between Ca2+ and TC implied that the element is essential for the growth of these microorganisms. Cl− also had a significant positive and moderate correlation with K+, indicating that the K+ in the groundwater samples was mainly in the form of potassium chloride (KCl). The pH was significantly moderately correlated with F−, suggesting that the higher the F− in groundwater, the higher the pH and vice versa. This also emphasised the fact that this element can be used to monitor the pH levels of the groundwater in this study.
It was concluded that the four extracted PCs, which explained 82.76% of the total variance, included all the information of the 17 selected groundwater quality indicators. As illustrated in Figure 4, Ca2+, Cl−, and N in the main principal component (PC 1) had high loading scores, indicating that they were the main chemical indicators that characterised the pollution of groundwater in Mankweng [45]. This component can be said to represent pollution from agricultural and domestic waste. The moderate positive correlations between Cl−, Ca2+, and N, as illustrated by the Pearson correlation, further confirmed this. The loadings of Na, pH, F−, DO, and SO42− values in PC 2 were relatively high, indicating that the parameters were great contributors to groundwater contamination. Therefore, the source of pollution in groundwater can be primarily geogenic, agricultural, and/or anthropogenic [46,47]. The negative correlation between DO and SO42− might be due to temporal changes [48].
In clustering, parameters are grouped in such a way that similar ones fall into the same class. The dendrogram clarified Cluster 1 as the abnormality observation, which had many sampling sites compared to Clusters 2 and 3, implying that Cluster 1 had sites with similar pollution levels in comparison to Clusters 2 and 3. The variation in Cluster 1 might be due to pollution from geogenic, agricultural, and artificial activities at different sites.
The WQI of groundwater from different sites fell within the range of 50.430–190.220, indicating a water rating of poor to unfit for consumption. The unfit for consumption category obtained at Site H was due mainly to very high turbidity and COD levels, which exceeded the WHO [9] recommended limits. The borehole at Site H was recently constructed; therefore, the high level of turbidity could have arisen from the presence of suspended solids such as clay or silt, whereas the COD levels might have been due to organic and inorganic pollutants [49]. Therefore, this borehole needs appropriate corrective measures to lower its turbidity and COD levels to desirable concentrations, consequently improving its groundwater quality. The very poor status of groundwater at Site C was attributed to high turbidity and F− levels and low DO content, which did not comply with WHO [9] guidelines. The poor status of water at Site C was due to low DO levels and high N concentrations, whereas the poor status of borehole water at Site D was due to low DO content and high EC and N levels. At Site F, the poor status of borehole water was attributed to the high EC and F− contents, whereas at Site G, it was due to low DO level and high EC concentration. Therefore, to improve the groundwater quality to good and excellent statuses, appropriate treatment methods such as in situ bioremediation and filtration should be implemented.
Mitigation strategies should be adopted to remediate the already polluted groundwater at some sites. Some of the strategies could include providing educational awareness of groundwater pollution. This can include factors that are generally known to cause pollution, which include appropriate distances between boreholes and toilets (septic tanks and latrines) as well as borehole depths. Moreover, enforcement of compulsory regular monitoring of groundwater and proper maintenance of boreholes can be some of the measures implemented to safeguard public health in Mankweng.
One of the important aspects of safeguarding human health is monitoring drinking water quality to assess its compliance with the safe guidelines set by different countries, which was the focus of this study. However, due to time constraints and resource availability, the study was limited to assessing groundwater quality once (June 2024), without looking into seasonal variations, which might have a significant impact. Moreover, factors such as distances of boreholes from septic tanks and latrines, the depths of the borehole, septic tanks and latrines and diameters of boreholes, were also not considered during sampling. However, despite these limitations, the findings underscored the need to regularly monitor groundwater and to properly maintain boreholes in order to have high-quality groundwater. Moreover, this study serves as a basis for future research on PCA integration and WQI evaluation of groundwater.
5. Conclusions
This study showed that the groundwater in Mankweng is vulnerable to physicochemical and microbiological contamination. Some of the boreholes were greatly affected by high levels of EC, turbidity, COD, F−, N, Ca2+, and Mg2+, and low DO, which did not comply with WHO [9] and SANS [10] standards for drinking water. Moreover, some groundwater samples were contaminated with high concentrations of E. coli and TC, rendering them unsafe for consumption. Based on the results of PCA, the four extracted PCs explained 82.69% of the total variance in the groundwater data. The WQI of groundwater from different sites fell within the range of 50.430–190.220%, indicating a water rating of poor to unfit for consumption. Further studies are needed to conclude whether the deterioration in the quality of groundwater is temporary or a progressive situation. Moreover, a health risk assessment of the impact of groundwater quality on human health is recommended.
Author Contributions
Conceptualization, T.N.S., T.S.M., and P.M.; formal analysis, T.S.M.; supervision, T.N.S.; investigation, N.M., F.M.M., N.N., and T.S.M.; writing—original draft preparation, T.S.M.; writing—review and editing, F.M.M. and T.S.M.; project administration, P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the staff and postgraduate students (Biofloc Group) in the Department of Water and Sanitation and the Department of Biochemistry, Microbiology and Biotechnology at the University of Limpopo for their outstanding support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anker, Y.; Gimburg, A.; Zilberbrand, M.; Livshitz, Y.; Mirlas, V. Groundwater recharge assessment for small karstic catchment basins with different extents of anthropogenic development. Environments 2023, 10, 158. [Google Scholar] [CrossRef]
- Akbar, H.; Nilsalab, P.; Silalertruksa, T.; Gheewala, S.H. Comprehensive review of groundwater scarcity, stress and sustainability index-based assessment. Groundw. Sustain. Dev. 2022, 18, 100782. [Google Scholar] [CrossRef]
- Makungo, R.; Odiyo, J.O. Groundwater quality and its distributions in Siloam Village, Limpopo Province, South Africa. WIT Trans. Ecol. Environ. 2018, 228, 35–44. [Google Scholar]
- Grönwall, J.; Danert, K. Regarding groundwater and drinking water access through a human rights lens: Self-supply as a norm. Water 2020, 12, 419. [Google Scholar] [CrossRef]
- Traoré, O.; Kpoda, D.S.; Dembélé, R.; Saba, C.K.S.; Cairns, J.; Barro, N.; Haukka, K. Microbiological and physicochemical quality of groundwater and risk factors for Its pollution in Ouagadougou, Burkina Faso. Water 2023, 15, 3734. [Google Scholar] [CrossRef]
- Gwenzi, W.; Marumure, J.; Makuvara, Z.; Simbanegavi, T.T.; Njomou-Ngounou, E.L.; Nya, E.L.; Kaetzl, K.; Noubactep, C.; Rzymski, P. The pit latrine paradox in low-income settings: A sanitation technology of choice or a pollution hotspot? Sci. Total Environ. 2023, 879, 163179. [Google Scholar] [CrossRef]
- Murei, A.; Mogane, B.; Mothiba, D.P.; Mochware, O.T.W.; Sekgobela, J.M.; Mudau, M.; Musumuvhi, N.; Khabo-Mmekoa, C.M.; Moropeng, R.C.; Momba, M.N.B. Barriers to Water and Sanitation Safety Plans in Rural Areas of South Africa—A Case Study in the Vhembe District, Limpopo Province. Water 2022, 14, 1244. [Google Scholar] [CrossRef]
- Mulaudzi, L.; Mudzielwana, R.; Gitari, M.W.; Ayinde, W.B.; Talabi, A.O.; Akinyemi, S.A. Hydrogeochemical and microbial constituents of groundwater in Lephalale Municipality, Limpopo Province, South Africa. Sci. Afr. 2024, 24, e02178. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First and Second Addenda; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240045064 (accessed on 21 March 2022).
- SANS. South African National Standards 241-1-2015; SANS: Pretoria, South Africa, 2015. [Google Scholar]
- Edokpayi, J.N.; Enitan, A.M.; Mutileni, N.; Odiyo, J.O. Evaluation of water quality and human risk assessment due to heavy metals in groundwater around Muledane area of Vhembe District, Limpopo Province, South Africa. Chem. Cent. J. 2018, 12, 1–16. [Google Scholar] [CrossRef]
- Varol, M. Use of water quality index and multivariate statistical methods for the evaluation of water quality of a stream affected by multiple stressors: A case study. Environ. Microbiol. 2020, 266, 115417. [Google Scholar] [CrossRef]
- Lukhabi, D.K.; Mensah, P.K.; Asare, N.K.; Pulumuka-Kamanga, T.; Ouma, K.O. Adapted water quality indices: Limitations and potential for water quality monitoring in Africa. Water 2023, 15, 1736. [Google Scholar] [CrossRef]
- Dandge, K.P.; Patil, S.S. Spatial distribution of ground water quality index using remote sensing and GIS techniques. Appl. Water Sci. 2022, 12, 7. [Google Scholar] [CrossRef]
- Ibrahim, M.N. Assessing groundwater quality for drinking purpose in Jordan: Application of water quality index. J. Ecol. Eng. 2019, 20, 101–111. [Google Scholar] [CrossRef]
- El Baba, M.; Kayastha, P.; Huysmans, M.; De Smedt, F. Evaluation of the groundwater quality using the water quality index and geostatistical analysis in the Dier al-Balah Governorate, Gaza Strip, Palestine. Water 2020, 12, 262. [Google Scholar] [CrossRef]
- Mashao, F.M.; Thaba, S.J.; Muyambo, N.P.; Tjale, C.R.; Zwane, P.S.M.; Munjonji, L.; Nkuna, D.; Ayisi, K.K.; Thamaga, K.H. Exploring laboratory-based spectroscopy for estimating NPK content in the hutton soils of Syferkuil Farmlands, South Africa. Geocarto Int. 2024, 39, 2339289. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Luvhimbi, N.; Tshitangano, T.G.; Mabunda, J.T.; Olaniyi, F.C.; Edokpayi, J.N. Water quality assessment and evaluation of human health risk of drinking water from source to point of use at Thulamela municipality, Limpopo Province. Sci. Rep. 2022, 12, 6059. [Google Scholar] [CrossRef]
- Taylor, M.; Elliott, H.A.; Navitsky, L.O. Relationship between total dissolved solids and electrical conductivity in Marcellus hydraulic fracturing fluids. Water Sci. Technol. 2018, 77, 1998–2004. [Google Scholar] [CrossRef]
- Venkateswaran, K.; Murakoshi, A.; Satake, M. Comparison of commercially available kits with standard methods for the detection of coliforms and Escherichia coli in foods. Appl. Environ. Microbiol. 1996, 62, 2236–2243. [Google Scholar] [CrossRef]
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; Tozer, R.G. A Water Quality Index: Do We Dare? Water Sew. Works 1970, 117, 339–343. [Google Scholar]
- Brown, R.M.; McClelland, N.I.; Deininger, R.A.; O’Connor, M.F. A water quality index—Crashing the psychological barrier. In Indicators of Environmental Quality; Springer: Boston, MA, USA, 1972; pp. 173–182. [Google Scholar]
- Ayeni, A.O.; Soneye, A.S.O. Interpretation of surface water quality using principal components analysis and cluster analysis. J. Geogr. Reg. Plann. 2013, 6, 132. [Google Scholar] [CrossRef]
- Edokpayi, J.N.; Rogawski, E.T.; Kahler, D.M.; Hill, C.L.; Reynolds, C.; Nyathi, E.; Dillingham, R. Challenges to sustainable safe drinking water: A case study of water quality and use across seasons in rural communities in Limpopo Province, South Africa. Water 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, B.I.; Shitu, T.; Zambuk, U.U.; Amamat, A.Y. Physicochemical characteristics of borehole water sources in a tertiary educational institution in Katsina, Katsina State, Nigeria. J. Appl. Sci. Environ. Manag. 2023, 27, 974–978. [Google Scholar] [CrossRef]
- Pratiwi, D.; Sumiarsa, D.; Oktavia, D.; Sunardi, S. Water quality influences self-purification in the Cihawuk and Majalaya Segments Upstream of the Citarum River, West Java, Indonesia. Water 2023, 15, 2998. [Google Scholar] [CrossRef]
- Soros, A.; Amburgey, J.E.; Stauber, C.E.; Sobsey, M.D.; Casanova, L.M. Turbidity reduction in drinking water by coagulation-flocculation with chitosan polymers. J. Water Health 2019, 17, 204–218. [Google Scholar] [CrossRef] [PubMed]
- Onipe, T.; Edokpayi, J.N.; Odiyo, J.O. Geochemical characterization and assessment of fluoride sources in groundwater of Siloam area, Limpopo Province, South Africa. Sci. Rep. 2021, 11, 14000. [Google Scholar] [CrossRef]
- Olivier, J.; Venter, J.S.; Jonker, C.Z. Thermal and chemical characteristics of thermal springs in the northern part of the Limpopo Province, South Africa. Water SA 2011, 34, 163–174. [Google Scholar] [CrossRef]
- Ali, A.A.H. Overview of the vital roles of macro minerals in the human body. J. Trace Elem. Min. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Richard, A.M.; Diaz, J.H.; Kaye, A.D. Reexamining the risks of drinking-water nitrates on public health. Ochsner J. 2014, 14, 392–398. [Google Scholar]
- Belzer, A.; Krasowski, M.D. Causes of acquired methemoglobinemia–A retrospective study at a large academic hospital. Toxicol. Rep. 2024, 12, 331–337. [Google Scholar] [CrossRef]
- Mutileni, N.; Mudau, M.; Edokpayi, J.N. Water quality, geochemistry and human health risk of groundwater in the Vyeboom region, Limpopo province, South Africa. Sci. Rep. 2023, 13, 19071. [Google Scholar] [CrossRef]
- Chove, L.M.; Mongi, R.; Chenge, L. Effect of depth and distance of the borehole from the septic tank on the physico-chemical quality of water. J. Food Stud. 2017, 7, 41–55. [Google Scholar]
- Yamada, S.; Inaba, M. Potassium metabolism and management in patients with CKD. Nutrients 2021, 13, 1751. [Google Scholar] [CrossRef] [PubMed]
- Robayo-Amortegui, H.; Quintero-Altare, A.; Florez-Navas, C.; Serna-Palacios, I.; Súarez-Saavedra, A.; Buitrago-Bernal, R.; Casallas-Barrera, J.O. Fluid dynamics of life: Exploring the physiology and importance of water in the critical illness. Front. Med. 2024, 11, 1368502. [Google Scholar] [CrossRef]
- Verlicchi, P.; Grillini, V. Surface water and groundwater quality in South Africa and mozambique—Analysis of the most critical pollutants for drinking purposes and challenges in water treatment selection. Water 2020, 12, 305. [Google Scholar] [CrossRef]
- Barbieri, M.; Ricolfi, L.; Vitale, S.; Muteto, P.V.; Nigro, A.; Sappa, G. Assessment of groundwater quality in the buffer zone of Limpopo National Park, Gaza Province, Southern Mozambique. Environ. Sci. Pollut. Res. Int. 2019, 26, 62–77. [Google Scholar] [CrossRef]
- Mishra, S.; Kumar, R.; Kumar, M. Use of treated sewage or wastewater as an irrigation water for agricultural purposes-Environmental, health, and economic impacts. Total Environ. Res. Themes 2023, 6, 100051. [Google Scholar] [CrossRef]
- Jaramillo, M.F.; Restrepo, I. Wastewater reuse in agriculture: A review about its limitations and benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Ali, A.S.; Gari, S.R.; Goodson, M.L.; Walsh, C.L.; Dessie, B.K.; Ambelu, A. Fecal contamination in the wastewater irrigation system and its health threat to wastewater-based farming households in Addis Ababa, Ethiopia. Environ. Health Insights 2023, 17, 11786302231181307. [Google Scholar] [CrossRef]
- Odiyo, J.O.; Makungo, R. Water quality problems and management in rural areas of Limpopo Province, South Africa. WIT Trans. Ecol. Environ. 2012, 164, 135–146. [Google Scholar]
- Chen, Y.; Su, Y.; Li, H.; Cheng, L.; Guo, L.; Zhang, L.; Ling, L. Spatial heterogeneity of water quality in a small watershed of an ionic rare earth mining area. Water Supply 2022, 22, 5575–5588. [Google Scholar] [CrossRef]
- Shaji, E.; Sarath, K.V.; Santosh, M.; Krishnaprasad, P.K.; Arya, B.K.; Babu, M.S. Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures. Geosci. Front. 2024, 15, 101734. [Google Scholar] [CrossRef]
- Spoelstra, J.; Leal, K.A.; Senger, N.D.; Schiff, S.L.; Post, R. Isotopic characterization of sulfate in a shallow aquifer impacted by agricultural fertilizer. Groundwater 2021, 59, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, H.; Sun, C.; Li, H.; Gao, Y. Multivariate statistical approaches to identify the major factors governing groundwater quality. Appl. Water Sci. 2018, 8, 215. [Google Scholar] [CrossRef]
- World Health Organization. Water Quality and Health-Review of Turbidity: Information for Regulators and Water Suppliers. 2017. Available online: https://apps.who.int/iris/handle/10665/254631 (accessed on 10 January 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).