Abstract
Physical rehabilitation and exercise training have emerged as promising solutions for improving health, restoring function, and preserving quality of life in populations that face disparate health challenges related to disability. Despite the immense potential for rehabilitation and exercise to help people with disabilities live longer, healthier, and more independent lives, people with disabilities can experience physical, psychosocial, environmental, and economic barriers that limit their ability to participate in rehabilitation, exercise, and other physical activities. Together, these barriers contribute to health inequities in people with disabilities, by disproportionately limiting their ability to participate in health-promoting physical activities, relative to people without disabilities. Therefore, there is great need for research and innovation focusing on the development of strategies to expand accessibility and promote participation in rehabilitation and exercise programs for people with disabilities. Here, we discuss how cutting-edge technologies related to telecommunications, wearables, virtual and augmented reality, artificial intelligence, and cloud computing are providing new opportunities to improve accessibility in rehabilitation and exercise for people with disabilities. In addition, we highlight new frontiers in digital health technology and emerging lines of scientific research that will shape the future of precision care strategies for people with disabilities.
1. Introduction
According to recent data from the Centers for Disease Control (CDC), 61 million (26%) adults in the United States are living with disability as defined as “serious difficulty walking, hearing, seeing, concentrating, remembering, or making decision”, with disability from mobility limitations being the most common (14%) [1]. Within this population, people with all types of disabilities experience physical, environmental, structural, social, and economic disadvantages that negatively impact their health and quality of life disproportionately, compared to those without disability. Specifically, when considering health disparities, people with disabilities are three times more likely to have comorbidities such as heart disease, stroke, and diabetes [2,3,4,5] and are more than four-fold more likely to experience mental distress compared to those without disability [6]. Moreover, physical disabilities that impact mobility can limit participation in physical activities and exercise [7,8], and, over time, chronic physical inactivity can lead to physiological deconditioning that further impairs physical function and cardiometabolic health [9,10,11,12,13]. In people with physical disabilities, research has demonstrated that the degree of cardiometabolic disease risk, dependence, anxiety, and depression worsens with the severity of mobility impairment [14,15,16,17,18,19,20]. Thus, there is a profound need for accessible and efficacious treatment strategies to improve physical and mental health in people with disabilities, particularly in those with mobility limitations.
Physical rehabilitation and exercise training have emerged as promising solutions to improving health, restoring function, and preserving quality of life in populations that face disparate health challenges related to disability [3,5,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37]. Specifically, clinical research studies have shown that rehabilitation and exercise programs provide an opportunity to simultaneously target multiple physiological systems and drive improvements in neural function, cardiopulmonary and metabolic capacity, muscle strength, endurance, flexibility, and vascular health [3,5,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] while also reducing mental distress and attenuating depression and anxiety [14,15,16,17,18,19,20] in these populations. Despite the enormous potential for rehabilitation and exercise to help people with disabilities live longer, healthier, and more independent lives, people with disabilities can experience physical, psychosocial, environmental, and economic barriers that limit their ability to participate in rehabilitation, exercise, and other health-promoting physical activities [38,39,40,41,42,43,44] (Figure 1). Here, we discuss how emerging technologies may provide new opportunities to overcome such barriers and establish more accessible and effective approaches to rehabilitation and exercise for people with disabilities.

Figure 1.
Emerging digital health technologies are providing opportunities to overcome barriers experienced by people with disabilities to participation in rehabilitation and exercise.
2. Telecommunications
Participation in physical rehabilitation and exercise often requires access to specific environments, specialized equipment, and professional instruction, which may not be safe, accessible, or relevant for people with disabilities and which can directly limit their ability to participate in such activities. For example, facilities that support rehabilitation and exercise such as clinics, gyms, parks, and trials are often physically inaccessible to people with disabilities who have mobility limitations, and inclement weather can further limit the utility of even the most accessible facilities [45,46,47,48,49,50,51]. In addition to physical barriers, public gyms and crowded spaces may also contribute to intrapersonal barriers, as people with disabilities commonly report barriers associated with self-efficacy, perception of risks, and fear of embarrassment [38,39,40,51,52,53]. Moreover, people with disabilities may also experience barriers associated with transportation and scheduling that negatively impact their ability to attend scheduled rehabilitation and exercise activities at specific locations. Outside of clinically-supervised rehabilitation programs, public-facing fitness organizations and exercise classes often lack expertise, which may limit the availability of safe instruction that is tailored to the needs and concerns of people with disabilities [45,46,47,54,55,56]. Together, these barriers contribute to health inequities in people with disabilities by disproportionately limiting their ability to participate in health-promoting physical activities such rehabilitation and exercise, relative to people without disabilities.
Tele-health has emerged as a promising solution to expanding access to quality care for individuals that are geographically, physically, cognitively, and economically disadvantaged [57,58,59,60]. “Tele-health” refers to strategies that leverage telecommunications technologies such as telephone, email, text, and the internet to provide healthcare services to individuals in remote locations outside the clinic [57,61,62,63,64,65]. Tele-health interventions can overcome many of the barriers related to facility access, scheduling, and transportation by offering a more convenient and economical solution that can be performed in one’s home or in an accessible location of one’s choosing [57,58,61,66,67,68,69]. As highlighted in this section, rehabilitation and exercise professionals have also adopted tele-health strategies to specifically address the accessibility issues faced by people with disabilities.
2.1. Tele-Rehabilitation
The need to deliver home-based rehabilitation programs has been a longstanding challenge for patients and clinicians. In addition to overcoming many of the barriers patients encounter when accessing rehabilitation services, home-based rehabilitation interventions also address fundamental treatment gaps that may ultimately improve outcomes for patients. For example, in response to a research survey administered to over 500 rehabilitation professionals, clinicians reported that 70% of their patients with disabilities required additional therapy after discharge, and more than 55% required additional therapy between in-clinic visits [70]. In this same cohort, 95% of the respondents indicated that tele-rehabilitation may provide an opportunity to address the need for additional treatment sessions [70]. Over the past 25 years, the utility and efficacy of tele-rehabilitation has been evaluated in several different clinical populations [71,72,73,74,75,76,77], and, according to the National Library of Medicine’s PubMed database, the number of peer-reviewed research articles featuring tele-rehabilitation in the title alone has increased more than 10-fold in the past decade. Empirical evidence from tele-rehabilitation research studies suggest that the use of these tele-health strategies may provide an accessible and effective option for deploying rehabilitation and exercise interventions for people with disabilities [57,61,64,65,69,78,79,80]. Recent systematic and thematic reviews of this body of literature have concluded that the available evidence demonstrates that tele-rehabilitation interventions are effective at improving physical function, cardiometabolic health, and quality of life in people with disabilities associated with neurologic, musculoskeletal, and cardiovascular disorders [57,59,61,64,65,69,79,80,81,82]. For example, studies evaluating the impact of tele-rehabilitation interventions in people with mobility impairment associated with neurologic conditions (stroke, multiple sclerosis, and spinal injury/disease) have found remote intervention strategies to be as effective as standard, in-person rehabilitation at improving measures of physical function and mobility [57,59,61,62,63,72,76,81,83,84,85].
Historically, home-based rehabilitation programs have been limited to using detailed written instructions and illustrations, before evolving into video-based instructions delivered through videotape or digital video disk [57,58,59,61,64,69,72,84]. More recently, the advent of high-speed internet, massive data storage capacity, and affordable digital videography have revolutionized in-home rehabilitation programming, through the emergence of web-based tele-rehabilitation [86,87,88,89]. Specifically, these advancements in telecommunications technology offer a more versatile approach to tele-exercise, which allows clinicians to efficiently create and upload large libraries of custom video content that can be accessed by users from any device with internet connectivity (i.e., tablet, smart phone, etc.). Furthermore, the increasing affordability and quality of real-time video streaming and video conferencing has also expanded the capabilities of tele-rehabilitation by enabling the live streaming of video content and allowing patients to interact with their healthcare providers through real-time two-way video conferencing in a secure, interactive environment [90,91]. While tele-rehabilitation delivered through various methods has demonstrated immense potential to make a substantial impact on health and function in people with disabilities, the available evidence also suggests that the effectiveness of tele-rehabilitation interventions may be limited by poor adherence and a lack of clinical supervision [57,59,61,64,65,69,75,79,80,81,82,92]. Undoubtedly, corrective and encouraging feedback is critical to the safety and effectiveness of rehabilitation, but this is difficult to provide during tele-rehabilitation sessions where direct clinical supervision is limited or absent altogether. Thus, future work is needed to address the limitations of tele-rehabilitation interventions and identify ways to improve sustained engagement and expand clinical evaluation into remote settings.
2.2. Tele-Exercise
In addition to clinician-guided rehabilitation interventions, mounting evidence has also demonstrated that regimented exercise can improve physical function and overall health in people with disabilities by increasing cardiopulmonary and metabolic capacity, muscle strength, coordination, and vascular health [21,33,36,93,94,95,96,97,98,99,100,101,102,103,104,105,106] while also reducing mental distress and attenuating depression and anxiety [2,25,107,108,109,110] in these populations. Yet, people with, and without, disabilities encounter a multitude of intra- and interpersonal barriers to participating in exercise programs that involve professional supervision, specialized facilities, or public spaces. To this end, telecommunications technologies have also been rapidly adopted by exercise professionals, and within the last half decade, the entire fitness industry has rapidly evolved into a hybrid ecosystem, as the popularity of online fitness programming has grown exponentially [111,112,113].
According to a survey of over 4000 fitness professionals conducted by the American College of Sport Medicine, online fitness climbed to the #1 most popular fitness trend in the fitness industry in 2021 [111,112]. However, the preponderance of public-facing tele-exercise platforms, such as the commercial tele-exercise programs designed by Peloton [114] and Tonal [115], provide content and user interfaces that are primarily curated for the general population and may not be suitable to the needs of people living with disabilities. Therefore, it may be difficult for someone living with a disability to identify commercially available exercise content that provides a safe and accessible effective tele-exercise platform that will help them attain their goals [116]. Furthermore, studies evaluating tele-exercise programs in clinical populations have consistently found that participants reported limited social engagement as a primary limitation to tele-exercise strategies [75,117,118,119]. Thus, while telecommunications technologies have immense potential to address health equity in people with disabilities, there is a need for more accessible and efficacious approaches to tele-exercise programs that are socially engaging and tailored to the needs of people with disabilities.
2.3. Expanding Tele-Health Solutions
Solutions that leverage emerging technologies to expand clinical evaluation into remote settings and integrate social connectivity into tele-health platforms will be a vital component in the future of tele-rehabilitation and tele-exercise. As discussed in detail below, a growing body of research is starting to reveal the many ways remote monitoring tools, such as wearable sensors and digital survey technologies, may be used to evaluate physical function and physiology in remote settings (see “remote monitoring”) [120,121,122]. In addition, some companies are exploring approaches to remote monitoring that directly integrate clinical evaluation into the tele-rehabilitation platform itself. For example, Kaia Health [123], is a tele-rehabilitation platform that leverages computer vision technology to map a patient’s biomechanics based on two-dimensional video data captured from a smartphone camera to evaluate a patient’s movements while they are viewing instructional video content through a mobile app. The software then provides automated, corrective feedback in real-time during their tele-rehabilitation activities, while also providing the opportunity for asynchronous clinician-mediated feedback, based on recordings. Other technologies, such as chatbots, are also being explored as a method to improve adherence in tele-rehabilitation by providing support in the form of tailored feedback and encouragement [124,125,126]. Specifically, some studies have shown how automated, SMS-mediated chatbot interactions can provide patients with information and encouragement, which may improve adherence to an assigned tele-rehabilitation [124,125,126]. Ultimately, establishing sustainable and efficacious approaches to tele-rehabilitation will require the integration of multiple different technologies such as those that data from wearable sensors, computer vision systems, and other remote monitoring technologies are used to drive automated feedback from by chatbots and other forms of digital communication.
The emergence of creator-driven and socially interactive digital health platforms may also provide novel solutions to address the limitations of modern tele-rehabilitation and tele-exercise programs [116,127]. Online tele-health platforms that emphasize social connectivity and allow users to directly communicate with each other and instructors could overcome limitations to tele-exercise associated with the lack of social interactions [2,52,127]. Moreover, tele-health platforms that provide an opportunity for rehabilitation and exercise professionals to organically publish original content may also establish diverse libraries of instructional content that are inclusive of the needs and goals of people with disabilities. For example, Burnalong [128], a digital wellness platform, offers an online community where credentialed creators can post video content such as exercise classes and programs. This model attracts hundreds of different creators with a wide range of expertise who generate a diverse library of tele-exercise video content that is tailored to the needs of various groups, including those with disabilities. The platform also allows users to interact with each other and the exercise instructors as well, through discussion forums and two-way video streams. Therefore, users with disabilities can find professionally curated content that is inclusive of their goals, ability level, and preferences, while also engaging with other individuals who may have similar lived experiences or health goals. Together, innovative tele-health platforms and remote monitoring technologies are starting to bridge the gap between the individual, clinic, and the communities of people living with disabilities. While these solutions have immense potential to collectively address many of the limitations of tele-rehabilitation and tele-exercise, future research is warranted to determine how, and to what extent, applications of remote monitoring and socially engaging platforms may improve adherence and outcomes for people with disabilities.
3. Remote Monitoring
While tele-rehabilitation and tele-exercise have emerged as potential approaches to overcoming many environmental and economic barriers to participating in health-promoting physical activities [57,61,62,63,64,65,80,118], remote interventions are limited by their inability to employ contemporary evaluation strategies and adaptive equipment that may greatly enhance the safety, accessibility, and precision of an intervention. For example, the deployment of safe and effective rehabilitation and exercise interventions requires individualized evaluation strategies that can identify changes in physical function and physiology over time, so that activity intensity and type can be adjusted as an individual adapts [97,129,130]. Yet, key clinical outcome measures, such as walking tests and cardiopulmonary assessments [131,132,133,134,135,136,137], require direct clinical supervision or costly, immobile instruments that cannot be used in remote settings outside the clinic, and therefore, it is difficult to conduct a rigorous evaluation of physical function and physiology of individuals participating in remote interventions, which may negatively impact risk and efficacy.
3.1. Wearable Sensors
Recent advancements in wearable sensor technologies have expanded our capacity to monitor various aspects of human movement and physiology [120,138,139,140,141,142,143,144,145,146,147,148]. Wireless, noninvasive instruments such as accelerometers and optical sensors can now be combined into a single, wearable electronic device, which measures multiple physiological systems in remote locations without any, or with little, clinical oversight required [120,121,122,139,149,150,151,152,153,154,155,156]. By integrating these measurement devices with contemporary system-on-a-chip technology, high-capacity lithium batteries, and Bluetooth connectivity, modern wearable devices can now generate and transmit continuous, high-resolution measurements for days or weeks at a time. Certainly, there is a great deal of consumer interest in wearables, and companies such as Fitbit [157,158,159], Apple [159,160], Garmin, and Whoop [161,162] provide commercially available, user-friendly products such as watches and wristbands that enable users to track their physical activity, cardiac function, sleep, and other aspects of their health, without any technical training required. However, the potential of wearables is also recognized by clinicians and researchers as well, and there has been a rapid expansion in research related to wearable devices over the past decade [120,138,139,140,141,142,143,144,145,146,147,148,163,164]. Moreover, similar to the trends observed in tele-rehabilitation research, the number of peer-reviewed research articles featuring “wearables”, in the title alone, has increased more than 12-fold in the past decade, according to the NLM’s PubMed database. These studies have demonstrated a wide range of potential use cases in which wearable sensors may overcome barriers to accessing evidence-based care by expanding clinical evaluation into remote settings; they provide new opportunities to remotely deliver rehabilitative interventions that require specialized equipment that was historically only available in the clinic [122,149,156,165,166,167,168,169].
Because wearable devices can incorporate many different instruments and may be integrated into many different types of wearable accessories, there are a wide range of potential clinical use cases for wearables. Within the wearable device ecosystem, sensors that use inertial measurement units (IMUs) to evaluate movement are amongst the common [120,121,122,141,143,170]. IMUs are noninvasive, electronic sensors that use accelerometers and gyroscopes to simultaneously measure linear acceleration and angular rotation. A wide range of different movements and biomechanics can be measured by pairing wearable IMU sensors with straps and adhesive to secure the devices to the wrist, ankle, hip, or other desired anatomical location. IMU sensors worn at different anatomical locations can provide reliable measures of general daily activity (i.e., steps) [143,151,171,172,173,174,175,176,177,178,179], gait metrics [145,180], spasticity [170,181], and specific upper- and lower-extremity movements [149,182,183,184,185,186,187,188]. Several research studies have used wearable IMUs to reveal important, multidirectional interactions among non-structured physical activity, rehabilitation, and exercise [148,151,179,189,190,191]. Specifically, studies have shown that reductions in daily, non-structured physical activities are related to increased risk of cardiometabolic disease and reduced mobility in people with disabilities, and that participation in rehabilitation and exercise can improve health outcomes, both directly and indirectly, through associated increases in physical activity.
In addition to research instruments, some commercially available devices also leverage IMUs to facilitate participation in home-based exercise activities. For example, Flint Rehab [192] has developed a system called FitMi [193,194,195] that integrates wearable IMU sensors with an interactive software program to monitor and provide feedback regarding movement quantity and quality during participation in tele-exercise activities. Systems such as the FitMi are creating opportunities for people with disabilities to engage in tailored exercise programs that are guided by real-time feedback based on their precise movements. Together, this work demonstrates how wearable IMU sensors may provide an opportunity to remotely evaluate the quantity and type of physical activities associated with tele-rehabilitation and exercise programs.
Measurements of walking function and gait mechanics are critical to evaluating the progression of physical disability and the impact of therapeutic strategies for people with mobility impairments [196,197]. While IMUs sensors embedded in wrist straps and smartphones can provide valuable information regarding physical activity and steps, these devices are limited in their capacity to evaluate lower-extremity biomechanics-associated gait kinetics. To this end, recent developments in wearable sensor technologies have started to incorporate small, wireless pressure sensors into footwear to capture lower-extremity pressure patterns generated by foot placement during gait cycles [198,199,200,201,202,203]. For example, Sensoria Health [204] has developed textile pressure-sensor-infused socks that can evaluate spatiotemporal parameters of gait associated with walking and running. Assessments of gait derived from smart socks have been validated by multiple independent research studies, which have found the sock sensor to be as, or more, accurate than gold-standard gait lab systems [202,203,205]. These preliminary studies have demonstrated exciting potential for smart socks to provide reliable measurements of gait in community-based settings, without the need for direct, clinical supervision. In addition to measuring specific spatiotemporal gait metrics associated with clinical disability, smart footwear may also provide a direct measurement of daily step count that is more inclusive to people with disabilities. The reliability of step counts derived from contemporary wrist-worn sensors is significantly reduced in clinical populations with lower gait speed, and wrist-worn trackers are unable to accurately count steps in populations that use assistive devices, such as walkers, which limit upper-extremity mobility during walking [190,191,206]. Establishing an outcome measure that can accurately evaluate daily steps in people with more severe mobility impairments will create new opportunities to explore the clinical importance of daily physical activity in people with disabilities, and will advance our knowledge of how various treatment strategies impact everyday life.
In addition to measuring human movement and biomechanics, wearable devices are also commonly used to monitor heart rate, which may be useful when delivering tele-rehabilitation and tele-exercise programs. For example, wearable heart-rate monitors can allow clinicians to monitor cardiac activity during participation in tele-rehabilitation, which may be critical to maintaining safety and identifying problems in patients with cardiac irregularities/impairments [66,139,154,156,179]. Furthermore, evidence-based guidelines for exercise in people with disabilities have been established, based on research showing that the intensity of exercise should be optimized to generate a specific physiological stimulus that can safely and effectively drive improvements in physical function, fatigue, and cardiometabolic health [97,129,130,207]. Thus, wearable sensors may also address this need by providing constant measures of heart rate that can be used to inform the calibration and progression of exercise intensity [107,129,208,209]. However, in populations with neurological conditions, autonomic dysregulation and/or the use of certain medications can disrupt the cardiovascular response to exercise [97,210,211,212,213]. In such cases, heart-rate measurements may not accurately reflect the intensity of physical exertion, and some individuals may not be able to achieve the target heart rate recommended by exercise guidelines. While exercise guidelines for people with neurological conditions can also be achieved by exercising, according to how hard someone perceives they are working [97,214], measuring perceived exertion [215,216] requires tools that can capture subjective, self-reported measures of individual experience.
3.2. Ecological Momentary Assessments (EMAs)
Advancements in mobile software technology have led to the development of digitally deployed surveys and questionnaires that can capture self-reported, ecological momentary assessments (EMAs) of symptom perception and mental health outcomes. EMAs evaluate perceptions and behaviors in real time in real-world environments [217,218,219,220,221] and have been used across a broad range of psychological research and clinical practice to evaluate psychological responses to experiences and mental health [217,218,219,220,222,223,224,225]. Software applications, such as RedCap [226] and ExamMed [227], allow researchers and clinicians to build custom EMAs that are sent directly to individuals through their email, text message, or mobile application. Because EMAs operate by prompting users to record their perceptions and thoughts in real time, in real-world environments, this approach minimizes error, such as recall bias, which might influence clinical assessment that requires recall over the course of several days or weeks. In addition, EMAs allows researchers and clinicians to better understand factors that influence symptom perception and mental health by evaluating events that occur in close temporal proximity (before and after) to EMA measurements [219,220,222,223]. Indeed, recent studies have demonstrated how information derived from EMAs can be integrated with activity and geolocation data from wearable sensors to identify key determinants of behavior in community settings, and these studies have started to reveal novel relationships between mood and free-living physical activity [217,219,220,222,228]. While similar evaluation strategies have great potential to inform rehabilitation and exercise strategies, there are currently limited methodologies established to integrate EMAs with multiplex data from multiple instruments in modern wearable sensors.
Integrated remote monitoring systems that combine quantitative data from wearable sensors with subjective data from EMAs could provide new opportunities to explore how physical and mental health are influenced by disease, disability, and related interventions in real-world environments. For example, people with disabilities often report fatigue and pain as significant barriers to participating in rehabilitation and exercise activities [4,229,230,231]. Fatigue and pain can be extremely variable from day to day, and even within a single day [232,233,234,235,236,237], and a few contemporary studies have used daily surveys and remote activity trackers to show that momentary feelings of fatigue and pain can have an immediate, negative impact on daily physical activity [232,236,237]. Yet, the preponderance of studies evaluating fatigue in people with disabilities have relied on questionnaires that ask individuals to provide a summative rating of the fatigue they have experienced over the past 7–28 days [238,239]. Therefore, it is difficult to delineate how fatigue impacts participation in physical activities on a day-to-day basis. Consequently, there is a lack of empirical scientific evidence regarding how daily fluctuations in fatigue and pain might acutely influence exercise participation in people with disabilities, and it is unclear whether long-term adaptations from exercise impact the relationship between fatigue, pain, and non-exercise physical activities in real-world settings in this population. Future studies implementing remote monitoring strategies that integrate physical activity and physiology metrics with perceived outcomes may help identify acute and chronic interactions among rehabilitation and exercise, fatigue, and pain in people with disabilities on a day-to-day basis in real-world settings. Such work could help clinicians develop and deploy more sustainable rehabilitation and exercise strategies for people with disabilities who experience elevated levels of fatigue and pain.
4. Digital Environments and Gaming
Safety, engagement, and adherence are critical components for delivering sustainable and effective rehabilitation and exercise programs. Undoubtedly, rehabilitation and exercise often require activities that are associated with a moderate risk of falling or injury, and people with deficits in motor control, balance, and cognition may be at greater risk during participation in such activities [97,130,240,241,242]. While clinicians can help mitigate risks by providing detailed instructions and physical assistance, remote strategies lack clinical oversight, which may lead to additional challenges related to safety and engagement and that lead to reduced adherence. Moreover, some intentional aspects of rehabilitation and exercise activities may present inherent safety concerns that persist even with professional oversight. For example, rehabilitation strategies that incorporate external stimuli and focus on walking around objects have been shown to improve walking function in people with disabilities [243], but such obstacles may increase the risk of an adverse event such as a fall and/or injury. The level of risk and an individual’s perception of safety during participation in rehabilitation and exercise programs may also contribute to hesitancy, and studies have shown that people with disabilities report safety concerns as a primary factor that contributes to low adherence [39,41,42,52,53]. Thus, maintaining environments that have reduced risk of adverse events and help patients feel safe during their participation in rehabilitation and exercise activities may also improve adherence and engagement.
In addition to safety, people with disabilities also commonly report a lack of motivation as another factor that may negatively impact adherence to rehabilitation and exercise programs [39,41,42,52,53]. During supervised rehabilitation and exercise activities, clinicians play a key role in keeping patients engaged in their activities by providing real-time instructive feedback and incorporating simple, goal-oriented tasks such as games. Specifically, studies have shown that rehabilitation strategies that incorporate feedback, games, and elements of entertainment may increase the intensity at which a specific activity is performed [244,245,246,247]. In this section, we highlight emerging technologies that are providing new opportunities to improve safety, engagement, and adherence in rehabilitation and exercise programs for people with disabilities.
4.1. Virtual and Augmented Reality
Virtual-reality (VR) and augmented-reality (AR) technologies provide an opportunity to address challenges associated with safety, engagement, and adherence in rehabilitation and exercise for people with disabilities [77,245,248,249,250]. VR and AR technologies use computer-generated imagery to create immersive and interactive digital environments. Specifically, VR establishes a digital environment that reflects real or fictional places, whereas AR is the real world viewed through a device, adding computer-generated objects or effects into one’s domain. Both VR and AR offer the opportunity to safely simulate walking or other daily activities, in various digital environments that may include obstacles or everyday scenarios, with a reduced risk of harm [249,251,252,253,254]. Moreover, VR and AR provide a method of integrating fantastical situations and gaming into rehabilitation and exercise activities, to make them more enjoyable and/or entertaining [253,254,255].
While multi-sensory digital experiences date back to the 1990s, recent developments in computer-generated imagery and microprocessors have expanded the quality and mobility of VR/AR technologies, and have consequently expanded their potential applications for rehabilitation and exercise [77,249,250,251,256,257]. Specifically, a growing body of research has demonstrated the safety and efficacy of integrating VR technologies into rehabilitation care plans in several rehabilitation populations, including stroke, multiple sclerosis, rheumatoid arthritis, fibromyalgia, and Parkinson’s disease, among others [77,249,250,251,256,257]. The use of VR technologies as a rehabilitation tool has been shown to improve mobility and function, decrease pain, and improve quality of life [77,249,250,251,256,257]. Moreover, VR may also decrease the burden on caregivers, while raising compliance, motivation, and adherence [250,253], and research indicates that skills learned in virtual environments can translate to the real world [250,253]. Regarding AR, some evidence suggests that AR-based interventions may improve physical function and reduce risk of fall when implemented in conventional physical therapy practice for people with disabilities [245,248]. However, there are a limited number of research studies investigating AR interventions, and the degree to which the integration of AR technology may contribute to additional improvements in outcomes, over and beyond traditional approaches to rehabilitation, remains unclear.
Contemporary VR and AR devices have many attributes that allow them to be used across a variety of rehabilitation and exercise strategies. For example, VR and AR can be combined with numerous accessories, such as omnidirectional treadmills and haptic suits, which incorporate physical activity and feedback with the virtual or augmented experience [258,259,260]. VR and AR devices are also becoming more mobile and integrated into accessories that enable everyday use, such as wireless eyewear, smartphones, and heads-up displays (HUDs) [261,262]. VR systems have also become increasingly accessible for individuals who use wheelchairs or have limited mobility. Specifically, some VR programs now offer seated experiences, making it suitable for those who prefer or require a stationary position. VR developers are also incorporating accessibility features, including adaptive controllers, customizable user interfaces, subtitles, and adjustable avatars.
Systematic reviews evaluating the usability of semi-immersive and fully immersive VR systems have reported strong usability in people with a wide range of neuromotor impairments, particularly when examining user ratings regarding ease-of-use and learnability [263]. Notwithstanding, VR and AR have limitations that reduce their clinical utility and require further development to establish more accessible and efficacious virtual environments for use in rehabilitation and exercise. For example, VR and AR use in rehabilitation and exercise can be associated with motion sickness, loss of human connections, safety issues for those with limited mobility, and cost [264,265,266] which may be particularly problematic for people with disabilities. Fortunately, recent development work has focused on overcoming many of the limitations of VR and AR. Companies such as Meta [267] and Apple [268] are in the process of developing more connected virtual environments, to enable users to remotely interact and exchange information in the metaverse [269,270]. Such innovations have the potential to open a world of possibilities for people with disabilities who have barriers to transportation or limited ability to spend long periods of time away from home. Google has also created Google Cardboard, a smartphone assembly for digital environments made of cardboard that has potential to reduce costs of VR and AR technology [271]. Furthermore, emerging products, such as Teslasuit [272] are now offering full-body haptic experiences that provide comprehensive feedback to multiple anatomical locations. The multidirectional feedback received from the suit is suggested to increase the body’s response to input from VR and/or AR, thus potentially stimulating neural pathways and propagating stronger synaptic connections [273] which are important targets for neurorehabilitation. Collectively, these advancements will lead to more realistic, affordable, and socially connected digital environments, which will establish more accessible and efficacious VR and AR solutions for rehabilitation and exercise.
4.2. Gaming
Digital gaming technologies have been around for over 50 years, and over the past decade, the gamification of rehabilitation and exercise has emerged as a promising strategy to improve engagement and adherence [167,244,248,264,274,275]. Gamification refers to the “use of game design elements in non-game contexts” [275]. In the context of rehabilitation and exercise, gamification may involve the use of quests, points, levels, leaderboards, or badges to guide and encourage users through a series of physical activities [167,244,248,264,274,275]. Users frequently report that gaming elements are fun, exciting, and reduces the monotony that patients may experience during their plan of care, which can increase adherence and performance during rehabilitation and exercise activities [167,244,248,264,274,275]. Moreover, the stimuli of gaming elements themselves may also initiate changes in specific neural adaptations [264]. Specifically, gaming has been shown to release dopamine with each achievement, facilitating neuroplasticity and potentiation of synaptic connections [244], and game-based therapies involve cognitive and motor components that recruit specific neurons of interest, leading to the formation of new neural networks [266].
The benefits of integrating non-immersive digital gaming into rehabilitation and exercise have been evaluated in different clinical populations [276,277], and there are several studies demonstrating how gaming platforms such as Microsoft Kinect and Nintendo Wii can improve physical function and overall wellbeing in people with disabilities [247,278,279,280,281,282]. Advancements in gaming are also offering new opportunities for people to engage in collaborative gaming activities through online, multiplayer games. Online games can overcome social barriers for people with disabilities, while also allowing people with mobility limitations to engage and compete in gamified activities that may be otherwise physically inaccessible [283,284,285]. More recently, clinicians and researchers have also started to explore how gamification of rehabilitation and exercise can be delivered using VR technologies [286,287,288]. Strategies that guide individuals through movements using gamified rehabilitation and exercise activities performed in immersive VR environments offer an approach that provides all of the benefits of gamification in addition to the enhanced user experience and psychophysiological stimuli generated by VR. Although future research is needed to determine the best practices for combining VR and gaming in a rehabilitation context, this integrated strategy has the potential to provide a safe, accessible, and motivating approach for people with disabilities, while also generating robust stimuli to drive adaptations within multiple physiological systems [248,255,274,278].
Taken together, the collective evidence demonstrates how telecommunications, wearables, VR, AR, and gaming may be used either individually, or in combination, to overcome many barriers people with disabilities face regarding rehabilitation and exercise. However, choosing the most effective combination of technological solutions for each individual is dependent on several factors related to personal goals, abilities, and preferences that are shaped by a myriad of physiological, psychological, sociological, and economical factors. In the next section, we highlight new frontiers in precision care strategies that are helping clinicians make data-driven decisions when it comes to selecting the most effective and efficient rehabilitation and exercise strategies for people with disabilities. Yet, the overall value of integrating VR and AR technologies into rehabilitation remains somewhat unclear. Future research focused on demonstrating how VR and AR may enhance outcomes and cost effectiveness, over and beyond conventional care strategies, may facilitate the translation of these technologies into clinical practice.
5. Promoting Accessibility with Co-Development
While digital health technology holds significant promise in increasing accessibility, adoption may not be universally seamless across diverse user groups, and it is crucial to recognize potential barriers that individuals with disabilities may encounter when utilizing technological devices and digital health services. For instance, individuals with mobility impairments or motor control challenges may encounter challenges when physically interacting with small buttons, straps, touchscreens, or complex clasp mechanisms that are often associated with wearable sensors and mobile telecommunications devices. Sensory impairments among individuals with visual or hearing deficits may also limit the usability of digital health technologies that do not offer alternative modalities for information presentation. In addition to physical barriers, people with disabilities may also experience cognitive impairments that make it difficult to navigate complex digital interfaces or manage complex protocols associated with operating and maintaining digital health devices and platforms. Thus, it is critical that future technological innovation efforts are informed by preemptive and parallel research agendas that are focused on understanding the potential barriers to implementing digital health technologies within the population of individuals with disabilities. Co-innovation strategies directly involve people with disabilities in the technological innovation process by conducting a series of focus groups, surveys, and pilot studies to identify the needs, barriers, and preferences of the intended user [117,289,290,291] and systematically evaluate the feasibility, usability, and safety of technologies. Through this iterative process, co-development strategies allow developers to design and refine digital health solutions that are not only effective, but also inclusive and accessible to individuals with disabilities (Figure 2).
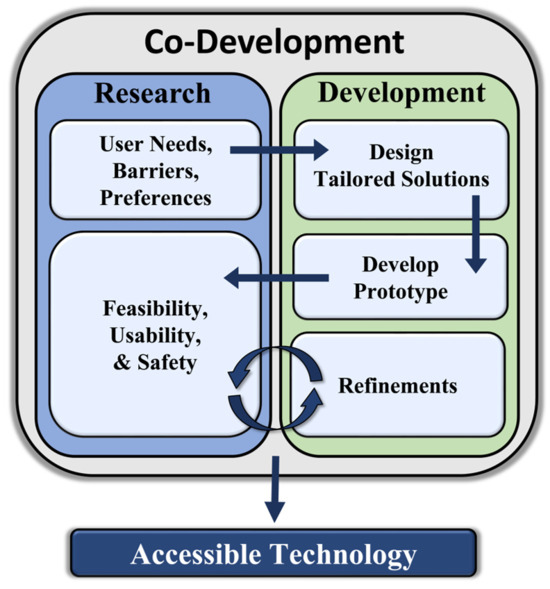
Figure 2.
Overview of co-development process.
6. Precision Care
While substantial progress has been made in establishing evidence-based guidelines for rehabilitation and exercise in people with disability [97,130,207], standardized guidelines only provide general recommendations, which are not directly tailored to the needs, abilities, and goals of each individual. Undoubtedly, people with disabilities can have a wide variety of mobility limitations and physiological disorders that differentially impact how they participate in, and how their body responds to, distinct types of interventions. Thus, there is increasing awareness in the field of physical medicine, rehabilitation, and exercise physiology surrounding the need for individualized, data-driven treatment strategies that are specifically tailored to an individual’s needs, goals, and abilities. To this end, the concept of precision care (i.e., precision medicine/rehabilitation) has emerged as an alternative approach to the generalized, broad application of evidence-based guidelines [292,293,294,295,296]. Precision rehabilitation leverages large, multiplex datasets to establish personalized, data-driven treatment strategies that are optimized to drive outcomes for individuals (Figure 3). For example, one approach to precision rehabilitation is to establish patient subtyping systems, in which subgroups of patients with the same diagnosis are identified and differentially assigned to specific treatments based on their integrated health profile [292,293,294,295,296]. In addition, precision rehabilitation strategies may also support more dynamic approaches to care that incorporate new health data as it is generated to make real-time adjustments to treatment strategies [165,292,294]. In addition to addressing barriers related to the lack of individualized care for people with disabilities, precision rehabilitation treatment strategies may also lead to more efficient and economical clinical practices, by creating data-driven models of care that concentrate resources on the specific rehabilitation services that are most likely to make an impact for each patient. Notwithstanding, precision rehabilitation is a relatively new concept, and, to date, only 13 peer-reviewed articles have been published with “precision rehabilitation” in the title. Substantial research and innovation efforts will be required in this area to establish pathology-specific best practices and develop robust technical solutions that can support the implementation of precision rehabilitation at scale.
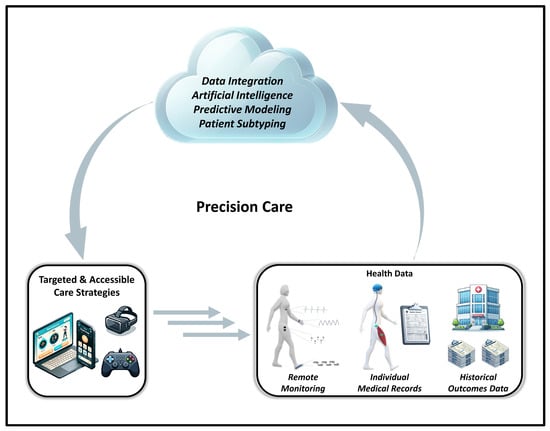
Figure 3.
Overview of how precision rehabilitation strategies can use integrated health data to inform targeted and accessible approaches to rehabilitation and exercise, for people with disabilities.
A primary limitation to precision rehabilitation strategies is the data collection, as well as the processing and analysis bottleneck that is created by establishing the large, multiplex datasets that result from integrating several sources of health information [292,297]. For example, precision rehabilitation strategies such as patient subtyping must be able to determine which types of treatments generate the most favorable outcomes for specific patient subtypes. Because patient outcomes depend on several physiological, functional, social, and environmental factors, generating accurate predictive models requires valid data and robust analytical approaches that can perform complex, iterative analyses to identify patterns and predictors within large, multiplex datasets that can cover several years of time. Therefore, the practical implementation of precision rehabilitation necessitates the development of automated, user-friendly data collection and analytical systems that can deliver actionable information to clinicians without the need for hours of manual or supervised data collection and analysis [292,297,298,299,300].
Artificial Intelligence and Cloud Computing
Emerging evidence is starting to reveal how applications of artificial intelligence (AI) may be used to automate analyses and identify patterns within large, integrated health datasets, which may be difficult for humans to otherwise detect. AI broadly refers to a form of computer science where algorithms are trained to perform complex tasks, such as pattern recognition. For example, applications of AI have made substantial contributions to the fields of oncology, neurology, and immunopathology, by revolutionizing, and automating, our capacity to rapidly and accurately analyze high-resolution images generated by medical imaging instruments [301,302,303,304,305,306,307]. Historically, biomedical imaging relied on manual analyses by physicians and scientists to identify and quantify biomarkers within large images containing many different relevant and irrelevant features. AI transformed this practice by using algorithm-based image analysis to drastically increase the rate, scale, and sensitivity of biomarker detection [301,307,308,309,310].
Similar to images, integrated health datasets used in precision rehabilitation strategies also include massive amounts of information that may or may not be relevant to an individuals’ prognosis. Therefore, it would follow that applications of AI also have the potential to drastically increase the rate, scale, and overall capacity to extract clinically meaningful information from datasets consisting of hundreds of metrics derived from multiple different sources. For example, recent work has demonstrated how AI algorithms can use multifactorial data derived from electronic medical records (EMRs) to predict hospitalization [311,312], drug efficacy [313], and the development of critical illness [314] in several different clinical populations. One limitation of this approach is that AI predictive models often provide little information about which factors may be driving the outcomes. Indeed, the algorithms used in medical applications of AI consider hundreds of factors, and systematically evaluate interactions and patterns among hundreds of factors including demographics, lab results, co-morbidities, social determinants of health, and medical images, among many others. However, identifying key factors associated with positive or negative outcomes is critical to designing targeted treatment strategies for people with disabilities. To this end, there have been recent efforts to employ “explainable” AI models that systematically identify key factors that are driving the predictive power of the algorithms [315,316]. For example, while it may be helpful to know which patients will adhere to their rehabilitation or exercise program, systems that use explanatory AI may help identify the primary reason patients are, or are not, adhering to protocols, which may inform future intervention strategies.
Although there is increasing interest surrounding the use of AI in precision rehabilitation [292,294,296], the application of AI in rehabilitation is still in the nascent stages of exploration. Several previous studies have demonstrated the utility of using machine learning to forecast outcomes related to walking function, assistive-device use, discharge potential, and falls risk in people with disabilities [315,317,318,319,320,321,322], and such applications of AI can certainly inform the design and management of individualized rehabilitation treatment strategies. Yet, further work is needed to systematically evaluate the safety and efficacy of using AI to direct an individual’s care through processes such as patient subtyping or AI-directed FES calibration [292]. Moreover, existing studies have focused on applying AI to datasets derived from a single source, such as EMRs, wearables, or specialized clinical measurement devices [315,317,318,319,320,321,322]. Undoubtedly, approaches that integrate data from these different sources, and others, would offer a more comprehensive profile of the patient, and may improve the precision of AI-generated predictive models aiming to forecast outcomes or determine the optimal care path for a particular patient subtype. In addition, data integration may also inform dynamic-precision rehabilitation strategies. For example, combining static EMR data with real-time data from remote patient-monitoring systems could provide deep insight regarding the acute and chronic impact of a rehabilitation treatment session if such data is integrated with the treatment schedule or information from tele-rehabilitation/tele-exercise platform [165]. Therefore, in addition to the need for complex, automated analytics, another substantial challenge for precision rehabilitation is the need for a robust technical infrastructure that can rapidly integrate, organize, and store large, multiplex datasets, in a readily accessible and analyzable format.
Cloud-based data repositories and computing systems have emerged as a promising solution to the data storage and processing demands of precision rehabilitation strategies that aim to leverage big data [323,324,325,326]. For example, EMR systems are often isolated from other sources of health information such as data derived from rehabilitation equipment and remote patient-monitoring systems. Moreover, even the most simplistic wearable devices can generate thousands of data points per day per patient, which can cause data storage and processing difficulties for consumer-level desktop computers. Contemporary cloud systems, such as those offered by Microsoft Azure [327] and Amazon Web Services [328], provide a robust, versatile infrastructure for addressing data integration, storage, and processing needs. Furthermore, the AI strategies highlighted above require immense computational power to support the complex, iterative analytical approaches used to identify patterns and predictors within large, multiplex datasets. While historical medical records contain a wealth of information related to patient outcomes, even the most sophisticated EMR systems lack the analytical tools and AI capacities needed to turn historical outcomes data into predictive algorithms for precision rehabilitation. Contemporary cloud-based computing services also address this need by offering access to nearly unlimited computational power on an as-needed basis. As healthcare technology continues to evolve, the complexity and size of the health data ecosystem will only continue to grow, and thus, data-driven approaches to healthcare also require versatile solutions that rapidly adapt and match the capacity of the continuously evolving data sources. To this end, the capabilities of cloud computing provide the technical infrastructure needed to establish scalable, big-data solutions to support contemporary and future approaches to precision medicine.
7. Conclusions
Emerging digital health technologies have the potential to significantly impact the accessibility and precision of rehabilitation and exercise strategies for people with disabilities. Telecommunications technologies are expanding access to care by providing remote rehabilitation and exercise services to individuals in remote or underserved areas, or to those who are unable to access in-person rehabilitation services, due to mobility or other accessibility barriers. Wearable devices and sensors can be used to monitor a person’s progress, provide real-time feedback, and generate data to inform the progression of rehabilitation and exercise programs. Furthermore, VR and AR offer immersive and interactive digital environments that can be used in rehabilitation and exercise to guide users through specific movements, simulate real-world scenarios, and integrate elements of gaming and entertainment into their training. Advancements in artificial intelligence and cloud computing are also improving rehabilitation and exercise for people with disabilities by enabling data-driven approaches to precision rehabilitation that are directly tailored to the needs and goals of the individual. Overall, emerging technologies have the potential to enhance and expand the capabilities of rehabilitation professionals, and to provide new and innovative ways to help individuals with disabilities or injuries achieve their goals and improve their quality of life.
Author Contributions
Conceptualization, T.B.W., J.S., G.C. and D.B.; methodology, T.B.W. and J.S.; formal analysis, T.B.W. and J.S.; investigation, T.B.W. and J.S.; resources, T.B.W., J.S., G.C. and D.B.; data curation, T.B.W. and J.S.; writing—original draft preparation, T.B.W. and J.S.; writing—review and editing, T.B.W., J.S., G.C. and D.B.; visualization, T.B.W.; supervision, T.B.W.; project administration, T.B.W.; funding acquisition, T.B.W., G.C. and D.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute on Disability, Independent Living and Rehabilitation Research grant number 90REGE0011.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- CDC. Disability and Health Data System (DHDS). Available online: https://www.cdc.gov/ncbddd/disabilityandhealth/dhds/index.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fncbddd%2Fdisabilityandhealth%2Fdhds.html (accessed on 17 May 2022).
- Lai, B.; Young, H.J.; Bickel, C.S.; Motl, R.W.; Rimmer, J.H. Current Trends in Exercise Intervention Research, Technology, and Behavioral Change Strategies for People with Disabilities: A Scoping Review. Am. J. Phys. Med. Rehabil. 2017, 96, 748–761. [Google Scholar] [CrossRef]
- Hassett, L.; Moseley, A.; Harmer, A. The Aetiology of Reduced Cardiorespiratory Fitness Among Adults with Severe Traumatic Brain Injury and the Relationship with Physical Activity: A Narrative Review. Brain Impair. 2016, 17, 43–54. [Google Scholar] [CrossRef]
- Klaren, R.E.; Motl, R.W.; Dlugonski, D.; Sandroff, B.M.; Pilutti, L.A. Objectively quantified physical activity in persons with multiple sclerosis. Arch. Phys. Med. Rehabil. 2013, 94, 2342–2348. [Google Scholar] [CrossRef]
- Carroll, D.D.; Courtney-Long, E.A.; Stevens, A.C.; Sloan, M.L.; Lullo, C.; Visser, S.N.; Fox, M.H.; Armour, B.S.; Campbell, V.A.; Brown, D.R.; et al. Vital Signs: Disability and Physical Activity—United States, 2009–2012. Morb. Mortal. Wkly. Rep. 2014, 63, 407. [Google Scholar]
- CDC. Many Adults with Disabilities Report Frequent Mental Distress. Available online: https://www.cdc.gov/ncbddd/disabilityandhealth/features/adults-with-disabilities-mental-distress.html (accessed on 20 April 2022).
- Motl, R.W. Physical activity and irreversible disability in multiple sclerosis. Exerc. Sport Sci. Rev. 2010, 38, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Arnett, P.A.; Smith, M.M.; Barwick, F.H.; Ahlstrom, B.; Stover, E.J. Worsening of symptoms is associated with lower physical activity levels in individuals with multiple sclerosis. Mult. Scler. J. 2008, 14, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Dlugonski, D.; Weikert, M.; Suh, Y.; Balantrapu, S.; Motl, R.W. Physical activity and multiple sclerosis: New insights regarding inactivity. Acta Neurol. Scand. 2012, 126, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Klaren, R.E.; Motl, R.W. Relationships among physical inactivity, deconditioning, and walking impairment in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2015, 39, 103–110. [Google Scholar] [CrossRef]
- Bruns, D.R.; Ehrlicher, S.E.; Khademi, S.; Biela, L.M.; Peelor, F.F.; Miller, B.F.; Hamilton, K.L. Long-duration bed rest modifies sympathetic neural recruitment strategies in male and female participants. J. Appl. Physiol. 2018, 124, 661–671. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Dempsey, L.; Fraser, D.; Zheng, D.; Hamilton, G.; Lambert, J.; Dohm, L. Changes in muscle strength, muscle fibre size and myofibrillar gene expression after immobilization and retraining in humans. J. Physiol. 2000, 524, 293–304. [Google Scholar] [CrossRef]
- Manganotti, P.; Stella, A.B.; Ajcevic, M.; di Girolamo, F.G.; Biolo, G.; Franchi, M.V.; Monti, E.; Sirago, G.; Marusic, U.; Simunic, B.; et al. Peripheral nerve adaptations to 10 days of horizontal bed rest in healthy young adult males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R495–R503. [Google Scholar] [CrossRef]
- Okoro, C.A.; McKnight-Eily, L.R.; Strine, T.W.; Crews, J.E.; Holt, J.B.; Balluz, L.S. State and local area estimates of depression and anxiety among adults with disabilities in 2006. Disabil. Health J. 2011, 4, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.H.; Jones, P.A.; Middleton, R.M.; Ford, D.V.; Tuite-Dalton, K.; Lockhart-Jones, H.; Peng, J.; Lyons, R.A.; John, A.; Noble, J.G. Physical Disability, Anxiety and Depression in People with MS: An Internet-Based Survey via the UK MS Register. PLoS ONE 2014, 9, e104604. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.C.; Roy, D.; Kontos, N.; Beach, S.R. Post-stroke depression: A 2020 updated review. Gen. Hosp. Psychiatry 2020, 66, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, H.; Zhao, X. Psychological morbidities and positive psychological outcomes in people with traumatic spinal cord injury in Mainland China. Spinal Cord 2018, 56, 704–711. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Middleton, J. Psychological morbidity and spinal cord injury: A systematic review. Spinal Cord 2009, 47, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Mckechnie, P.S.; John, A. Anxiety and depression following traumatic limb amputation: A systematic review. Injury 2014, 45, 1859–1866. [Google Scholar] [CrossRef]
- Christensen, J.; Ipsen, T.; Doherty, P.; Langberg, H. Physical and social factors determining quality of life for veterans with lower-limb amputation(s): A systematic review. Disabil. Rehabil. 2016, 38, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Martin Ginis, K.A.; Jörgensen, S.; Stapleton, J. Exercise and Sport for Persons with Spinal Cord Injury. PMR 2012, 4, 894–900. [Google Scholar] [CrossRef]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Ginis, K.A.M.; Fenuta, A.M.; Mackibbon, K.A.; Motl, R.W. Effects of Exercise Training on Fitness, Mobility, Fatigue, and Health-Related Quality of Life Among Adults with Multiple Sclerosis: A Systematic Review to Inform Guideline Development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828. [Google Scholar] [CrossRef]
- Mora, J.C.; Valencia, W.M. Exercise and Older Adults. Clin. Geriatr. Med. 2018, 34, 145–162. [Google Scholar] [CrossRef]
- Carbone, S.; del Buono, M.G.; Ozemek, C.; Lavie, C.J. Obesity, risk of diabetes and role of physical activity, exercise training and cardiorespiratory fitness. Prog. Cardiovasc. Dis. 2019, 62, 327–333. [Google Scholar] [CrossRef]
- Kandola, A.; Vancampfort, D.; Herring, M.; Rebar, A.; Hallgren, M.; Firth, J.; Stubbs, B. Moving to Beat Anxiety: Epidemiology and Therapeutic Issues with Physical Activity for Anxiety. Curr. Psychiatry Rep. 2018, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine–Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Klaren, R.E.; Pilutti, L.A.; Dlugonski, D.; Benedict, R.H.B.; Motl, R.W. Randomized controlled trial of physical activity, cognition, and walking in multiple sclerosis. J. Neurol. 2013, 261, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, C.Y.; Greguol, M. Physical activity, quality of life, and functional autonomy of adults with spinal cord injuries. Adapt. Phys. Act. Q. APAQ 2013, 30, 317–337. [Google Scholar] [CrossRef] [PubMed]
- Sancassiani, F.; Machado, S.; Preti, A. Physical Activity, Exercise and Sport Programs as Effective Therapeutic Tools in Psychosocial Rehabilitation. Clin. Pract. Epidemiol. Ment. Health 2018, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Sandroff, B.M.; Motl, R.W.; Scudder, M.R.; DeLuca, J. Systematic, Evidence-Based Review of Exercise, Physical Activity, and Physical Fitness Effects on Cognition in Persons with Multiple Sclerosis. Neuropsychol. Rev. 2016, 26, 271–294. [Google Scholar] [CrossRef]
- Durstine, J.L.; Painter, P.; Franklin, B.A.; Morgan, D.; Pitetti, K.H.; Roberts, S.O. Physical activity for the chronically ill and disabled. Sports Med. 2000, 30, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Nash, M.S. Exercise as a health-promoting activity following spinal cord injury. J. Neurol. Phys. Ther. 2005, 29, 87–106. [Google Scholar] [CrossRef]
- Naschitz, J.E.; Lenger, R. Why traumatic leg amputees are at increased risk for cardiovascular diseases. Int. J. Med. 2008, 101, 251–259. [Google Scholar] [CrossRef]
- Zhang, Q.; Schwade, M.; Smith, Y.; Wood, R.; Young, L. Exercise-based interventions for post-stroke social participation: A systematic review and network meta-analysis. Int. J. Nurs. Stud. 2020, 111, 103738. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Zhang, W.; Kang, L.; Ma, Y.; Fu, L.; Jia, L.; Yu, H.; Chen, X.; Hou, L.; Wang, L.; et al. Clinical Evidence of Exercise Benefits for Stroke. Adv. Exp. Med. Biol. 2017, 1000, 131–151. [Google Scholar] [CrossRef]
- Vanderbeken, I.; Kerckhofs, E. A systematic review of the effect of physical exercise on cognition in stroke and traumatic brain injury patients. NeuroRehabilitation 2017, 40, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Duquette, P.; Andersen, R.; Lapierre, Y.; Mayo, N.E. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil. Rehabil. 2013, 35, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.M.; Newman, M.A.; Hirsch, M.A. Perceived Barriers to Exercise in Adults with Traumatic Brain Injury Vary by Age. J. Funct. Morphol. Kinesiol. 2018, 3, 47. [Google Scholar] [CrossRef]
- Jaarsma, E.A.; Dijkstra, P.U.; Geertzen, J.H.B.; Dekker, R. Barriers to and facilitators of sports participation for people with physical disabilities: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, 871–881. [Google Scholar] [CrossRef]
- Diblasio, C.; Novack, T.; Bogner, J.; Johnson-Greene, D.; Felix, E.; Mellick, D.; Corrigan, J.; Kennedy, R.; Cox, M.; Dreer, L.; et al. Barriers to Regular Exercise from Pre-Injury Across 2-Year Recovery Among TBI Survivors: A NIDILRR Investigation. Arch. Phys. Med. Rehabil. 2019, 100, e46. [Google Scholar] [CrossRef]
- Vissers, M.; van den Berg-Emons, R.; Sluis, T.; Bergen, M.; Stam, H.; Bussmann, H. Barriers to and facilitators of everyday physical activity in persons with a spinal cord injury after discharge from the rehabilitation centre. J. Rehabil. Med. 2008, 40, 461–467. [Google Scholar] [CrossRef]
- Martin, J.J. Benefits and barriers to physical activity for individuals with disabilities: A social-relational model of disability perspective. Disabil. Rehabil. 2013, 35, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, C.E.; Gerber, E.G.; Smith, B.C. Designed to deter. Community barriers to physical activity for people with visual or motor impairments. Am. J. Prev. Med. 2008, 34, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Dolbow, D.R.; Figoni, S.F. Accommodation of wheelchair-reliant individuals by community fitness facilities. Spinal Cord 2015, 53, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Rimmer, J.H.; Riley, B.; Wang, E.; Rauworth, A. Development and validation of AIMFREE: Accessibility Instruments Measuring Fitness and Recreation Environments. Disabil. Rehabil. 2004, 26, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Arbour-Nicitopoulos, K.P.; Ginis, K.A.M. Universal accessibility of “accessible” fitness and recreational facilities for persons with mobility disabilities. Adapt. Phys. Act. Q. APAQ 2011, 28, 1–15. [Google Scholar] [CrossRef]
- Rosenberg, D.E.; Huang, D.L.; Simonovich, S.D.; Belza, B. Outdoor built environment barriers and facilitators to activity among midlife and older adults with mobility disabilities. Gerontologist 2013, 53, 268–279. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Padalabalanarayanan, S.; Malone, L.A.; Mehta, T. Fitness facilities still lack accessibility for people with disabilities. Disabil. Health J. 2017, 10, 214–221. [Google Scholar] [CrossRef]
- Perry, M.A.; Devan, H.; Fitzgerald, H.; Han, K.; Liu, L.T.; Rouse, J. Accessibility and usability of parks and playgrounds. Disabil. Health J. 2018, 11, 221–229. [Google Scholar] [CrossRef]
- Martin Ginis, K.A.; Ma, J.K.; Latimer-Cheung, A.E.; Rimmer, J.H. A systematic review of review articles addressing factors related to physical activity participation among children and adults with physical disabilities. Health Psychol. Rev. 2016, 10, 478–494. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Riley, B.; Wang, E.; Rauworth, A.; Jurkowski, J. Physical activity participation among persons with disabilities: Barriers and facilitators. Am. J. Prev. Med. 2004, 26, 419–425. [Google Scholar] [CrossRef]
- Stroud, N.; Minahan, C.; Sabapathy, S. The perceived benefits and barriers to exercise participation in persons with multiple sclerosis. Disabil. Rehabil. 2009, 31, 2216–2222. [Google Scholar] [CrossRef]
- Anderson, C.; Grant, R.L.; Hurley, M.V. Exercise facilities for neurologically disabled populations—Perceptions from the fitness industry. Disabil. Health J. 2017, 10, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Stoelzle, H.Y.; Finco, K.; Foss, S.E.; Carstens, K. ADA Compliance and Accessibility of Fitness Facilities in Western Wisconsin. Top. Spinal Cord. Inj. Rehabil. 2012, 18, 340. [Google Scholar] [CrossRef] [PubMed]
- Calder, A.; Sole, G.; Mulligan, H. The accessibility of fitness centers for people with disabilities: A systematic review. Disabil. Health J. 2018, 11, 525–536. [Google Scholar] [CrossRef]
- Peretti, A.; Amenta, F.; Tayebati, S.K.; Nittari, G.; Mahdi, S.S. Telerehabilitation: Review of the State-of-the-Art and Areas of Application. JMIR Rehabil. Assist. Technol. 2017, 4, e7. [Google Scholar] [CrossRef] [PubMed]
- Kairy, D.; Lehoux, P.; Vincent, C.; Visintin, M. A systematic review of clinical outcomes, clinical process, healthcare utilization and costs associated with telerehabilitation. Disabil. Rehabil. 2009, 31, 427–447. [Google Scholar] [CrossRef] [PubMed]
- Suso-Martí, L.; La Touche, R.; Herranz-Gómez, A.; Angulo-Díaz-Parreño, S.; Paris-Alemany, A.; Cuenca-Martínez, F. Effectiveness of Telerehabilitation in Physical Therapist Practice: An Umbrella and Mapping Review with Meta-Meta-Analysis. Phys. Ther. 2021, 101, pzab075. [Google Scholar] [CrossRef]
- Jones, M.; Deruyter, F.; Morris, J. The Digital Health Revolution and People with Disabilities: Perspective from the United States. Int. J. Environ. Res. Public Health 2020, 17, 381. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Kesselring, J.; Galea, M.P.G. Telerehabilitation for persons with multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, 311–325. [Google Scholar] [CrossRef]
- Amatya, B.; Galea, M.P.; Kesselring, J.; Khan, F. Effectiveness of telerehabilitation interventions in persons with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2015, 4, 358–369. [Google Scholar] [CrossRef]
- Di Tella, S.; Pagliari, C.; Blasi, V.; Mendozzi, L.; Rovaris, M.; Baglio, F. Integrated telerehabilitation approach in multiple sclerosis: A systematic review and meta-analysis. J. Telemed. Telecare 2020, 26, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Young, P.; Kiecker, J. Telerehabilitation for Amputee Care. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Sarfo, F.S.; Ulasavets, U.; Opare-Sem, O.K.; Ovbiagele, B. Tele-Rehabilitation after Stroke: An Updated Systematic Review of the Literature. J. Stroke Cerebrovasc. Dis. 2018, 27, 2306–2318. [Google Scholar] [CrossRef] [PubMed]
- Hwang, R.; Gane, E.M.; Morris, N.R. No transport? No worries! Cardiac telerehabilitation is a feasible and effective alternative to centre-based programs. Heart Fail. Rev. 2023, 28, 1277–1284. [Google Scholar] [CrossRef] [PubMed]
- Tate, D.F.; Finkelstein, E.A.; Khavjou, O.; Gustafson, A. Cost effectiveness of internet interventions: Review and recommendations. Ann. Behav. Med. 2009, 38, 40–45. [Google Scholar] [CrossRef]
- Richardson, C.R.; Buis, L.R.; Janney, A.W.; Goodrich, D.E.; Sen, A.; Hess, M.L.; Mehari, K.S.; Fortlage, L.A.; Resnick, P.J.; Zikmund-Fisher, B.J.; et al. An online community improves adherence in an internet-mediated walking program. Part 1: Results of a randomized controlled trial. J. Med. Internet Res. 2010, 12, e71. [Google Scholar] [CrossRef]
- Subbarao, B.S.; Stokke, J.; Martin, S.J. Telerehabilitation in Acquired Brain Injury. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 223–238. [Google Scholar] [CrossRef]
- Morris, J.; Jones, M.; Thompson, N.; Wallace, T.; Deruyter, F. Clinician Perspectives on mRehab Interventions and Technologies for People with Disabilities in the United States: A National Survey. Int. J. Environ. Res. Public Health 2019, 16, 4220. [Google Scholar] [CrossRef]
- Chae, S.H.; Kim, Y.; Lee, K.S.; Park, H.S. Development and Clinical Evaluation of a Web-Based Upper Limb Home Rehabilitation System Using a Smartwatch and Machine Learning Model for Chronic Stroke Survivors: Prospective Comparative Study. JMIR Mhealth Uhealth 2020, 8, e17216. [Google Scholar] [CrossRef]
- Tchero, H.; Teguo, M.T.; Lannuzel, A.; Rusch, E. Telerehabilitation for Stroke Survivors: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2018, 20, e10867. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Kaur, I.; Baynton, S.L.; Fairchild, T.; Paul, L.; van Rens, F. Changing Behaviour towards Aerobic and Strength Exercise (BASE): Design of a randomised, phase I study determining the safety, feasibility and consumer-evaluation of a remotely-delivered exercise programme in persons with multiple sclerosis. Contemp. Clin. Trials. 2021, 102, 106281. [Google Scholar] [CrossRef] [PubMed]
- Uslu, A.S.; Gerber, S.M.; Schmidt, N.; Röthlisberger, C.; Wyss, P.; Vanbellingen, T.; Schaller, S.; Wyss, C.; Koenig-Bruhin, M.; Berger, T.; et al. Investigating a new tablet-based telerehabilitation app in patients with aphasia: A randomised, controlled, evaluator-blinded, multicentre trial protocol. BMJ Open 2020, 10, e037702. [Google Scholar] [CrossRef] [PubMed]
- Tallner, A.; Pfeifer, K.; Mäurer, M. Web-based interventions in multiple sclerosis: The potential of tele-rehabilitation. Ther. Adv. Neurol. Disord. 2016, 9, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Del Pino, R.; Díez-Cirarda, M.; Ustarroz-Aguirre, I.; Gonzalez-Larragan, S.; Caprino, M.; Busnatu, S.; Gand, K.; Schlieter, H.; Gabilondo, I.; Gómez-Esteban, J.C. Costs and effects of telerehabilitation in neurological and cardiological diseases: A systematic review. Front. Med. 2022, 9, 832229. [Google Scholar] [CrossRef]
- Hao, J.; Pu, Y.; Chen, Z.; Siu, K.C. Effects of virtual reality-based telerehabilitation for stroke patients: A systematic review and meta-analysis of randomized controlled trials. J. Stroke Cerebrovasc. Dis. 2023, 32, 106960. [Google Scholar] [CrossRef]
- Annaswamy, T.M.; Pradhan, G.N.; Chakka, K.; Khargonkar, N.; Borresen, A.; Prabhakaran, B. Using Biometric Technology for Telehealth and Telerehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 437–449. [Google Scholar] [CrossRef]
- Yeroushalmi, S.; Maloni, H.; Costello, K.; Wallin, M.T. Telemedicine and multiple sclerosis: A comprehensive literature review. J. Telemed. Telecare. 2020, 26, 400–413. [Google Scholar] [CrossRef]
- Rimmer, J.H.; Wilroy, J.; Galea, P.; Jeter, A.; Lai, B.W. Retrospective evaluation of a pilot eHealth/mHealth telewellness program for people with disabilities: Mindfulness, Exercise, and Nutrition to Optimize Resilience (MENTOR). Mhealth 2022, 8, 15. [Google Scholar] [CrossRef]
- Ownsworth, T.; Arnautovska, U.; Beadle, E.; Shum, D.H.K.; Moyle, W. Efficacy of Telerehabilitation for Adults with Traumatic Brain Injury: A Systematic Review. J. Head Trauma Rehabil. 2018, 33, E33–E46. [Google Scholar] [CrossRef]
- McDermott, M.M.; Spring, B.; Berger, J.S.; Treat-Jacobson, D.; Conte, M.S.; Creager, M.A.; Criqui, M.H.; Ferrucci, L.; Gornik, H.L.; Guralnik, J.M.; et al. Effect of a Home-Based Exercise Intervention of Wearable Technology and Telephone Coaching on Walking Performance in Peripheral Artery Disease: The HONOR Randomized Clinical Trial. JAMA 2018, 319, 1665–1676. [Google Scholar] [CrossRef]
- Nussbaum, R.; Kelly, C.; Quinby, E.; Mac, A.; Parmanto, B.; Dicianno, B.E. Systematic Review of Mobile Health Applications in Rehabilitation. Arch. Phys. Med. Rehabil. 2019, 100, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Agostini, M.; Moja, L.; Banzi, R.; Pistotti, V.; Tonin, P.; Venneri, A.; Turolla, A. Telerehabilitation and recovery of motor function: A systematic review and meta-analysis. J. Telemed. Telecare 2015, 21, 202–213. [Google Scholar] [CrossRef]
- Huijgen, B.C.H.; Vollenbroek-Hutten, M.M.R.; Zampolini, M.; Opisso, E.; Bernabeu, M.; van Nieuwenhoven, J.; Ilsbroukx, S.; Magni, R.; Giacomozzi, C.; Marcellari, V.; et al. Feasibility of a home-based telerehabilitation system compared to usual care: Arm/hand function in patients with stroke, traumatic brain injury and multiple sclerosis. J. Telemed. Telecare 2008, 14, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Paul, L.; Coulter, E.H.; Miller, L.; McFadyen, A.; Dorfman, J.; Mattison, P.G.G. Web-based physiotherapy for people moderately affected with Multiple Sclerosis; quantitative and qualitative data from a randomized, controlled pilot study. Clin. Rehabil. 2014, 28, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Larsson-Lund, M.; Månsson Lexell, E.; Nyman, A. Strategies for Empowering activities in Everyday life (SEE 1.0): Study protocol for a feasibility study of an Internet-based occupational therapy intervention for people with stroke. Pilot Feasibility Stud. 2021, 7, 187. [Google Scholar] [CrossRef]
- Mirbaha, S.; Morgan, A.; Tang, A.; Smith-Turchyn, J.; Richardson, J. Models of Telehealth Service Delivery in Adults with Spinal Cord Injuries: Scoping Review. JMIR Rehabil. Assist. Technol. 2023, 10, e41186. [Google Scholar] [CrossRef]
- Jekauc, D.; Rayling, S.; Klopp, S.; Schmidt, D.; Rittmann, L.M.; Fritsch, J. Effects of a web-based rehabilitation aftercare on subjective health, work ability and motivation: A partially randomized controlled trial. BMC Musculoskelet. Disord. 2021, 22, 366. [Google Scholar] [CrossRef]
- Menengiç, K.N.; Yeldan, İ.; Çınar, N.; Şahiner, T. Effectiveness of motor-cognitive dual-task exercise via telerehabilitation in Alzheimer’s disease: An online pilot randomized controlled study. Clin. Neurol. Neurosurg. 2022, 223, 107501. [Google Scholar] [CrossRef]
- Celikel, R.; Ramazanoglu, E.; Talu, B. The effect of motor learning-based telerehabilitation on quality of life of children with cerebral palsy during the COVID-19 pandemic. Arch. Pediatr. 2023, 30, 383–388. [Google Scholar] [CrossRef]
- Bricker, J.; Miao, Z.; Mull, K.; Santiago-Torres, M.; Vock, D.M. Can a Single Variable Predict Early Dropout from Digital Health Interventions? Comparison of Predictive Models from Two Large Randomized Trials. J. Med. Internet Res. 2023, 25, e43629. [Google Scholar] [CrossRef]
- Tan, C.O.; Meehan, W.P.; Iverson, G.L.; Taylor, J.A. Cerebrovascular regulation, exercise, and mild traumatic brain injury. Neurology 2014, 83, 1665–1672. [Google Scholar] [CrossRef] [PubMed]
- Learmonth, Y.C.; Motl, R.W. Physical activity and exercise training in multiple sclerosis: A review and content analysis of qualitative research identifying perceived determinants and consequences. Disabil. Rehabil. 2016, 38, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Edwards, T.; Pilutti, L.A. The effect of exercise training in adults with multiple sclerosis with severe mobility disability: A systematic review and future research directions. Mult. Scler. Relat. Disord. 2017, 16, 31–39. [Google Scholar] [CrossRef]
- Rimmer, J.; Lai, B. Framing new pathways in transformative exercise for individuals with existing and newly acquired disability. Disabil. Rehabil. 2017, 39, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lai, B.; Mehta, T.; Thirumalai, M.; Padalabalanarayanan, S.; Rimmer, J.H.; Motl, R.W. Exercise Training Guidelines for Multiple Sclerosis, Stroke, and Parkinson Disease: Rapid Review and Synthesis. Am. J. Phys. Med. Rehabil. 2019, 98, 613–621. [Google Scholar] [CrossRef]
- Wise, E.K.; Hoffman, J.M.; Powell, J.M.; Bombardier, C.H.; Bell, K.R. Benefits of exercise maintenance after traumatic brain injury. Arch. Phys. Med. Rehabil. 2012, 93, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Willingham, T.B.; McCully, K.K. In Vivo Assessment of Mitochondrial Dysfunction in Clinical Populations Using Near-Infrared Spectroscopy. Front. Physiol. 2017, 8, 689. [Google Scholar] [CrossRef]
- Willingham, T.B.; Backus, D.; McCully, K.K. Muscle Dysfunction and Walking Impairment in Women with Multiple Sclerosis. Int. J. MS Care 2019, 21, 249–256. [Google Scholar] [CrossRef]
- Willingham, T.B.; Melbourn, J.; Moldavskiy, M.; McCully, K.K.; Backus, D. Effects of Treadmill Training on Muscle Oxidative Capacity and Endurance in People with Multiple Sclerosis with Significant Walking Limitations. Int. J. MS Care 2019, 21, 166. [Google Scholar] [CrossRef]
- Backus, D. Increasing Physical Activity and Participation in People with Multiple Sclerosis: A Review. Arch. Phys. Med. Rehabil. 2016, 97 (Suppl. S9), S210–S217. [Google Scholar] [CrossRef]
- Backus, D.; Moldavskiy, M.; Sweatman, W.M. Effects of Functional Electrical Stimulation Cycling on Fatigue and Quality of Life in People with Multiple Sclerosis Who Are Nonambulatory. Int. J. MS Care 2020, 22, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Willingham, T.B.; Melbourn, J.; Moldavskiy, M.; McCully, K.K.; Backus, D. Case Report: Effect of Antigravity Treadmill Training on Muscle Oxidative Capacity, Muscle Endurance, and Walking Function in a Person with Multiple Sclerosis. Int. J. MS Care 2018, 20, 186–190. [Google Scholar] [CrossRef]
- Reynolds, M.A.; McCully, K.; Burdett, B.; Manella, C.; Hawkins, L.; Backus, D. Pilot study: Evaluation of the effect of functional electrical stimulation cycling on muscle metabolism in nonambulatory people with multiple sclerosis. Arch. Phys. Med. Rehabil. 2015, 96, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Backus, D.; Burdett, B.; Hawkins, L.; Manella, C.; McCully, K.K.; Sweatman, M. Outcomes After Functional Electrical Stimulation Cycle Training in Individuals with Multiple Sclerosis Who Are Nonambulatory. Int. J. MS Care 2017, 19, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Negaresh, R.; Motl, R.; Mokhtarzade, M.; Ranjbar, R.; Majdinasab, N.; Khodadoost, M.; Zimmer, P.; Baker, J.S.; Patel, D. Effect of Short-Term Interval Exercise Training on Fatigue, Depression, and Fitness in Normal Weight vs. Overweight Person with Multiple Sclerosis. Explore 2019, 15, 134–141. [Google Scholar] [CrossRef]
- Motl, R.W.; Sandroff, B.M. Randomized controlled trial of physical activity intervention effects on fatigue and depression in multiple sclerosis: Secondary analysis of data from persons with elevated symptom status. Contemp. Clin. Trials Commun. 2020, 17, 100521. [Google Scholar] [CrossRef] [PubMed]
- Motl, R.W.; Sandroff, B.M. Benefits of Exercise Training in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2015, 15, 62. [Google Scholar] [CrossRef]
- Perry, S.A.; Coetzer, R.; Saville, C.W.N. The effectiveness of physical exercise as an intervention to reduce depressive symptoms following traumatic brain injury: A meta-analysis and systematic review. Neuropsychol. Rehabil. 2020, 30, 564–578. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide Survey of Fitness Trends for 2021. ACSMs Health Fit J. 2021, 25, 10–19. [Google Scholar] [CrossRef]
- Thompson, W.R. Worldwide Survey of Fitness Trends for 2020. ACSMs Health Fit J. 2019, 23, 10–18. [Google Scholar] [CrossRef]
- Kalgotra, P.; Raja, U.; Sharda, R. Growth in the development of health and fitness mobile apps amid COVID-19 pandemic. Digit. Health. 2022, 8, 20552076221129070. [Google Scholar] [CrossRef]
- Peloton®. Workouts Streamed Live & On-Demand. Available online: https://www.onepeloton.com/ (accessed on 16 July 2023).
- Tonal. The World’s Smartest Home Gym Machine for Strength & Fitness. Available online: https://www.tonal.com/ (accessed on 16 July 2023).
- Jones, M.; Morris, J.; Deruyter, F. Mobile Healthcare and People with Disabilities: Current State and Future Needs. Int. J. Environ. Res. Public Health 2018, 15, 515. [Google Scholar] [CrossRef] [PubMed]
- Mays, A.M.; Kim, S.; Rosales, K.; Au, T.; Rosen, S. The Leveraging Exercise to Age in Place (LEAP) Study: Engaging Older Adults in Community-Based Exercise Classes to Impact Loneliness and Social Isolation. Am. J. Geriatr. Psychiatry 2021, 29, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Ramage, E.R.; Burke, M.; Galloway, M.; Graham, I.D.; Janssen, H.; Marsden, D.L.; Patterson, A.J.; Pollack, M.; Said, C.M.; Lynch, E.A.; et al. Fit for purpose. Co-production of complex behavioural interventions. A practical guide and exemplar of co-producing a telehealth-delivered exercise intervention for people with stroke. Health Res. Policy Syst. 2022, 20, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Young, H.J.; Mehta, T.; Kim, Y.; Padalabalanarayanan, S.; Chiu, C.Y.; Rimmer, J.H.; Thirumalai, M. The Spinal Cord Injury Program in Exercise (SCIPE) study: Study protocol for a randomized controlled trial evaluating teleexercise programs for people with spinal cord injury. Trials 2021, 22, 551. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhang, J.; Xie, Y.; Gao, F.; Xu, S.; Wu, X.; Ye, Z. Wearable Health Devices in Health Care: Narrative Systematic Review. JMIR Mhealth Uhealth 2020, 8, e18907. [Google Scholar] [CrossRef]
- Wang, Q.; Markopoulos, P.; Yu, B.; Chen, W.; Timmermans, A. Interactive wearable systems for upper body rehabilitation: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 20. [Google Scholar] [CrossRef]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PMR 2018, 10 (Suppl. S2), S220–S232. [Google Scholar] [CrossRef]
- Kaia Health. Digital Health Treatment for Musculoskeletal Pain. Available online: https://kaiahealth.com/ (accessed on 24 May 2023).
- Rabinowitz, A.R.; Collier, G.; Vaccaro, M.; Wingfield, R. Development of RehaBot-A Conversational Agent for Promoting Rewarding Activities in Users with Traumatic Brain Injury. J. Head Trauma Rehabil. 2022, 37, 144–151. [Google Scholar] [CrossRef]
- Federici, S.; de Filippis, M.L.; Mele, M.L.; Borsci, S.; Bracalenti, M.; Gaudino, G.; Cocco, A.; Amendola, M.; Simonetti, E. Inside pandora’s box: A systematic review of the assessment of the perceived quality of chatbots for people with disabilities or special needs. Disabil. Rehabil. Assist. Technol. 2020, 15, 832–837. [Google Scholar] [CrossRef]
- Hauser-Ulrich, S.; Künzli, H.; Meier-Peterhans, D.; Kowatsch, T. A Smartphone-Based Health Care Chatbot to Promote Self-Management of Chronic Pain (SELMA): Pilot Randomized Controlled Trial. JMIR Mhealth Uhealth 2020, 8, e15806. [Google Scholar] [CrossRef] [PubMed]
- Lai, B.; Wilroy, J.; Young, H.J.; Howell, J.; Rimmer, J.H.; Mehta, T.; Thirumalai, M. A Mobile App to Promote Adapted Exercise and Social Networking for People with Physical Disabilities: Usability Study. JMIR Form. Res. 2019, 3, e11689. [Google Scholar] [CrossRef]
- Burnalong. Corporate Wellness Platform and Online Wellness Company. Available online: https://www.burnalong.com/ (accessed on 13 June 2023).
- Schlagheck, M.L.; Joisten, N.; Walzik, D.; Wolf, F.; Neil-Sztramko, S.E.; Bansi, J.; Rademacher, A.; Zimmer, P. Systematic Review of Exercise Studies in Persons with Multiple Sclerosis: Exploring the Quality of Interventions According to the Principles of Exercise Training. Neurol. Ther. 2021, 10, 585. [Google Scholar] [CrossRef] [PubMed]
- Martin Ginis, K.A.; van der Scheer, J.W.; Latimer-Cheung, A.E.; Barrow, A.; Bourne, C.; Carruthers, P.; Bernardi, M.; Ditor, D.S.; Gaudet, S.; de Groot, S.; et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: An update and a new guideline. Spinal Cord 2017, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Mossberg, K.A. Reliability of a timed walk test in persons with acquired brain injury. Am. J. Phys. Med. Rehabil. 2003, 82, 385–390. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-minute walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef]
- Hansen, D.; Wens, I.; Kosten, L.; Verboven, K.; Eijnde, B.O. Slowed exercise-onset Vo2 kinetics during submaximal endurance exercise in subjects with multiple sclerosis. Neurorehabil. Neural Repair 2013, 27, 87–95. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Keytsman, C.; Eijnde, B.O.; Hansen, D.; Verboven, K.; Wens, I. Elevated cardiovascular risk factors in multiple sclerosis. Mult. Scler. Relat. Disord. 2017, 17, 220–223. [Google Scholar] [CrossRef]
- Scally, J.B.; Baker, J.S.; Rankin, J.; Renfrew, L.; Sculthorpe, N. Evaluating functional electrical stimulation (FES) cycling on cardiovascular, musculoskeletal and functional outcomes in adults with multiple sclerosis and mobility impairment: A systematic review. Mult. Scler. Relat. Disord. 2020, 37, 101485. [Google Scholar] [CrossRef]
- Lavis, T.D.; Scelza, W.M.; Bockenek, W.L. Cardiovascular health and fitness in persons with spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, A.; Mikalsen, M.H.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet Res. 2018, 20, e110. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Cowie, M.R. The Role of Wearables in Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 125–132. [Google Scholar] [CrossRef]
- Düking, P.; Giessing, L.; Frenkel, M.O.; Koehler, K.; Holmberg, H.C.; Sperlich, B. Wrist-Worn Wearables for Monitoring Heart Rate and Energy Expenditure While Sitting or Performing Light-to-Vigorous Physical Activity: Validation Study. JMIR Mhealth Uhealth 2020, 8, e16716. [Google Scholar] [CrossRef] [PubMed]
- Aroganam, G.; Manivannan, N.; Harrison, D. Review on Wearable Technology Sensors Used in Consumer Sport Applications. Sensors 2019, 19, 1983. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Ha, Y.; won Park, H. Accuracy of Wearable Devices for Measuring Heart Rate During Conventional and Nordic Walking. PMR 2021, 13, 379–386. [Google Scholar] [CrossRef]
- Evenson, K.R.; Goto, M.M.; Furberg, R.D. Systematic review of the validity and reliability of consumer-wearable activity trackers. Int. J. Behav. Nutr. Phys. Act. 2015, 12, 159. [Google Scholar] [CrossRef]
- Lujan, M.R.; Perez-Pozuelo, I.; Grandner, M.A. Past, Present, and Future of Multisensory Wearable Technology to Monitor Sleep and Circadian Rhythms. Front. Digit. Health 2021, 3, 721919. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, J.; Yu, B.; Shull, P.B. Validity of FitBit, Jawbone, UP, Nike+ and other wearable devices for level and stair walking. Gait Posture 2016, 48, 36–41. [Google Scholar] [CrossRef]
- Santos-Gago, J.M.; Ramos-Merino, M.; Vallarades-Rodriguez, S.; Álvarez-Sabucedo, L.M.; Fernández-Iglesias, M.J.; García-Soidán, J.L. Innovative use of wrist-worn wearable devices in the sports domain: A systematic review. Electronics 2019, 8, 1257. [Google Scholar] [CrossRef]
- Xie, J.; Wen, D.; Liang, L.; Jia, Y.; Gao, L.; Lei, J. Evaluating the Validity of Current Mainstream Wearable Devices in Fitness Tracking Under Various Physical Activities: Comparative Study. JMIR Mhealth Uhealth 2018, 6, e94. [Google Scholar] [CrossRef] [PubMed]
- Fuller, D.; Colwell, E.; Low, J.; Orychock, K.; Ann Tobin, M.; Simango, B.; Buote, R.; van Heerden, D.; Luan, H.; Cullen, K.; et al. Reliability and Validity of Commercially Available Wearable Devices for Measuring Steps, Energy Expenditure, and Heart Rate: Systematic Review. JMIR Mhealth Uhealth 2020, 8, e18694. [Google Scholar] [CrossRef]
- Alhammad, N.; Al-Dossari, H. Recognizing Physical Activities for Spinal Cord Injury Rehabilitation Using Wearable Sensors. Sensors 2021, 21, 5479. [Google Scholar] [CrossRef] [PubMed]
- Balestra, N.; Sharma, G.; Riek, L.M.; Busza, A. Automatic Identification of Upper Extremity Rehabilitation Exercise Type and Dose Using Body-Worn Sensors and Machine Learning: A Pilot Study. Digit. Biomark. 2021, 5, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Van Remoortel, H.; Giavedoni, S.; Raste, Y.; Burtin, C.; Louvaris, Z.; Gimeno-Santos, E.; Langer, D.; Glendenning, A.; Hopkinson, N.S.; Vogiatzis, I.; et al. Validity of activity monitors in health and chronic disease: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2012, 9, 84. [Google Scholar] [CrossRef] [PubMed]
- Anwer, S.; Li, H.; Antwi-Afari, M.F.; Umer, W.; Wong, A.Y.L. Cardiorespiratory and Thermoregulatory Parameters Are Good Surrogates for Measuring Physical Fatigue during a Simulated Construction Task. Int. J. Environ. Res. Public Health 2020, 17, 5418. [Google Scholar] [CrossRef]
- Andersen, T.O.; Langstrup, H.; Lomborg, S. Experiences with Wearable Activity Data During Self-Care by Chronic Heart Patients: Qualitative Study. J. Med. Internet Res. 2020, 22, e15873. [Google Scholar] [CrossRef]
- Medina Quero, J.; Fernández Olmo, M.R.; Peláez Aguilera, M.D.; Espinilla Estévez, M. Real-Time Monitoring in Home-Based Cardiac Rehabilitation Using Wrist-Worn Heart Rate Devices. Sensors 2017, 17, 2892. [Google Scholar] [CrossRef]
- O’Driscoll, R.; Turicchi, J.; Beaulieu, K.; Scott, S.; Matu, J.; Deighton, K.; Finlayson, G.; Stubbs, J. How well do activity monitors estimate energy expenditure? A systematic review and meta-analysis of the validity of current technologies. Br. J. Sports Med. 2020, 54, 332–340. [Google Scholar] [CrossRef]
- Weeks, D.L.; Sprint, G.L.; Stilwill, V.; Meisen-Vehrs, A.L.; Cook, D.J. Implementing Wearable Sensors for Continuous Assessment of Daytime Heart Rate Response in Inpatient Rehabilitation. Telemed. e-Health 2018, 24, 1014–1020. [Google Scholar] [CrossRef]
- Ringeval, M.; Wagner, G.; Denford, J.; Paré, G.; Kitsiou, S. Fitbit-Based Interventions for Healthy Lifestyle Outcomes: Systematic Review and Meta-Analysis. J. Med. Internet Res. 2020, 22, e23954. [Google Scholar] [CrossRef] [PubMed]
- Haghayegh, S.; Khoshnevis, S.; Smolensky, M.H.; Diller, K.R. Accuracy of PurePulse photoplethysmography technology of Fitbit Charge 2 for assessment of heart rate during sleep. Chronobiol. Int. 2019, 36, 927–933. [Google Scholar] [CrossRef]
- Hajj-Boutros, G.; Landry-Duval, M.A.; Comtois, A.S.; Gouspillou, G.; Karelis, A.D. Wrist-worn devices for the measurement of heart rate and energy expenditure: A validation study for the Apple Watch 6, Polar Vantage V and Fitbit Sense. Eur. J. Sport Sci. 2022, 23, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Pipek, L.Z.; Nascimento, R.F.V.; Acencio, M.M.P.; Teixeira, L.R. Comparison of SpO2 and heart rate values on Apple Watch and conventional commercial oximeters devices in patients with lung disease. Sci. Rep. 2021, 11, 18901. [Google Scholar] [CrossRef]
- Støve, M.P.; Hansen, E.C.K. Accuracy of the Apple Watch Series 6 and the Whoop Band 3.0 for assessing heart rate during resistance exercises. J. Sports Sci. 2022, 40, 2639–2644. [Google Scholar] [CrossRef]
- Bellenger, C.R.; Miller, D.; Halson, S.L.; Roach, G.D.; Maclennan, M.; Sargent, C. Evaluating the Typical Day-to-Day Variability of WHOOP-Derived Heart Rate Variability in Olympic Water Polo Athletes. Sensors 2022, 22, 6723. [Google Scholar] [CrossRef] [PubMed]
- Alzueta, E.; de Zambotti, M.; Javitz, H.; Dulai, T.; Albinni, B.; Simon, K.C.; Sattari, N.; Zhang, J.; Shuster, A.; Mednick, S.C.; et al. Tracking Sleep, Temperature, Heart Rate, and Daily Symptoms Across the Menstrual Cycle with the Oura Ring in Healthy Women. Int. J. Womens Health 2022, 14, 491–503. [Google Scholar] [CrossRef]
- Niotis, K.; Saif, N.; Simonetto, M.; Wu, X.; Yan, P.; Lakis, J.P.; Ariza, I.E.; Buckholz, A.P.; Sharma, N.; Fink, M.E.; et al. Feasibility of a wearable biosensor device to characterize exercise and sleep in neurology residents. Expert. Rev. Med. Dev. 2021, 18, 1123–1131. [Google Scholar] [CrossRef]
- Adans-Dester, C.; Hankov, N.; O’Brien, A.; Vergara-Diaz, G.; Black-Schaffer, R.; Zafonte, R.; Dy, J.; Lee, S.I.; Bonato, P. Enabling precision rehabilitation interventions using wearable sensors and machine learning to track motor recovery. NPJ Digit. Med. 2020, 3, 121. [Google Scholar] [CrossRef]
- Lee, S.I.; Adans-Dester, C.P.; Obrien, A.T.; Vergara-Diaz, G.P.; Black-Schaffer, R.; Zafonte, R.; Dy, J.G.; Bonato, P. Predicting and Monitoring Upper-Limb Rehabilitation Outcomes Using Clinical and Wearable Sensor Data in Brain Injury Survivors. IEEE Trans. Biomed. Eng. 2021, 68, 1871–1881. [Google Scholar] [CrossRef]
- Meijer, H.A.; Graafland, M.; Goslings, J.C.; Schijven, M.P. Systematic Review on the Effects of Serious Games and Wearable Technology Used in Rehabilitation of Patients with Traumatic Bone and Soft Tissue Injuries. Arch. Phys. Med. Rehabil. 2018, 99, 1890–1899. [Google Scholar] [CrossRef] [PubMed]
- Okita, S.; De Lucena, D.S.; Chan, V.; Reinkensmeyer, D.J. Measuring Movement Quality of the Stroke-Impaired Upper Extremity with a Wearable Sensor: Toward a Smoothness Metric for Home Rehabilitation Exercise Programs. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 6691–6694. [Google Scholar] [CrossRef] [PubMed]
- Lavelle, G.; Norris, M.; Flemming, J.; Harper, J.; Bradley, J.; Johnston, H.; Fortune, J.; Stennett, A.; Kilbride, C.; Ryan, J.M. Validity and Acceptability of Wearable Devices for Monitoring Step-Count and Activity Minutes Among People with Multiple Sclerosis. Front. Rehabil. Sci. 2022, 2, 737384. [Google Scholar] [CrossRef] [PubMed]
- Weizman, Y.; Tirosh, O.; Fuss, F.K.; Tan, A.M.; Rutz, E. Recent State of Wearable IMU Sensors Use in People Living with Spasticity: A Systematic Review. Sensors 2022, 22, 1791. [Google Scholar] [CrossRef]
- Block, V.J.; Zhao, C.; Hollenbach, J.A.; Olgin, J.E.; Marcus, G.M.; Pletcher, M.J.; Henry, R.; Gelfand, J.M.; Cree, B.A.C. Validation of a consumer-grade activity monitor for continuous daily activity monitoring in individuals with multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2019, 5, 2055217319888660. [Google Scholar] [CrossRef]
- Sola-Valls, N.; Blanco, Y.; Sepúlveda, M.; Llufriu, S.; Martínez-Lapiscina, E.H.; la Puma, D.; Graus, F.; Villoslada, P.; Saiz, A. Walking function in clinical monitoring of multiple sclerosis by telemedicine. J. Neurol. 2015, 262, 1706–1713. [Google Scholar] [CrossRef]
- Stuart, C.M.; Varatharaj, A.; Domjan, J.; Philip, S.; Galea, I.; Adams, E.; Ali, R.; Cader, S.; Chartres, S.; Cornick, F.; et al. Physical activity monitoring to assess disability progression in multiple sclerosis. Mult. Scler. J. Exp. Transl. Clin. 2020, 6, 2055217320975185. [Google Scholar] [CrossRef]
- Block, V.J.; Lizée, A.; Crabtree-Hartman, E.; Bevan, C.J.; Graves, J.S.; Bove, R.; Green, A.J.; Nourbakhsh, B.; Tremblay, M.; Gourraud, P.A.; et al. Continuous daily assessment of multiple sclerosis disability using remote step count monitoring. J. Neurol. 2017, 264, 316–326. [Google Scholar] [CrossRef]
- Supratak, A.; Datta, G.; Gafson, A.R.; Nicholas, R.; Guo, Y.; Matthews, P.M. Remote Monitoring in the Home Validates Clinical Gait Measures for Multiple Sclerosis. Front. Neurol. 2018, 9, 561. [Google Scholar] [CrossRef]
- Block, V.J.; Bove, R.; Zhao, C.; Garcha, P.; Graves, J.; Romeo, A.R.; Green, A.J.; Allen, D.D.; Hollenbach, J.A.; Olgin, J.E.; et al. Association of Continuous Assessment of Step Count by Remote Monitoring with Disability Progression Among Adults with Multiple Sclerosis. JAMA Netw. Open 2019, 2, e190570. [Google Scholar] [CrossRef]
- Tsang, K.; Hiremath, S.V.; Crytzer, T.M.; Dicianno, B.E.; Ding, D. Validity of activity monitors in wheelchair users: A systematic review. J. Rehabil. Res. Dev. 2016, 53, 641–658. [Google Scholar] [CrossRef]
- Boudreaux, B.D.; Hebert, E.P.; Hollander, D.B.; Williams, B.M.; Cormier, C.L.; Naquin, M.R.; Gillan, W.W.; Gusew, E.E.; Kraemer, R.R. Validity of Wearable Activity Monitors during Cycling and Resistance Exercise. Med. Sci. Sports Exerc. 2018, 50, 624–633. [Google Scholar] [CrossRef] [PubMed]
- Vetrovsky, T.; Siranec, M.; Marencakova, J.; Tufano, J.J.; Capek, V.; Bunc, V.; Belohlavek, J. Validity of six consumer-level activity monitors for measuring steps in patients with chronic heart failure. PLoS ONE 2019, 14, e0222569. [Google Scholar] [CrossRef]
- Moon, Y.; Motl, R.W.; Sosnoff, J.J. Monitoring Gait in Multiple Sclerosis with Novel Wearable Motion Sensors. Arch. Phys. Med. Rehabil. 2016, 97, e99–e100. [Google Scholar] [CrossRef][Green Version]
- Wang, S.J.; Park, H.S.; Park, J.; Albert, M. Wearable-based Spasticity Prediction and Validation Using Machine Learning. Arch. Phys. Med. Rehabil. 2020, 101, e72. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Zhu, J.; Chen, Z.; Liu, L.; Yang, S.; Zhu, G.; Zhu, B.; Li, J.; Jin, R.; et al. Upper-Limb Motion Recognition Based on Hybrid Feature Selection: Algorithm Development and Validation. JMIR Mhealth Uhealth 2021, 9, e24402. [Google Scholar] [CrossRef] [PubMed]
- Morrow, M.M.B.; Lowndes, B.; Fortune, E.; Kaufman, K.R.; Hallbeck, M.S. Validation of Inertial Measurement Units for Upper Body Kinematics. J. Appl. Biomech. 2017, 33, 227. [Google Scholar] [CrossRef]
- Hua, A.; Chaudhari, P.; Johnson, N.; Quinton, J.; Schatz, B.; Buchner, D.; Hernandez, M.E. Evaluation of Machine Learning Models for Classifying Upper Extremity Exercises Using Inertial Measurement Unit-Based Kinematic Data. IEEE J. Biomed. Health Inform. 2020, 24, 2452–2460. [Google Scholar] [CrossRef]
- Montoye, A.H.K.; Westgate, B.S.; Fonley, M.R.; Pfeiffer, K.A. Cross-validation and out-of-sample testing of physical activity intensity predictions with a wrist-worn accelerometer. J. Appl. Physiol. 2018, 124, 1284–1293. [Google Scholar] [CrossRef]
- Soro, A.; Brunner, G.; Tanner, S.; Wattenhofer, R. Recognition and Repetition Counting for Complex Physical Exercises with Deep Learning. Sensors 2019, 19, 714. [Google Scholar] [CrossRef]
- Abbott, J.C.; Wagle, J.P.; Sato, K.; Painter, K.; Light, T.J.; Stone, M.H. Validation of Inertial Sensor to Measure Barbell Kinematics across a Spectrum of Loading Conditions. Sports 2020, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Clemente, F.M.; Akyildiz, Z.; Pino-Ortega, J.; Rico-González, M. Validity and Reliability of the Inertial Measurement Unit for Barbell Velocity Assessments: A Systematic Review. Sensors 2021, 21, 2511. [Google Scholar] [CrossRef]
- Tudor-Locke, C., Jr.; Schuna, J.M. Steps to preventing type 2 diabetes: Exercise, walk more, or sit less? Front. Endocrinol. 2012, 3, 142. [Google Scholar] [CrossRef] [PubMed]
- Farmer, C.; van den Berg, M.E.L.; Vuu, S.; Barr, C.J. A study of the accuracy of the Fitbit Zip in measuring steps both indoors and outdoors in a mixed rehabilitation population. Clin. Rehabil. 2022, 36, 125–132. [Google Scholar] [CrossRef]
- Ummels, D.; Beekman, E.; Theunissen, K.; Braun, S.; Beurskens, A.J. Counting Steps in Activities of Daily Living in People with a Chronic Disease Using Nine Commercially Available Fitness Trackers: Cross-Sectional Validity Study. JMIR Mhealth Uhealth 2018, 6, e70. [Google Scholar] [CrossRef]
- Flint Rehab: Neurorehabilitation Devices for the Home & Clinic. Available online: https://www.flintrehab.com/ (accessed on 25 May 2023).
- Swanson, V.A.; Chan, V.; Cruz-Coble, B.; Alcantara, C.M.; Scott, D.; Jones, M.; Zondervan, D.K.; Khan, N.; Ichimura, J.; Reinkensmeyer, D.J. A Pilot Study of a Sensor Enhanced Activity Management System for Promoting Home Rehabilitation Exercise Performed during the COVID-19 Pandemic: Therapist Experience, Reimbursement, and Recommendations for Implementation. Int. J. Environ. Res. Public Health 2021, 18, 10186. [Google Scholar] [CrossRef] [PubMed]
- Swanson, V.A.; Johnson, C.; Zondervan, D.K.; Bayus, N.; McCoy, P.; Ng, Y.F.J.; Schindele, J.; Reinkensmeyer, D.J.; Shaw, S. Optimized Home Rehabilitation Technology Reduces Upper Extremity Impairment Compared to a Conventional Home Exercise Program: A Randomized, Controlled, Single-Blind Trial in Subacute Stroke. Neurorehabil. Neural Repair. 2023, 37, 53–65. [Google Scholar] [CrossRef]
- Ramos Muñoz, E.D.J.; Swanson, V.A.; Johnson, C.; Anderson, R.K.; Rabinowitz, A.R.; Zondervan, D.K.; Collier, G.H.; Reinkensmeyer, D.J. Using Large-Scale Sensor Data to Test Factors Predictive of Perseverance in Home Movement Rehabilitation: Optimal Challenge and Steady Engagement. Front. Neurol. 2022, 13, 896298. [Google Scholar] [CrossRef]
- Inojosa, H.; Schriefer, D.; Ziemssen, T. Clinical outcome measures in multiple sclerosis: A review. Autoimmun. Rev. 2020, 19, 102512. [Google Scholar] [CrossRef]
- Motl, R.W.; Learmonth, Y.C. Neurological disability and its association with walking impairment in multiple sclerosis: Brief review. Neurodegener. Dis. Manag. 2014, 4, 491–500. [Google Scholar] [CrossRef]
- Mokhlespour Esfahani, M.I.; Nussbaum, M.A. Classifying Diverse Physical Activities Using “Smart Garments”. Sensors 2019, 19, 3133. [Google Scholar] [CrossRef] [PubMed]
- Soroudi, A.; Hernández, N.; Berglin, L.; Nierstrasz, V. Electrode placement in electrocardiography smart garments: A review. J. Electrocardiol. 2019, 57, 27–30. [Google Scholar] [CrossRef]
- Angelucci, A.; Cavicchioli, M.; Cintorrino, I.A.; Lauricella, G.; Rossi, C.; Strati, S.; Aliverti, A. Smart Textiles and Sensorized Garments for Physiological Monitoring: A Review of Available Solutions and Techniques. Sensors 2021, 21, 814. [Google Scholar] [CrossRef] [PubMed]
- Yeung, J.; Catolico, D.; Fullmer, N.; Daniel, R.; Lovell, R.; Tang, R.; Pearson, E.M.; Rosenberg, S.S. Evaluating the Sensoria Smart Socks Gait Monitoring System for Rehabilitation Outcomes. PMR 2019, 11, 512–521. [Google Scholar] [CrossRef] [PubMed]
- D’Addio, G.; Iuppariello, L.; Pagano, G.; Biancardi, A.; Lanzillo, B.; Pappone, N.; Cesarelli, M. New posturographic assessment by means of novel e-textile and wireless socks device. In Proceedings of the 2016 IEEE International Symposium on Medical Measurements and Applications, MeMeA 2016, Benevento, Italy, 15–18 May 2016. [Google Scholar] [CrossRef]
- Navalta, J.W.; Ramirez, G.G.; Maxwell, C.; Radzak, K.N.; McGinnis, G.R. Validity and Reliability of Three Commercially Available Smart Sports Bras during Treadmill Walking and Running. Sci. Rep. 2020, 10, 7397. [Google Scholar] [CrossRef] [PubMed]
- Sensoria® Socks–Sensoria Health. Available online: https://www.sensoriahealth.com/sensoria-socks (accessed on 14 May 2023).
- Walha, R.; Lebel, K.; Gaudreault, N.; Dagenais, P.; Cereatti, A.; della Croce, U.; Boissy, P. The Accuracy and Precision of Gait Spatio-Temporal Parameters Extracted from an Instrumented Sock during Treadmill and Overground Walking in Healthy Subjects and Patients with a Foot Impairment Secondary to Psoriatic Arthritis. Sensors 2021, 21, 6179. [Google Scholar] [CrossRef]
- Hergenroeder, A.L.; Gibbs, B.B.; Kotlarczyk, M.P.; Perera, S.; Kowalsky, R.J.; Brach, J.S. Accuracy and Acceptability of Commercial-Grade Physical Activity Monitors in Older Adults. J. Aging Phys. Act. 2019, 27, 222. [Google Scholar] [CrossRef]
- Hassett, L.; Moseley, A.M.; Harmer, A.R. Fitness training for cardiorespiratory conditioning after traumatic brain injury. Cochrane Database Syst. Rev. 2017, 2017, CD006123. [Google Scholar] [CrossRef]
- Kjølhede, T.; Dalgas, U.; Gade, A.B.; Bjerre, M.; Stenager, E.; Petersen, T.; Vissing, K. Acute and chronic cytokine responses to resistance exercise and training in people with multiple sclerosis. Scand. J. Med. Sci. Sports 2016, 26, 824–834. [Google Scholar] [CrossRef]
- Sandroff, B.M.; Balto, J.M.; Klaren, R.E.; Sommer, S.K.; DeLuca, J.; Motl, R.W. Systematically developed pilot randomized controlled trial of exercise and cognition in persons with multiple sclerosis. Neurocase 2016, 22, 443–450. [Google Scholar] [CrossRef]
- Huang, M.; Jay, O.; Davis, S.L. Autonomic dysfunction in multiple sclerosis: Implications for exercise. Auton. Neurosci. 2015, 188, 82–85. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Senaratne, M.P.; Carroll, D.; Warren, K.G.; Kappagoda, T. Evidence for cardiovascular autonomic nerve dysfunction in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 1984, 47, 947–952. [Google Scholar] [CrossRef]
- Jacobs, P.L.; Nash, M.S. Exercise recommendations for individuals with spinal cord injury. Sports Med. 2004, 34, 727–751. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Messerli, F.H. Beta-blockers and exercise. J. Am. Coll. Cardiol. 2006, 48, 1284–1285. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.V.; Miller, R.G.; Gelinas, D.; Kent-Braun, J.A. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve Off. J. Am. Assoc. Electrodiagn. 2004, 29, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Morrison, E.H.; Cooper, D.M.; White, L.J.; Larson, J.; Leu, S.-Y.; Zaldivar, F.; Ng, A. V Ratings of Perceived Exertion During Aerobic Exercise in Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2008, 89, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent Validation of the OMNI Perceived Exertion Scale for Resistance Exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Bui, Q.; Kaufman, K.J.; Pham, V.; Lenze, E.J.; Lee, J.-M.; Mohr, D.C.; Fong, M.W.M.; Metts, C.L.; Tomazin, S.E.; Wong, A.W.K. Ecological Momentary Assessment of Real-World Functional Behaviors in Individuals with Stroke: A Longitudinal Observational Study. Arch. Phys. Med. Rehabil. 2022, 103, 1327–1337. [Google Scholar] [CrossRef]
- Rabinowitz, A.; Hart, T.; Wilson, J. Ecological momentary assessment of affect in context after traumatic brain injury. Rehabil. Psychol. 2021, 66, 442–449. [Google Scholar] [CrossRef]
- Liao, Y.; Chou, C.P.; Huh, J.; Leventhal, A.; Dunton, G. Associations of Affective Responses During Free-Living Physical Activity and Future Physical Activity Levels: An Ecological Momentary Assessment Study. Int. J. Behav. Med. 2017, 24, 513–519. [Google Scholar] [CrossRef]
- Liao, Y.; Chou, C.P.; Huh, J.; Leventhal, A.; Dunton, G. Examining acute bi-directional relationships between affect, physical feeling states, and physical activity in free-living situations using electronic ecological momentary assessment. J. Behav. Med. 2017, 40, 445–457. [Google Scholar] [CrossRef]
- Trull, T.J.; Ebner-Priemer, U.W. Using Experience Sampling Methods/Ecological Momentary Assessment (ESM/EMA) in Clinical Assessment and Clinical Research: Introduction to the Special Section. Psychol. Assess. 2009, 21, 457. [Google Scholar] [CrossRef] [PubMed]
- Do, B.; Mason, T.B.; Yi, L.; Yang, C.H.; Dunton, G.F. Momentary associations between stress and physical activity among children using ecological momentary assessment. Psychol. Sport Exerc. 2021, 55, 101935. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.J.H.; Liossi, C.; Schlotz, W.; Moss-Morris, R. Tracking daily fatigue fluctuations in multiple sclerosis: Ecological momentary assessment provides unique insights. J. Behav. Med. 2017, 40, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Henwood, B.F.; Redline, B.; Dzubur, E.; Madden, D.R.; Rhoades, H.; Dunton, G.F.; Rice, E.; Semborski, S.; Tang, Q.; Intille, S.S. Investigating Health Risk Environments in Housing Programs for Young Adults: Protocol for a Geographically Explicit Ecological Momentary Assessment Study. JMIR Res. Protoc. 2019, 8, e12112. [Google Scholar] [CrossRef]
- Liao, Y.; Skelton, K.; Dunton, G.; Bruening, M. A Systematic Review of Methods and Procedures Used in Ecological Momentary Assessments of Diet and Physical Activity Research in Youth: An Adapted STROBE Checklist for Reporting EMA Studies (CREMAS). J. Med. Internet Res. 2016, 18, e151. [Google Scholar] [CrossRef]
- REDCap. Available online: https://www.project-redcap.org/ (accessed on 5 July 2023).
- ExamMed. Available online: https://www.exammed.com/ (accessed on 5 July 2023).
- Ponnada, A.; Thapa-Chhetry, B.; Manjourides, J.; Intille, S. Measuring Criterion Validity of Microinteraction Ecological Momentary Assessment (Micro-EMA): Exploratory Pilot Study with Physical Activity Measurement. JMIR Mhealth Uhealth 2021, 9, e23391. [Google Scholar] [CrossRef]
- Motl, R.W.; Snook, E.M.; Schapiro, R.T. Symptoms and physical activity behavior in individuals with multiple sclerosis. Res. Nurs. Health 2008, 31, 466–475. [Google Scholar] [CrossRef]
- Motl, R.W.; McAuley, E.; Snook, E.M. Physical activity and multiple sclerosis: A meta-analysis. Mult. Scler. 2005, 11, 459–463. [Google Scholar] [CrossRef]
- Motl, R.W.; McAuley, E.; Wynn, D.; Suh, Y.; Weikert, M.; Dlugonski, D. Symptoms and physical activity among adults with relapsing-remitting multiple sclerosis. J. Nerv. Ment. Dis. 2010, 198, 213–219. [Google Scholar] [CrossRef]
- Kratz, A.L.; Murphy, S.L.; Braley, T.J. Ecological Momentary Assessment of Pain, Fatigue, Depressive, and Cognitive Symptoms Reveals Significant Daily Variability in Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2017, 98, 2142–2150. [Google Scholar] [CrossRef] [PubMed]
- Katrine, W.; Ritzel, S.B.; Caroline, K.; Marie, L.; Olsgaard, B.S.; Lasse, S. Potentials and barriers of using digital tools for collecting daily measurements in multiple sclerosis research. Digit. Health 2021, 7, 20552076211055552. [Google Scholar] [CrossRef] [PubMed]
- Block, V.J.; Bove, R.; Nourbakhsh, B. The Role of Remote Monitoring in Evaluating Fatigue in Multiple Sclerosis: A Review. Front. Neurol. 2022, 13, 878313. [Google Scholar] [CrossRef]
- Tyszka, E.E.; Bozinov, N.; Briggs, F.B.S. Characterizing Relationships Between Cognitive, Mental, and Physical Health and Physical Activity Levels in Persons with Multiple Sclerosis. Int. J. MS Care 2022, 24, 243–249. [Google Scholar] [CrossRef]
- Kratz, A.L.; Fritz, N.E.; Braley, T.J.; Scott, E.L.; Foxen-Craft, E.; Murphy, S.L. Daily Temporal Associations Between Physical Activity and Symptoms in Multiple Sclerosis. Ann. Behav. Med. 2019, 53, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.L.; Braley, T.J.; Foxen-Craft, E.; Scott, E.; Murphy, J.F.; Murphy, S.L. How Do Pain, Fatigue, Depressive, and Cognitive Symptoms Relate to Well-Being and Social and Physical Functioning in the Daily Lives of Individuals with Multiple Sclerosis? Arch. Phys. Med. Rehabil. 2017, 98, 2160–2166. [Google Scholar] [CrossRef]
- Elbers, R.G.; Rietberg, M.B.; van Wegen, E.E.H.; Verhoef, J.; Kramer, S.F.; Terwee, C.B.; Kwakkel, G. Self-report fatigue questionnaires in multiple sclerosis, Parkinson’s disease and stroke: A systematic review of measurement properties. Qual. Life Res. 2012, 21, 925. [Google Scholar] [CrossRef]
- Flachenecker, P.; Kümpfel, T.; Kallmann, B.; Gottschalk, M.; Grauer, O.; Rieckmann, P.; Trenkwalder, C.; Toyka, K.V. Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Mult. Scler. 2002, 8, 523–526. [Google Scholar] [CrossRef]
- Marzolini, S.; Robertson, A.D.; Oh, P.; Goodman, J.M.; Corbett, D.; Du, X.; MacIntosh, B.J. Aerobic Training and Mobilization Early Post-stroke: Cautions and Considerations. Front. Neurol. 2019, 10, 1187. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Ronai, P.; La Fontaine, T.; Bollinger, L. Exercise guidelines for persons with multiple sclerosis. Strength Cond. J. 2011, 33, 30–33. [Google Scholar] [CrossRef]
- Hornby, T.G.; Reisman, D.S.; Ward, I.G.; Scheets, P.L.; Miller, A.; Haddad, D.; Fox, E.J.; Fritz, N.E.; Hawkins, K.; Henderson, C.E.; et al. Clinical Practice Guideline to Improve Locomotor Function Following Chronic Stroke, Incomplete Spinal Cord Injury, and Brain Injury. J. Neurol. Phys. Ther. 2020, 44, 49–100. [Google Scholar] [CrossRef]
- Janssen, J.; Verschuren, O.; Renger, W.J.; Ermers, J.; Ketelaar, M.; Van Ee, R. Gamification in Physical Therapy: More Than Using Games. Pediatr. Phys. Ther. 2017, 29, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.J.V.; Gonzalez-Medina, G.; Lucena-Anton, D.; Perez-Cabezas, V.; Del Carmen Ruiz-Molinero, M.; Martín-Valero, R. Augmented Reality in Physical Therapy: Systematic Review and Meta-analysis. JMIR Serious Games 2021, 9, e30985. [Google Scholar] [CrossRef]
- Ambrosino, P.; Fuschillo, S.; Papa, A.; DI Minno, M.N.D.; Maniscalco, M. Exergaming as a Supportive Tool for Home-Based Rehabilitation in the COVID-19 Pandemic Era. Games Health J. 2020, 9, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Malone, L.A.; Thirumalai, M.; Padalabalanarayanan, S.; Neal, W.N.; Bowman, S.; Mehta, T. Energy Expenditure and Enjoyment During Active Video Gaming Using an Adapted Wii Fit Balance Board in Adults with Physical Disabilities: Observational Study. JMIR Serious Games 2019, 7, e11326. [Google Scholar] [CrossRef]
- Berton, A.; Longo, U.G.; Candela, V.; Fioravanti, S.; Giannone, L.; Arcangeli, V.; Alciati, V.; Berton, C.; Facchinetti, G.; Marchetti, A.; et al. Virtual Reality, Augmented Reality, Gamification, and Telerehabilitation: Psychological Impact on Orthopedic Patients’ Rehabilitation. J. Clin. Med. 2020, 9, 2567. [Google Scholar] [CrossRef]
- Rutkowski, S.; Kiper, P.; Cacciante, L.; Cieślik, B.; Mazurek, J.; Turolla, A.; Szczepańska-Gieracha, J. Use of virtual reality-based training in different fields of rehabilitation: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00121. [Google Scholar] [CrossRef]
- Cortés-Pérez, I.; Sánchez-Alcalá, M.; Nieto-Escámez, F.A.; Castellote-Caballero, Y.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Virtual Reality-Based Therapy Improves Fatigue, Impact, and Quality of Life in Patients with Multiple Sclerosis. A Systematic Review with a Meta-Analysis. Sensors 2021, 21, 7389. [Google Scholar] [CrossRef]
- Chen, J.; Or, C.K.; Chen, T. Effectiveness of Using Virtual Reality-Supported Exercise Therapy for Upper Extremity Motor Rehabilitation in Patients With Stroke: Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Med. Internet Res. 2022, 24, e24111. [Google Scholar] [CrossRef]
- Alashram, A.R.; Padua, E.; Annino, G. Virtual reality for balance and mobility rehabilitation following traumatic brain injury: A systematic review of randomized controlled trials. J. Clin. Neurosci. 2022, 105, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Li, C.; Liu, J.; Wang, L.; Ma, J.; Li, G.; Gan, L.; Shang, X.; Wu, Z. Virtual Reality Rehabilitation Versus Conventional Physical Therapy for Improving Balance and Gait in Parkinson’s Disease Patients: A Randomized Controlled Trial. Med. Sci. Monit. 2019, 25, 4186. [Google Scholar] [CrossRef] [PubMed]
- Calafiore, D.; Invernizzi, M.; Ammendolia, A.; Marotta, N.; Fortunato, F.; Paolucci, T.; Ferraro, F.; Curci, C.; Cwirlej-Sozanska, A.; de Sire, A. Efficacy of Virtual Reality and Exergaming in Improving Balance in Patients with Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 773459. [Google Scholar] [CrossRef] [PubMed]
- Faric, N.; Potts, H.W.W.; Hon, A.; Smith, L.; Newby, K.; Steptoe, A.; Fisher, A. What Players of Virtual Reality Exercise Games Want: Thematic Analysis of Web-Based Reviews. J. Med. Internet Res. 2019, 21, e13833. [Google Scholar] [CrossRef] [PubMed]
- Dockx, K.; Bekkers, E.M.J.; Van den Bergh, V.; Ginis, P.; Rochester, L.; Hausdorff, J.M.; Mirelman, A.; Nieuwboer, A. Virtual reality for rehabilitation in Parkinson’s disease. Cochrane Database Syst. Rev. 2016, 12, CD010760. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.C.; De Amorim Lima, A.M.; Ferraz, K.M.; Benedetti Rodrigues, M.A. Use of virtual reality in gait recovery among post stroke patients—A systematic literature review. Disabil. Rehabil. Assist. Technol. 2013, 8, 357–362. [Google Scholar] [CrossRef]
- Farhad, A.; Pyun, J.Y. Terahertz Meets AI: The State of the Art. Sensors 2023, 23, 5034. [Google Scholar] [CrossRef]
- Collins, J.D.; Markham, A.; Service, K.; Reini, S.; Wolf, E.; Sessoms, P. A systematic literature review of the use and effectiveness of the Computer Assisted Rehabilitation Environment for research and rehabilitation as it relates to the wounded warrior. Work 2015, 50, 121–129. [Google Scholar] [CrossRef]
- Van Wegen, M.; Herder, J.L.; Adelsberger, R.; Pastore-Wapp, M.; van Wegen, E.E.H.; Bohlhalter, S.; Nef, T.; Krack, P.; Vanbellingen, T. An Overview of Wearable Haptic Technologies and Their Performance in Virtual Object Exploration. Sensors 2023, 23, 1563. [Google Scholar] [CrossRef]
- Willett, A.; Haq, M.; Holland, J.; Bridwell, E. Commentary: Augmented reality in neurosurgery, state of art and future projections. A systematic review. Front. Surg. 2023, 10, 1218308. [Google Scholar] [CrossRef]
- Palumbo, A. Microsoft HoloLens 2 in Medical and Healthcare Context: State of the Art and Future Prospects. Sensors 2022, 22, 7709. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, F.; Giardini, A.; Pierobon, A.; D’Addario, M.; Steca, P. A systematic review on the usability of robotic and virtual reality devices in neuromotor rehabilitation: Patients’ and healthcare professionals’ perspective. Health Serv. Res. 2021, 22, 523. [Google Scholar] [CrossRef]
- Alfieri, F.M.; da Silva Dias, C.; de Oliveira, N.C.; Battistella, L.R. Gamification in Musculoskeletal Rehabilitation. Curr. Rev. Musculoskelet. Med. 2022, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Havran, M.A.; Bidelspach, D.E. Virtual Physical Therapy and Telerehabilitation. Phys. Med. Rehabil. Clin. N. Am. 2021, 32, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.K. Virtual environments for motor rehabilitation: Review. Cyberpsychol. Behav. 2005, 8, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Meta Quest VR Headsets, Accessories & Equipment. Meta Quest. Meta Store. Available online: https://www.meta.com/quest/?utm_source=www.google.com&utm_medium=oculusredirect (accessed on 17 July 2023).
- Apple Vision Pro–Apple. Available online: https://www.apple.com/apple-vision-pro/ (accessed on 17 July 2023).
- Cho, K.H.; Park, J.B.; Kang, A. Metaverse for Exercise Rehabilitation: Possibilities and Limitations. Int. J. Environ. Res. Public Health 2023, 20, 5483. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Kumari, S.; Senapati, A.; Ambasta, R.K.; Kumar, P. New era of artificial intelligence and machine learning-based detection, diagnosis, and therapeutics in Parkinson’s disease. Ageing Res. Rev. 2023, 90, 102013. [Google Scholar] [CrossRef]
- Yeolekar, A.M.; Shinde, K.J.; Qadri, H. Innovative Use of Google Cardboard in Clinical Examination of Patients of Vertigo. Clin. Med. Insights Ear Nose Throat 2019, 12, 117955061988201. [Google Scholar] [CrossRef]
- Teslasuit. Meet our Haptic VR Suit and Glove with Force Feedback. Available online: https://teslasuit.io/ (accessed on 17 July 2023).
- Caserman, P.; Krug, C.; Göbel, S. Recognizing Full-Body Exercise Execution Errors Using the Teslasuit. Sensors 2021, 21, 8389. [Google Scholar] [CrossRef]
- Lange, B.S.; Requejo, P.; Flynn, S.M.; Rizzo, A.A.; Valero-Cuevas, F.J.; Baker, L.; Winstein, C. The potential of virtual reality and gaming to assist successful aging with disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 339–356. [Google Scholar] [CrossRef] [PubMed]
- Sardi, L.; Idri, A.; Fernández-Alemán, J.L. A systematic review of gamification in e-Health. J. Biomed. Inform. 2017, 71, 31–48. [Google Scholar] [CrossRef] [PubMed]
- Rica, R.L.; Shimojo, G.L.; Gomes, M.C.S.S.; Alonso, A.C.; Pitta, R.M.; Santa-Rosa, F.A.; Pontes Junior, F.L.; Ceschini, F.; Gobbo, S.; Bergamin, M.; et al. Effects of a Kinect-based physical training program on body composition, functional fitness and depression in institutionalized older adults. Geriatr. Gerontol. Int. 2020, 20, 195–200. [Google Scholar] [CrossRef]
- Pham, T.N.; Wong, J.N.; Terken, T.; Gibran, N.S.; Carrougher, G.J.; Bunnell, A. Feasibility of a Kinect ®-based rehabilitation strategy after burn injury. Burns 2018, 44, 2080–2086. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Hondori, H.; Khademi, M. A Review on Technical and Clinical Impact of Microsoft Kinect on Physical Therapy and Rehabilitation. J. Med. Eng. 2014, 2014, 846514. [Google Scholar] [CrossRef] [PubMed]
- Wall, T.; Feinn, R.; Chui, K.; Cheng, M.S. The effects of the NintendoTM Wii Fit on gait, balance, and quality of life in individuals with incomplete spinal cord injury. J. Spinal Cord. Med. 2015, 38, 777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hao, J.; He, Z.; Yu, X.; Remis, A. Comparison of immersive and non-immersive virtual reality for upper extremity functional recovery in patients with stroke: A systematic review and network meta-analysis. Neurol. Sci. 2023, 44, 2679–2691. [Google Scholar] [CrossRef]
- Santos, P.; Scaldaferri, G.; Santos, L.; Ribeiro, N.; Neto, M.; Melo, A. Effects of the Nintendo Wii training on balance rehabilitation and quality of life of patients with Parkinson’s disease: A systematic review and meta-analysis. NeuroRehabilitation 2019, 44, 569–577. [Google Scholar] [CrossRef]
- Karasu, A.U.; Batur, E.B.; Karatas, G.K. Effectiveness of Wii-based rehabilitation in stroke: A randomized controlled study. J. Rehabil. Med. 2018, 50, 406–412. [Google Scholar] [CrossRef]
- Redepenning, D.H.; Huss, S.A.; Maddali, S. Influence of adaptive video gaming on quality of life and social relationships. Assist. Technol. 2023, 35, 339–346. [Google Scholar] [CrossRef]
- Gonçalves, D.; Rodrigues, A.; Guerreiro, T. Playing with Others: Depicting Multiplayer Gaming Experiences of People with Visual Impairments. In Proceedings of the ASSETS 2020–22nd International ACM SIGACCESS Conference on Computers and Accessibility, Online, 26–28 October 2020; Volume 12. [Google Scholar] [CrossRef]
- Baltzar, P.; Hassan, L.; Turunen, M. Social accessibility in multiplayer games: Theory and praxis. Entertain. Comput. 2023, 47, 100592. [Google Scholar] [CrossRef]
- Shah, S.H.H.; Karlsen, A.S.T.; Solberg, M.; Hameed, I.A. A social VR-based collaborative exergame for rehabilitation: Codesign, development and user study. Virtual Real. 2022, 27, 3403–3420. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, J.L.; Wong, A.M.K.; Liang, K.C.; Tseng, K.C. Game-Based Virtual Reality System for Upper Limb Rehabilitation After Stroke in a Clinical Environment: Systematic Review and Meta-Analysis. Games Health J. 2022, 11, 277–297. [Google Scholar] [CrossRef] [PubMed]
- Charles, D.; Holmes, D.; Charles, T.; McDonough, S. Virtual Reality Design for Stroke Rehabilitation. Adv. Exp. Med. Biol. 2020, 1235, 53–87. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.R.; Berta, W.; Kothari, A.; Boyko, J.; Urquhart, R. Integrated knowledge translation (IKT) in health care: A scoping review. Implement. Sci. 2016, 11, 38. [Google Scholar] [CrossRef] [PubMed]
- Vereenooghe, L.; Westermann, K. Co-development of an interactive digital intervention to promote the well-being of people with intellectual disabilities. Int. J. Dev. Disabil. 2019, 65, 128–134. [Google Scholar] [CrossRef]
- Aflatoony, L.; Lee, S.J.; Sanford, J. Collective making: Co-designing 3D printed assistive technologies with occupational therapists, designers, and end-users. Assist. Technol. 2023, 35, 153–162. [Google Scholar] [CrossRef]
- Cotton, R.J.; Segal, R.L.; Seamon, B.A.; Sahu, A.; McLeod, M.M.; Davis, R.D.; Ramey, S.L.; French, M.A.; Roemmich, R.T.; Daley, K.; et al. Precision Rehabilitation: Optimizing Function, Adding Value to Health Care. Arch. Phys. Med. Rehabil. 2022, 103, 1883–1884. [Google Scholar] [CrossRef] [PubMed]
- Shields, R.K. Precision Rehabilitation: How Lifelong Healthy Behaviors Modulate Biology, Determine Health, and Affect Populations. Phys. Ther. 2022, 102, pzab248. [Google Scholar] [CrossRef]
- Lin, D.J.; Stein, J. Stepping Closer to Precision Rehabilitation. JAMA Neurol. 2023, 80, 339–341. [Google Scholar] [CrossRef]
- O’Donnell, D.E.; Neder, J.A. Chronic respiratory diseases: The dawn of precision rehabilitation. Respirology 2019, 24, 826–827. [Google Scholar] [CrossRef]
- French, M.A.; Daley, K.; Lavezza, A.; Roemmich, R.T.; Wegener, S.T.; Raghavan, P.; Celnik, P. A Learning Health System Infrastructure for Precision Rehabilitation After Stroke. Am. J. Phys. Med. Rehabil. 2023, 102 (Suppl. S1), S56–S60. [Google Scholar] [CrossRef]
- Malhotra, A.; Molloy, E.J.; Bearer, C.F.; Mulkey, S.B. Emerging role of artificial intelligence, big data analysis and precision medicine in pediatrics. Pediatr. Res. 2023, 93, 281–283. [Google Scholar] [CrossRef]
- Macias, C.G.; Remy, K.E.; Barda, A.J. Utilizing big data from electronic health records in pediatric clinical care. Pediatr. Res. 2022, 93, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Thirunavukarasu, A.J.; Ting, D.S.J.; Elangovan, K.; Gutierrez, L.; Tan, T.F.; Ting, D.S.W. Large language models in medicine. Nat. Med. 2023, 29, 1930–1940. [Google Scholar] [CrossRef]
- He, J.; Baxter, S.L.; Xu, J.; Xu, J.; Zhou, X.; Zhang, K. The practical implementation of artificial intelligence technologies in medicine. Nat. Med. 2019, 25, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J.W.L. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Budhdeo, S.; William, W.; Cerrato, P.; Shuaib, H.; Sood, H.; Ashrafian, H.; Halamka, J.; Teo, J.T. Moving towards vertically integrated artificial intelligence development. NPJ Digit. Med. 2022, 5, 143. [Google Scholar] [CrossRef]
- Cadario, R.; Longoni, C.; Morewedge, C.K. Understanding, explaining, and utilizing medical artificial intelligence. Nat. Human Behav. 2021, 5, 1636–1642. [Google Scholar] [CrossRef]
- Koelzer, V.H.; Sirinukunwattana, K.; Rittscher, J.; Mertz, K.D. Precision immunoprofiling by image analysis and artificial intelligence. Virchows Arch. 2019, 474, 511–522. [Google Scholar] [CrossRef]
- Baxi, V.; Edwards, R.; Montalto, M.; Saha, S. Digital pathology and artificial intelligence in translational medicine and clinical practice. Mod. Pathol. 2021, 35, 23–32. [Google Scholar] [CrossRef]
- Bera, K.; Schalper, K.A.; Rimm, D.L.; Velcheti, V.; Madabhushi, A. Artificial intelligence in digital pathology–New tools for diagnosis and precision oncology. Nat. Rev. Clin. Oncol. 2019, 16, 703–715. [Google Scholar] [CrossRef]
- Shoeibi, A.; Khodatars, M.; Jafari, M.; Moridian, P.; Rezaei, M.; Alizadehsani, R.; Khozeimeh, F.; Gorriz, J.M.; Heras, J.; Panahiazar, M. Applications of deep learning techniques for automated multiple sclerosis detection using magnetic resonance imaging: A review. Comput. Biol. Med. 2021, 136, 104697. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Dickson, D.M.; Akram, M.U. Survey of explainable artificial intelligence techniques for biomedical imaging with deep neural networks. Comput. Biol. Med. 2023, 156, 106668. [Google Scholar] [CrossRef]
- Bera, K.; Braman, N.; Gupta, A.; Velcheti, V.; Madabhushi, A. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat. Rev. Clin. Oncol. 2022, 19, 132–146. [Google Scholar] [CrossRef]
- Dey, D.; Slomka, P.J.; Leeson, P.; Comaniciu, D.; Shrestha, S.; Sengupta, P.P.; Marwick, T.H. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 1317–1335. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.M.; Lee, K.H.; Tsai, M.T.; Tseng, W.C.; Chu, Y.C.; Tarng, D.C. Artificial Intelligence for Risk Prediction of Rehospitalization with Acute Kidney Injury in Sepsis Survivors. J. Pers. Med. 2022, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Croon, P.M.; Selder, J.L.; Allaart, C.P.; Bleijendaal, H.; Chamuleau, S.A.J.; Hofstra, L.; Išgum, I.; Ziesemer, K.A.; Winter, M.M. Current state of artificial intelligence-based algorithms for hospital admission prediction in patients with heart failure: A scoping review. Eur. Heart J. Digit. Health 2022, 3, 415–425. [Google Scholar] [CrossRef]
- Badwan, B.A.; Liaropoulos, G.; Kyrodimos, E.; Skaltsas, D.; Tsirigos, A.; Gorgoulis, V.G. Machine learning approaches to predict drug efficacy and toxicity in oncology. Cell Rep. Methods 2023, 3, 100413. [Google Scholar] [CrossRef]
- Lauritsen, S.M.; Kristensen, M.; Olsen, M.V.; Larsen, M.S.; Lauritsen, K.M.; Jørgensen, M.J.; Lange, J.; Thiesson, B. Explainable artificial intelligence model to predict acute critical illness from electronic health records. Nat. Commun. 2020, 11, 3852. [Google Scholar] [CrossRef]
- Gandolfi, M.; Galazzo, I.B.; Pavan, R.G.; Cruciani, F.; Vale, N.; Picelli, A.; Storti, S.F.; Smania, N.; Menegaz, G. eXplainable AI Allows Predicting Upper Limb Rehabilitation Outcomes in Sub-Acute Stroke Patients. IEEE J. Biomed. Health Inform. 2023, 27, 263–273. [Google Scholar] [CrossRef]
- Kokkotis, C.; Moustakidis, S.; Tsatalas, T.; Ntakolia, C.; Chalatsis, G.; Konstadakos, S.; Hantes, M.E.; Giakas, G.; Tsaopoulos, D. Leveraging explainable machine learning to identify gait biomechanical parameters associated with anterior cruciate ligament injury. Sci. Rep. 2022, 12, 6647. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Kawakami, M.; Kondo, K.; Tsujikawa, M.; Honaga, K.; Suzuki, K.; Tsuji, T. Improvement of predictive accuracies of functional outcomes after subacute stroke inpatient rehabilitation by machine learning models. PLoS ONE 2023, 18, e0286269. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.; Chung, B.; Panchaseelan, H.P.; Kim, T.; Park, H.; Oh, B.; Chun, M.; Won, S.; Kim, D.; Beom, J.; et al. Forecasting the Walking Assistance Rehabilitation Level of Stroke Patients Using Artificial Intelligence. Diagnostics 2021, 11, 1096. [Google Scholar] [CrossRef] [PubMed]
- Imura, T.; Toda, H.; Iwamoto, Y.; Inagawa, T.; Imada, N.; Tanaka, R.; Inoue, Y.; Araki, H.; Araki, O. Comparison of Supervised Machine Learning Algorithms for Classifying of Home Discharge Possibility in Convalescent Stroke Patients: A Secondary Analysis. J. Stroke Cerebrovasc. Dis. 2021, 30, 106011. [Google Scholar] [CrossRef]
- Juneau, P.; Baddour, N.; Burger, H.; Bavec, A.; Lemaire, E.D. Amputee Fall Risk Classification Using Machine Learning and Smartphone Sensor Data from 2-Minute and 6-Minute Walk Tests. Sensors 2022, 22, 1749. [Google Scholar] [CrossRef]
- Hu, W.; Combden, O.; Jiang, X.; Buragadda, S.; Newell, C.J.; Williams, M.C.; Critch, A.L.; Ploughman, M. Machine learning classification of multiple sclerosis patients based on raw data from an instrumented walkway. Biomed. Eng. Online 2022, 21, 21. [Google Scholar] [CrossRef]
- Santilli, V.; Mangone, M.; Diko, A.; Alviti, F.; Bernetti, A.; Agostini, F.; Palagi, L.; Servidio, M.; Paoloni, M.; Goffredo, M.; et al. The Use of Machine Learning for Inferencing the Effectiveness of a Rehabilitation Program for Orthopedic and Neurological Patients. Int. J. Environ. Res. Public Health 2023, 20, 5575. [Google Scholar] [CrossRef]
- Rajendran, S.; Obeid, J.S.; Binol, H.; D’Agostino, R.; Foley, K.; Zhang, W.; Austin, P.; Brakefield, J.; Gurcan, M.N.; Topaloglu, U. Cloud-Based Federated Learning Implementation Across Medical Centers. JCO Clin. Cancer Inform. 2021, 5, 1–11. [Google Scholar] [CrossRef]
- Palumbo, A.; Ielpo, N.; Calabrese, B.; Corchiola, D.; Garropoli, R.; Gramigna, V.; Perri, G. SIMpLE: A Mobile Cloud-Based System for Health Monitoring of People with ALS. Sensors 2021, 21, 7239. [Google Scholar] [CrossRef]
- Calabrese, B.; Cannataro, M. Cloud Computing in Healthcare and Biomedicine. Scalable Comput. Pract. Exp. 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Hsieh, J.C.; Li, A.H.; Yang, C.C. Mobile, cloud, and big data computing: Contributions, challenges, and new directions in telecardiology. Int. J. Environ. Res. Public Health 2013, 10, 6131–6153. [Google Scholar] [CrossRef] [PubMed]
- Microsoft Azure. Azure for Healthcare–Healthcare Solutions. Available online: https://azure.microsoft.com/en-us/solutions/industries/healthcare/#overview (accessed on 10 July 2023).
- AWS for Health. Healthcare & Life Sciences. AWS. Available online: https://aws.amazon.com/health/?nc2=h_ql_sol_ind_hcl (accessed on 10 July 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).