Abstract
Illness cognitions (IC) influence how a patient adapts to a chronic disease. The aim was (1) to determine if training for a handcycling mountain time trial (HandbikeBattle) improves IC and (2) to identify factors associated with IC change scores. Persons with a chronic disability (N = 220; including N = 151 with spinal cord disorder) trained 5 months and participated in the time trial. The IC Questionnaire measured helplessness, acceptance, perceived benefits and was assessed before training (T1), after training (T2), and four months after the event (T3). Age, sex, body mass index (BMI), time since injury (TSI), disability characteristics, self-efficacy, mental health (MH) and musculoskeletal pain were obtained at T1. Multilevel regression analyses showed that helplessness decreased (from 11.96 to 11.28, p < 0.01) and perceived benefits increased (from 16.91 to 17.58, p < 0.01) from T1 to T2. For helplessness this decrease persisted during follow-up (11.16 at T3). Changes in helplessness were associated with self-efficacy (p = 0.02), MH (p = 0.02) and lesion completeness (p = 0.02), and were independent of disability type (p = 0.66), lesion level (p = 0.30) and demographics such as sex (p = 0.29) and age (p = 0.67). Training with peers may improve helplessness and perceived benefits in individuals with a chronic disability. Especially individuals with MH problems might benefit from training for an athletic challenge with peers to improve illness cognitions, and ultimately, quality of life.
1. Introduction
When individuals acquire a severe chronic disease, such as spinal cord injury (SCI), they are faced with life-long physical health consequences. In addition, life satisfaction is reduced and mental health problems are more common in individuals with SCI compared to the general population [1,2,3,4]. However, how individuals with SCI rate their quality of life is diverse and not necessarily related to the severity of the SCI [1,5]. It is assumed that the way individuals perceive their illness accounts for much of the individual differences in quality of life [6,7,8,9,10]. This is referred to as “illness cognitions”, “illness representations” or “appraisals”. These illness cognitions can be adaptive or maladaptive. Evers et al. [6] proposed three generic illness cognitions: “helplessness as a way of emphasizing the aversive meaning of the disease, acceptance as a way to diminish the aversive meaning, and perceived benefits as a way of adding a positive meaning to the disease.” Helplessness is a maladaptive illness cognition with negative impact on health-related behaviors and outcomes [6]. Acceptance and perceived benefits are positive adaptations with beneficial impact [6].
It is important to know more about illness cognitions and how to possibly influence illness cognitions as research in individuals with SCI showed strong negative associations between helplessness and well-being and strong positive associations between acceptance and well-being [11,12]. Two previous observational studies on change in illness cognitions in individuals with SCI showed inconclusive results. One study showed no changes in helplessness, acceptance and perceived benefits from inpatient rehabilitation to 6 months after discharge [13]. However, the other study showed a decrease of experienced illness threat associated with having SCI according to the illness representations at discharge compared to first inpatient rehabilitation admission [14]. In addition, older age and having a complete SCI were associated with higher threat at admission, but not at discharge [14].
Interventions targeting illness cognition have improved health behaviors and outcomes in diverse populations [15]. These interventions are often grounded in cognitive paradigms and have utilized motivational interviewing [16], mindfulness-based cognitive therapy [17], disease education [18], and education on illness perception [19]. Unfortunately, there is a lack of research into physical activity-types of interventions to improve illness cognition, whereas previous studies showed that such interventions are associated with improved self-efficacy [20], increased acceptance of disability [21], improved physical and mental empowerment [22], a greater sense of regaining control over the body [22], improved body satisfaction [23] and life satisfaction [24].
In addition, few studies have examined the associations between demographic and health-related factors and responsiveness to an illness cognition intervention. From previous, primarily cross-sectional, studies it is known that poor mental health and pain are consistently associated with maladaptive illness cognition in multiple populations [12,25,26,27,28,29]. In contrast, factors such as age [6,30,31,32], sex [6,32], illness duration [6,30], and Body Mass Index (BMI) [33,34] have shown inconsistent associations with illness cognition.
The purpose of this study is to assess if participation in training for a challenging handcycling event improves illness cognition in persons with a physical disability. Our hypothesis is that training for a handcycling mountain time trial (the HandbikeBattle) improves illness cognition. Our aims are twofold; (i) to describe changes in illness cognition during training and after participation, and (ii) to identify which participant-centered factors are associated with changes in illness cognition.
2. Materials and Methods
This study utilized data collected in the ongoing HandbikeBattle project.
2.1. The HandbikeBattle
The HandbikeBattle event is an annual 20.2 km mountain time trial each June. It was founded in 2013 by participating rehabilitation centers with the purpose of encouraging persons with chronic disabilities to participate in regular physical activity. Twelve Dutch rehabilitation centers participate in the event each year. Each center competes with a team consisting of former rehabilitation patients with a chronic disability, for example SCI or amputation. The 5-month training period is free-living, that is, no specific training program is provided by the researchers. Connected to the HandbikeBattle is an observational cohort study that was initiated to monitor effects of participation in the training period and the event. All HandbikeBattle participants were asked to participate in the study. They enrolled voluntarily. The study was approved by the local ethics committee of the Center for Human Movement Sciences, University Medical Center Groningen, The Netherlands (ECB/2012_12.04_l_rev/Ml). Each participant signed an informed consent before the first assessment.
2.2. Participants
Participants in the current study were former patients of twelve rehabilitation centers who participated in the HandbikeBattle between 2013 to 2018. A physician medically screened each participant prior to the start of training. Exclusion criteria included any contraindication for exercise according to the American College of Sports Medicine guidelines [35]. In addition, sufficient knowledge of the Dutch language was a prerequisite to understand the instructions and questionnaires. Participants were included in the current study if they filled out the questionnaires during at least two out of three time points.
2.3. Data Collection
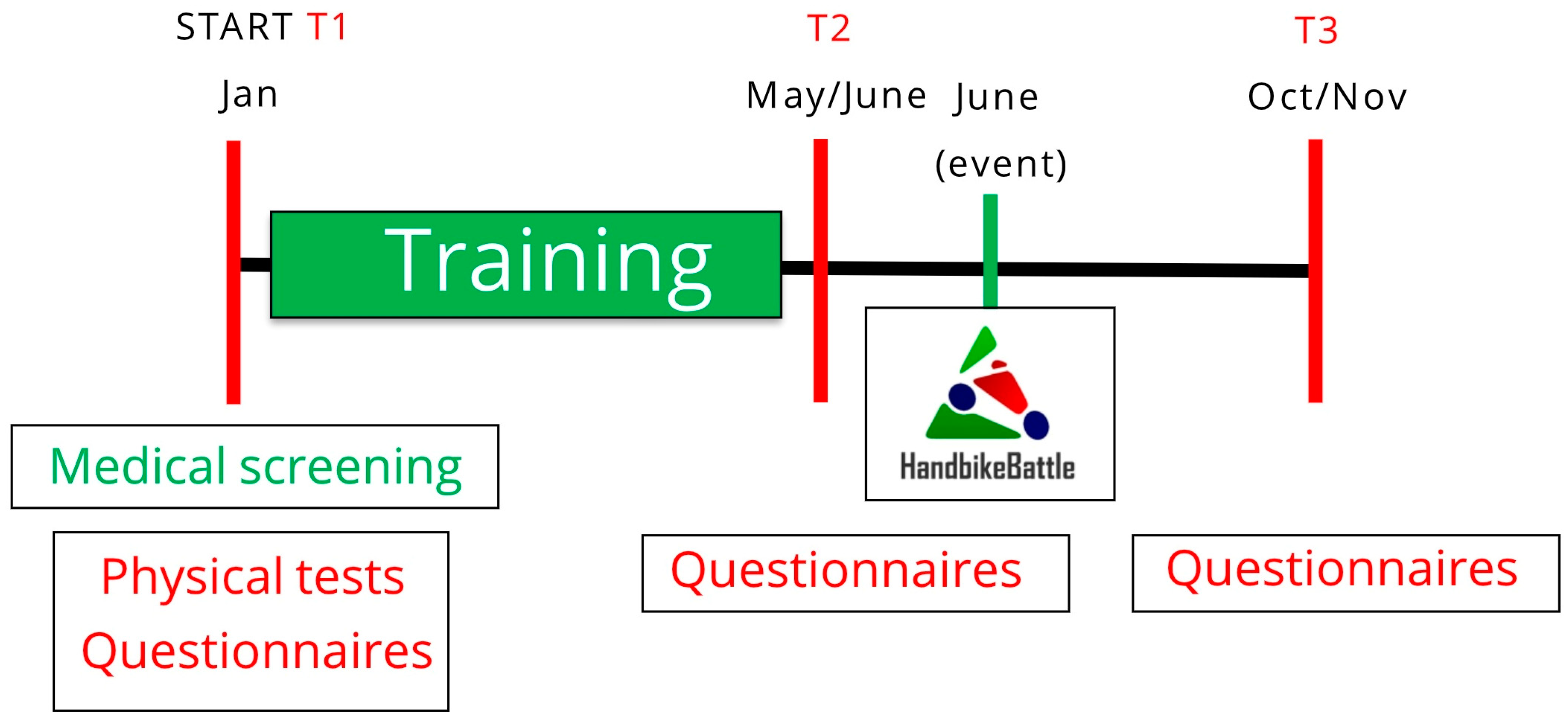
Testing occurred at each participating center’s designated test sites in January (T1) and June (T2) (Figure 1). These months, respectively, correspond to pre-training and post-training (just before the event). At T1, demographic information (age and sex), disability characteristics, physical fitness and BMI were collected. Psychological questionnaires were administered at T1, T2 and October/November (T3), i.e., post-participation. The questionnaires were part of a set of questionnaires which took participants 30–45 min to complete in total. Participants were invited by e-mail with a link and could fill out the questionnaires in their own time at home.
Figure 1.
Study design. Measurements are performed at the start of the training period (January, T1); after the training period, prior to the event (June, T2); and follow-up, four months after the event (October/November, T3).
2.4. Questionnaires
2.4.1. Illness Cognition Questionnaire
Illness cognition was quantified at T1, T2, and T3 with the self-reported Illness Cognition Questionnaire (ICQ), which consists of 18 items that are scored on a 4-point scale (1 = not at all, 2 = somewhat, 3 = to a large extent, 4 = completely) (Supplementary Table S1). The ICQ has three sub-scales; helplessness (6 items), acceptance (6 items), and perceived benefits (6 items) [6]. Helplessness includes cognitions emphasizing the aversive meaning of the illness (e.g., “My illness limits me in everything that is important to me”), acceptance includes cognitions diminishing the aversive meaning of the illness (e.g., “I have learned to accept the limitations imposed by my illness”), and perceived benefits includes cognitions giving a positive meaning to the illness (e.g., “Dealing with my illness has made me a stronger person”) [6]. Each subscale ranges from 6 to 24 and is computed by summing responses to each sub-scale item. Helplessness is computed by summing questions 1, 5, 7, 9, 12 and 15. Acceptance is computed by summing questions 2, 3, 10, 13, 14 and 17. Perceived benefits is computed by summing questions 4, 6, 8, 11, 16 and 18. Higher scores correspond to greater levels of the construct. ICQ validity and reliability have been demonstrated in individuals with multiple sclerosis and rheumatoid arthritis [6]. Previous studies have administered the ICQ to individuals with SCI [11,12,13,36].
2.4.2. Mental Health Inventory
The self-reported 5-item Mental Health Inventory (MHI-5) of the SF-36 quantified mental health at T1. It requires respondents to indicate the frequency of nervousness, sadness, peacefulness, depressed mood, and happiness during the previous 4 weeks using a 6-point Likert scale (1 = all the time to 6 = none of the time). A total score between 0 (lowest mental health) and 100 (highest mental health) was computed. MHI-5 scores at or below 72 indicate mental health problems are present [37]. For analyses, participants were dichotomized as having (MHI-5 ≤ 72) or not having (MHI-5 > 72) mental health problems. The MHI has been shown to be reliable and valid in individuals with SCI [38].
2.4.3. Self-Efficacy
Self-efficacy was measured with the self-reported general self-efficacy scale [39,40] at T1. It consists of 16 items graded on a 5-point Likert scale ranging from strongly disagree to strongly agree. A total score between 16 (low self-efficacy) and 80 (high self-efficacy) was computed. It has good re-test reliability and internal consistency [39,40] and has been used in other SCI studies [11,13].
2.4.4. Pain Assessment
Participants were asked in a standardized self-reported questionnaire whether they experienced pain in the joints or muscles of both upper extremities at T1. If pain was present, participants were asked to indicate the location and rate the severity (1 = no pain, 2 = very mild, 3 = mild, 4 = moderate, 5 = severe, and 6 = very severe). For analyses participants were dichotomized into no/mild pain (rated 0–3) or moderate/severe pain (rated ≥ 4) [41].
2.4.5. Sports Participation
The hours of sports participation per week were self-reported by the participants at T2 and T3.
2.5. Physical Determinants
2.5.1. Disability Etiology
Participants with SCI and spina bifida were grouped together for analysis. Participants with other injury types (e.g., amputation and multi trauma) were categorized as “other”.
2.5.2. SCI Lesion Characteristics
SCI was characterized according to the 2011 International Standards for Neurological Classification of Spinal Cord Injury [42]. Paraplegia was defined as neurologic levels at or below T2 and tetraplegia was defined as neurologic levels at or above T1. Motor complete corresponded to AIS grades A and B and motor incomplete corresponded to AIS grades C and D.
2.5.3. Time Since Injury
For all participants, time since injury (TSI) was determined as the time between occurrence of injury and the first measurement time (T1).
2.5.4. Physical Fitness
Physical fitness was defined as peak power output (POpeak, watts (W)) determined during a synchronous arm crank aerobic exercise test to volitional exhaustion. Either a 1-min step protocol or continuous ramp protocol was used and was individualized for each participant. For the 1-min protocol, POpeak was defined as the highest PO that was maintained for at least 30 seconds. For the ramp protocol, the highest PO achieved during the test was considered POpeak.
2.5.5. Body Mass Index
BMI was calculated for each participant with the following formula: body mass (kg)/height (m)2. Body mass was measured with a wheelchair scale and height was recalled by the participants.
2.6. Statistical Analysis
The analyses were performed with SPSS (Version 28.0; IBM Corp., Armonk, NY, USA) and MLwiN version 2.36 [43]. Demographic and health-related factors of the study participants were analyzed by using descriptive statistics, including mean and percentage. Data were tested for normality with the Kolmogorov-Smirnov test with Lilliefors significance correction, combined with z scores for skewness and kurtosis. To ascertain possible response bias, characteristics of included participants in the present study were compared with non-participants (i.e., drop-outs or those who did not fill out all questionnaires) using t-tests, Mann-Whitney U tests, and chi-square tests.
Multilevel analyses were conducted to be able to make adjustments for the dependency of the observations within participants and participants within centers. An additional advantage of multilevel analyses is the robustness for missing data [44].
To test the first aim, multilevel models with three levels were created; with observations within participants as first level, participant as second level, and rehabilitation center as third level. Three models were created to examine the longitudinal trajectory of illness cognition: one with helplessness, one with acceptance and one with perceived benefits as outcome measure. Time (T1, T2, T3) was included as a categorical variable with two dummies.
To test the second aim, exploratory analyses were performed in which interaction terms with the time dummies were investigated in a series of separate models for each of the following T1 determinants: age (years), sex (male = 1, female = 2), BMI (kg/m2), disability etiology (SCI/SB = 0, other = 1), TSI (years), lesion completeness (motor complete = 0, motor incomplete = 1), level of injury (tetraplegia = 0, paraplegia = 1), POpeak (W), musculoskeletal pain (no-mild = 0, moderate-severe = 1), self-efficacy, mental health (mental health problems MHI-5 ≤ 72 = 0, no mental health problems MHI-5 > 72 = 1).
3. Results
In total, 319 individuals with chronic physical disabilities started training. Forty-three individuals dropped out due to medical reasons (N = 20), motivational problems (N = 12), being too busy with work/family (N = 5) or personal/unknown reasons (N = 6). Fifty-six individuals did not fill out questionnaires at any time-point (N = 25), or only at one time-point (N = 31). Hence, 99 out of 319 individuals were not included in the present study and defined as non-participants. This resulted in 220 participants to be included. The sample for this study included 151 individuals with SCI or spina bifida, and 69 with other disabilities. Participants were on average older and had a higher POpeak than non-participants (Table 1). Descriptive data of the IC outcome measures at all time-points are depicted in Table 2. The mean hours of sports participation at T2 and T3 was 8.2 ± 3.2 h/week and 5.8 ± 4.0 h/week, respectively.

Table 1.
Demographic characteristics, injury-related characteristics, and baseline assessments of participants and non-participants.

Table 2.
Illness cognition of participants at all time points.
3.1. Longitudinal Changes in Illness Cognition
Helplessness showed a significant improvement between T1 (start of training) and T2 (after training), whereas no significant change was found during follow-up (T3) (Table 3). When the model was recalculated with T1 as reference category, there was a significant improvement between T1 and T3 (Table 3). Acceptance showed no significant changes over time (Table 3). Perceived benefits showed a significant improvement between T1 and T2, whereas no significant change was found during follow-up (T3) (Table 3). When the model was recalculated with T1 as reference category, there was no significant change between T1 and T3 (Table 3).

Table 3.
Longitudinal trajectory of illness cognition.
3.2. Factors Associated with Change in Illness Cognition
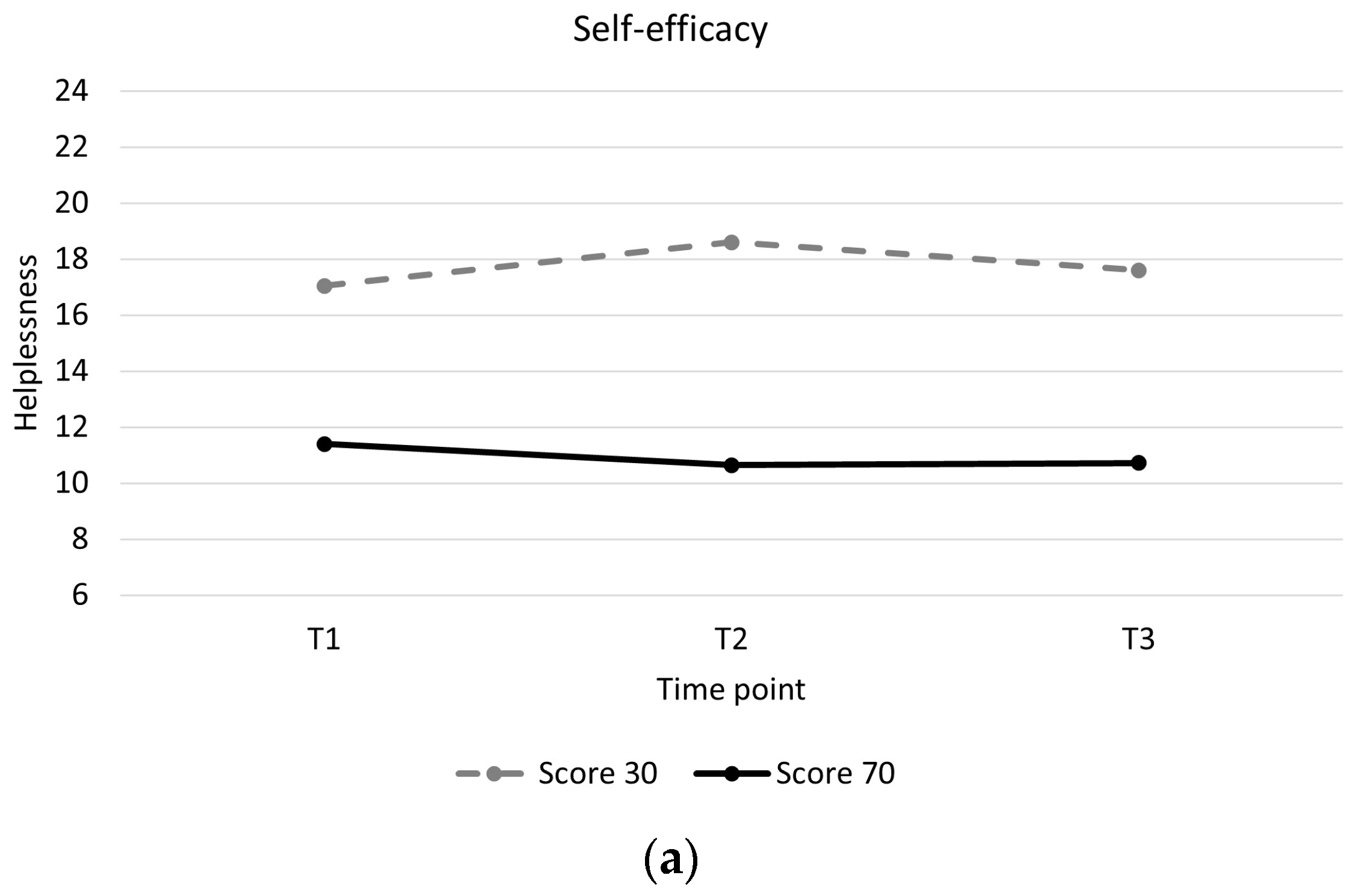
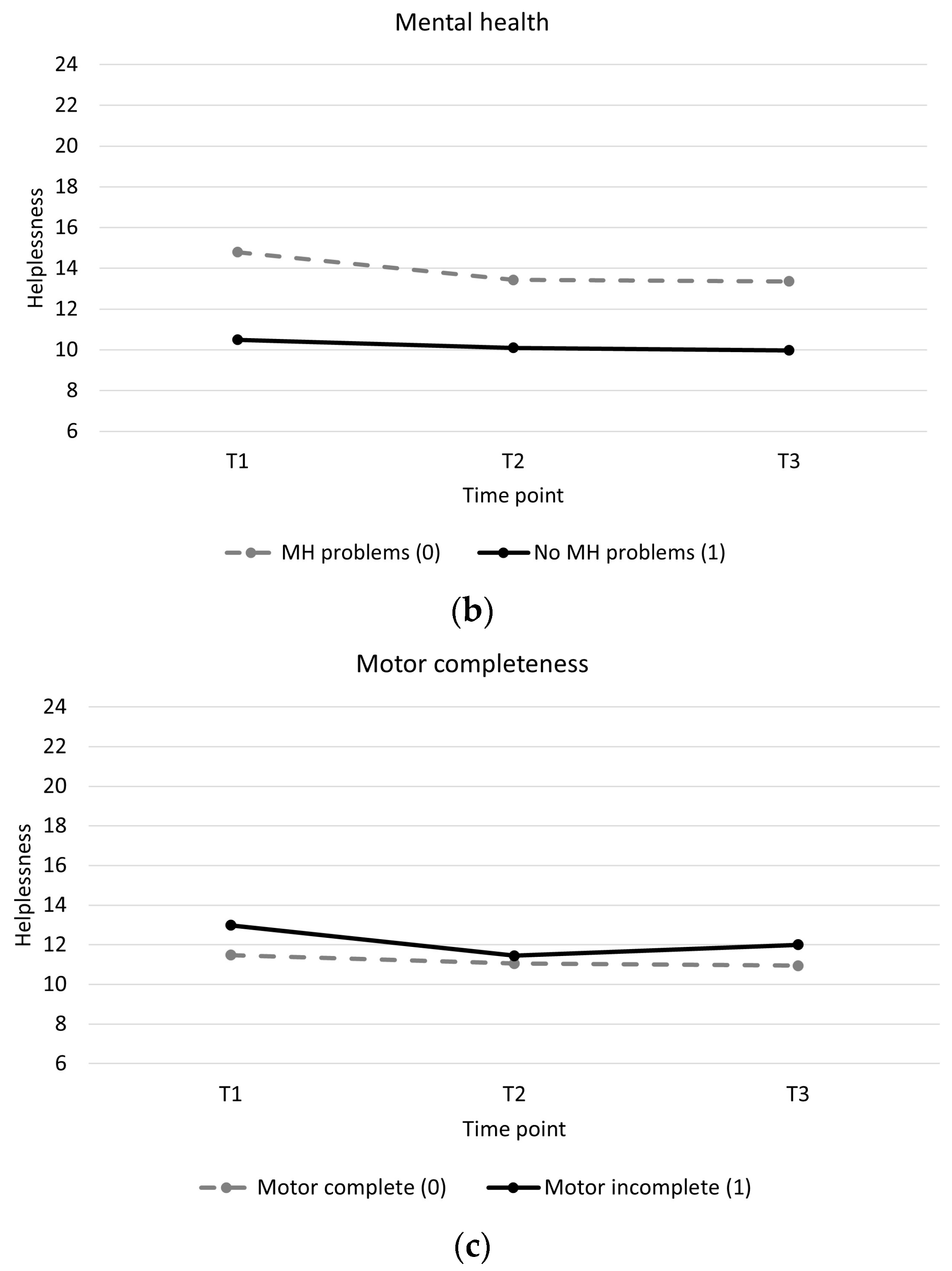
Individuals with low baseline self-efficacy showed higher levels of helplessness throughout and showed a slight increase in helplessness during training compared to individuals with high baseline self-efficacy, who showed a slight decrease in helplessness (Table 4, Figure 2a). Individuals with mental health problems showed higher levels of helplessness throughout and greater decreases in helplessness between pre and post training than individuals without mental health problems (Table 4, Figure 2b). Individuals with a motor incomplete injury showed greater decreases in helplessness than individuals with motor complete injury (Table 4, Figure 2c). There were no significant associations between changes in acceptance and perceived benefits over time and baseline participant characteristics (Supplementary Material Tables S2 and S3).

Table 4.
Longitudinal trajectory of helplessness with interaction effects.
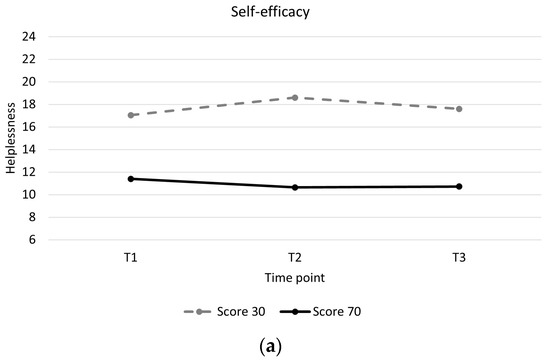
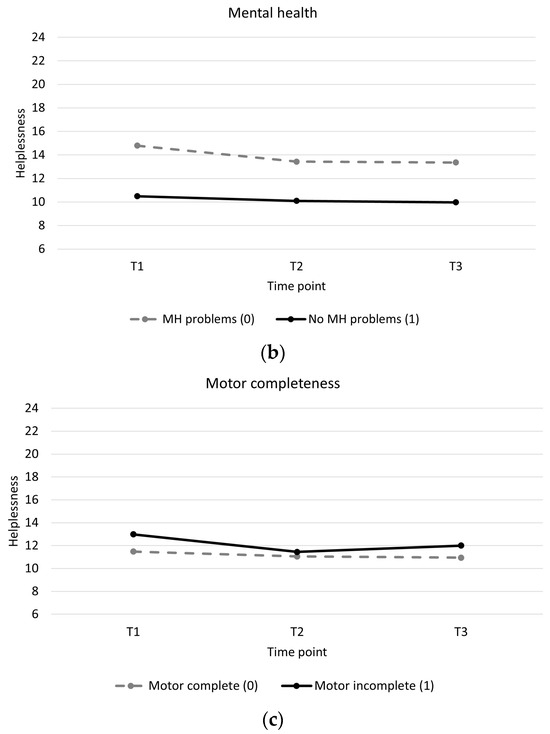
Figure 2.
Longitudinal trajectory of helplessness with interaction effects. (a): Helplessness for a participant with a self-efficacy score of 30 (low self-efficacy) versus a participant with a self-efficacy score of 70 (high self-efficacy). (b): Helplessness for participants with mental health problems (0) versus participants without mental health problems (1). (c): Helplessness for participants with a motor complete spinal cord injury (0) versus participants with a motor incomplete spinal cord injury (1).
4. Discussion
We investigated the effect of training for a handcycling mountain time trial on illness cognition in individuals with chronic disability. It appears that participation in training for the HandbikeBattle can improve illness cognition specifically by decreasing helplessness and increasing perceived benefits. For helplessness, this effect appears to persist for several months after participation in the time trial. Self-efficacy, mental health and lesion completeness at baseline were associated with change in helplessness during the training period, whereas, among others, disability etiology (SCI/SB versus other) was not.
Participants in the current study showed helplessness scores within the same range found in previous studies with patients with SCI, stroke, multiple sclerosis and rheumatoid arthritis (median and mean ranging from 11.3 to 13.4) [6,13,45]. The acceptance scores were within the same range as scores in previous studies as well (median and mean ranging from 15.8 to 19.0) [6,13,45]. The pre-training perceived benefits scores were higher than in previous studies (median and mean ranging from 15.0 to 15.5) [6,13,45]. Possible differences might be due to TSI. The participants in the current study had an average TSI of 12 years and were in the chronic phase of their disease, whereas some of the previous studies included patients within or shortly after inpatient rehabilitation.
We observed small but significant decreases in helplessness and small but significant increases in perceived benefits after five months of free-living handbike training and these improvements were sustained four months after participation in the HandbikeBattle. The improvement in helplessness was 4.4% for the total group from pre-training (T1) to follow-up (T3), whereas this improvement was 8.0% for the subgroup with mental health problems. Although improvements are small, previous studies on illness cognition interventions showed similar results. In the study by Callahan et al. [46] effects of a 6-week walking program for adults with arthritis were evaluated. They found a significant improvement in helplessness of 5.5% with an effect size of 0.24–0.28. Sararoudi et al. [18] studied an illness perception intervention given by a mental health counselor in patients with myocardial infarction. They reported an improvement of 7.1% in illness perception, 3 months after discharge.
The effects that were found in the present study may be explained by the role of exercise in alleviating stress and pain, and improving resiliency, personal control, self-efficacy, social integration and functional independence [47,48,49,50,51,52,53]. Anecdotal evidence suggests that social integration and peer support play an important role during training for and participating in the HandbikeBattle event. Unfortunately, we did not collect data with the participants in the current study to support this.
It is interesting that the decrease in helplessness was sustained several months after the event despite evidence that the stress-relieving benefits of exercise significantly decline after two weeks of exercise cessation [47]. Based on the self-reported hours of sports participation, it seems that most participants still participate in sports after the HandbikeBattle, but spend less hours on sports participation than during the training period. More detailed information about participants’ exercise habits following event completion would help clarify whether maintenance of decreased helplessness is mediated by continued exercise.
Our findings regarding associations between pre-training participant-centered factors and change in illness cognition are both supported and contradicted by the literature. Cross-sectional associations between illness cognition and mental health [12,45,54,55] and self-efficacy [56,57,58] are consistently reported in the existing literature. The results in the present study are in line with these findings. However, it is unclear whether the association with motor incompleteness is supported by literature. Kuiper et al. [14] showed that motor complete SCI was associated with higher threat at admission. At discharge there was no association with motor completeness. The findings of Kuiper et al. [14] could suggest that those with motor complete SCI experience a stronger decrease of threat compared to those with motor incomplete SCI. Though one would indeed expect individuals with less severe SCI to have lower levels of helplessness than those with more severe injury, the findings in the current study supports research that demonstrates poorer psychological outcomes in individuals with motor incomplete injuries [59]. Previously cited reasons for this finding include shorter acute rehabilitation stays, frustration with slowness of ambulation, anxiety about reinjury, and feeling misunderstood due to having less visible deficits [59]. More research is needed to confirm the role of lesion completeness in illness cognition.
4.1. Practical Implications
Overall we can conclude that in individuals with a chronic disability, small improvements in illness cognition can be achieved with training for a challenging handcycling event with peers. Therefore, physical activity-types of interventions might help to improve illness cognition, alongside cognitive interventions. In addition, we showed that changes in illness cognitions are more or less independent of disability type (SCI/SB versus other), lesion level and demographics such as sex and age. It might be that the way individuals perceive their illness is not necessarily dependent on the type and severity of the disease, but on other (psychological) factors. We showed that especially individuals with mental health problems showed a decrease in helplessness. Therefore, especially this group might benefit from training for and participating in an athletic challenge with peers to improve illness cognitions, and ultimately, quality of life. These findings concur with previous longitudinal HandbikeBattle studies that showed improvements in body satisfaction and life satisfaction during training [23,24]. A change in illness cognition might be another piece in the multifactorial puzzle of improvements in quality of life during training and physical activity.
4.2. Study Limitations
There might be a selection bias, as individuals who dropped out before or during the training period were not included in the analyses. Therefore, individuals with a very low cardiorespiratory fitness might be less prominent in the present study. In addition, individuals who are well-adjusted to their disability may be more likely to sign up for the HandbikeBattle. It is, however, important to mention that the event was created to push physical and mental boundaries for individuals who might need this and that participants were not professional athletes. Second, we only measured musculoskeletal pain and no other causes of pain such as neuropathic pain. Therefore, we are unsure whether pain in general might effect changes in illness cognition. Third, we are unable to comment on what social aspects of the training, such as social integration and peer support, might have contributed to the observed changes. Last, there is a potential risk of bias with self-reported questionnaires. For example, it has been described that individuals might overestimate their level of physical activity and sports participation [60]. In that respect objective measures are more appropriate to measure physical activity. In future studies, qualitative methods might aid in unraveling more details on the change in illness cognition, associated factors and whether individuals perceive this change as meaningful.
5. Conclusions
Training with peers may improve helplessness and perceived benefits in individuals with a chronic disability. For helplessness, this change appears to persist for several months during follow-up. Changes in illness cognitions are more or less independent of disability type, lesion level and demographics such as sex and age. Especially mental health and self-efficacy are important determinants for (change in) helplessness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph21010058/s1, Table S1: Illness Cognition Questionnaire; Table S2: Longitudinal trajectory of acceptance with interaction effects; Table S3: Longitudinal trajectory of perceived benefits with interaction effects.
Author Contributions
Conceptualization, J.M.D., R.E.C., L.J.M.V., S.d.G., C.M.C.v.L. and M.W.M.P.; data collection, HandbikeBattle Group and I.K.; methodology, J.M.D., R.E.C., I.K., S.d.G., M.W.M.P. and H.W.; formal analysis, I.K. and H.W.; writing—original draft preparation, J.M.D. and I.K.; writing—review and editing, S.d.G., M.W.M.P., L.J.M.V., C.M.C.v.L., H.W. and R.E.C.; funding acquisition, L.J.M.V. and S.d.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by HandicapNL (project no. R2015028), Stichting Mitialto, Stichting Beatrixoord Noord-Nederland (project no. 210.175), University Medical Center Groningen, Heliomare Rehabilitation Center and Stichting Handbike Events.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the local ethics committee of the Center for Human Movement Sciences, University Medical Center Groningen, the Netherlands (protocol code: ECB/2012_12.04_l_rev/Ml, approval date: 28 January 2013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
HandbikeBattle group: Paul Dobbelsteijn, Adelante Zorggroep, Hoensbroek, The Netherlands. Eric Helmantel, University Medical Center Groningen, Center for Rehabilitation Beatrixoord, Groningen, The Netherlands. Esther Belser, Heliomare Rehabilitation Center, Wijk aan Zee, The Netherlands. Linda van Vliet, Rehabilitation Center De Hoogstraat, Utrecht, The Netherlands. Christian van Niekerk, Libra Rehabilitation and Audiology, Eindhoven, The Netherlands. Lise Wilders, Sint Maartenskliniek, Nijmegen, The Netherlands. Ludwine van Orsouw, Amsterdam Rehabilitation Research Center|Reade, Amsterdam, The Netherlands. Wilbert Snoek, Rehabilitation Center Revant, Breda, The Netherlands. Saskia Maas, Rijndam Rehabilitation Center, Rotterdam, The Netherlands. Bram van Gemeren, Roessingh Rehabilitation Center, Enschede, The Netherlands. Edwin Blom, Rehabilitation center Tolbrug, Den Bosch, The Netherlands. Anniek van Vilsteren, Vogellanden, Zwolle, The Netherlands.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Van Koppenhagen, C.F.; Post, M.W.M.; van der Woude, L.H.V.; de Witte, L.P. Changes and Determinants of Life Satisfaction After Spinal Cord Injury: A Cohort Study in The Netherlands. Arch. Phys. Med. Rehabil. 2008, 89, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- Dijkers, M.P.J.M. Quality of life of individuals with spinal cord injury: A review of conceptualization, measurement, and research findings. JRRD 2005, 42, 87–110. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.; Tran, Y.; Middleton, J. Psychological morbidity and spinal cord injury: A systematic review. Spinal Cord 2009, 47, 108–114. [Google Scholar] [CrossRef] [PubMed]
- North, N.T. The psychological effects of spinal cord injury: A review. J. Spinal Cord Med. 1999, 37, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Petrie, K.J.; Broadbent, E.; Kydd, R. Illness perceptions in mental health: Issues and potential applications. J. Ment. Health 2008, 17, 559–564. [Google Scholar] [CrossRef]
- Evers, A.W.M.; Kraaimaat, F.W.; van Lankveld, W.; Jongen, P.J.H.; Jacobs, J.W.G.; Bijlsma, J.W.J. Beyond unfavorable thinking: The Illness Cognition Questionnaire for chronic diseases. J. Consult. Clin. Psychol. 2001, 69, 1026–1036. [Google Scholar] [CrossRef]
- Broadbent, E.; Petrie, K.J.; Main, J.; Weinman, J. The Brief Illness Perception Questionnaire. J. Psychosom. Res. 2006, 60, 631–637. [Google Scholar] [CrossRef]
- Shabany, M.; Ghodsi, S.M.; Arejan, R.H.; Baigi, V.; Ghodsi, Z.; Rakhshani, F.; Gholami, M.; Sharif, P.M.; Shool, S.; Vaccaro, A.R.; et al. Cognitive appraisals of disability in persons with traumatic spinal cord injury: A scoping review. Spinal Cord 2022, 60, 954–962. [Google Scholar] [CrossRef]
- Russell, M.; Ames, H.; Dunn, C.; Beckwith, S.; Holmes, S.A. Appraisals of disability and psychological adjustment in veterans with spinal cord injuries. J. Spinal Cord Med. 2021, 44, 958–965. [Google Scholar] [CrossRef]
- Kennedy, P.; Evans, M.; Sandhu, N. Psychological adjustment to spinal cord injury: The contribution of coping, hope and cognitive appraisals. Psychol. Health Med. 2009, 14, 17–33. [Google Scholar] [CrossRef]
- Van Leeuwen, C.M.C.; Post, M.W.M.; Westers, P.; van der Woude, L.H.V. Relationships Between Activities, Participation, Personal Factors, Mental Health, and Life Satisfaction in Persons with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2012, 93, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Wollaars, M.M.; Post, M.W.M.; van Asbeck, F.W.A.; Brand, N. Spinal Cord Injury Pain: The Influence of Psychologic Factors and Impact on Quality of Life. Clin. J. Pain 2007, 23, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, C.M.C.; Edelaar-Peeters, Y.; Peter, C.; Stiggelbout, A.M.; Post, M.W.M. Psychological factors and mental health in persons with spinal cord injury: An exploration of change or stability. J. Rehabil. Med. 2015, 47, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, H.; van Leeuwen, C.M.C.; Stolwijk-Swüste, J.M.; Post, M.W.M. Illness perception of individuals with spinal cord injury (SCI) during inpatient rehabilitation: A longitudinal study. Spinal Cord 2022, 60, 831–836. [Google Scholar] [CrossRef]
- Jones, C.J.; Smith, H.E.; Llewellyn, C.D. A systematic review of the effectiveness of interventions using the Common Sense Self-Regulatory Model to improve adherence behaviours. J. Health Psychol. 2016, 21, 2709–2724. [Google Scholar] [CrossRef]
- Mülhauser, S.; Bonhote Börner, M.; Saner, H.; Zumstein-Shaha, M. The impact of motivational interviewing on illness perception in patients with stable coronary artery disease-a randomised controlled study. Pflege 2018, 31, 75–85. [Google Scholar] [CrossRef]
- Dalili, Z.; Bayazi, M.H. The effectiveness of Mindfulness-Based Cognitive Therapy on the illness perception and Psychological Symptoms in patients with Rheumatoid Arthritis. Complement. Ther. Clin. Pract. 2019, 34, 139–144. [Google Scholar] [CrossRef]
- Sararoudi, R.B.; Motmaen, M.; Maracy, M.R.; Pishghadam, E.; Kheirabadi, G.R. Efficacy of illness perception focused intervention on quality of life, anxiety, and depression in patients with myocardial infarction. J. Res. Med. Sci. 2016, 21, 125. [Google Scholar] [CrossRef]
- Weldam, S.W.M.; Schuurmans, M.J.; Zanen, P.; Heijmans, M.J.W.M.; Sachs, A.P.E.; Lammers, J.W.J. The effectiveness of a nurse-led illness perception intervention in COPD patients: A cluster randomised trial in primary care. ERJ Open Res. 2017, 3, 115–2016. [Google Scholar] [CrossRef]
- Côté-Leclerc, F.; Boileau Duchesne, G.; Bolduc, P.; Gëlinas-Lafreniere, A.; Santerre, C.; Desrosiers, J.; Levasseur, M. How does playing adapted sports affect quality of life of people with mobility limitations? Results from a mixed-method sequential explanatory study. Health Qual. Life Outcomes 2017, 15, 1–8. [Google Scholar] [CrossRef]
- Sporner, M.L.; Fitzgerald, S.G.; DiCianno, B.E.; Collins, D.; Teodorski, E.; Pasquina, P.F.; Cooper, R.A. Psychosocial impact of participation in the National Veterans Wheelchair Games and Winter Sports Clinic. Disabil. Rehabil. 2009, 31, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Ashton-Shaeffer, C.; Gibson, H.; Holt, M.; Willming, C. Women’s resistance and empowerment through wheelchair sport. World Leisure 2001, 4, 11–21. [Google Scholar] [CrossRef]
- Kouwijzer, I.; de Groot, S.; van Leeuwen, C.M.C.; Valent, L.J.M.; Stolwijk-Swüste, J.M.; HandbikeBattle group; van der Woude, L.H.V.; Post, M.W.M. Changes in body satisfaction during and after a 5-month handcycle training period and associations with physical capacity and body composition in individuals with a physical impairment. Disabil. Rehabil. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Kouwijzer, I.; de Groot, S.; van Leeuwen, C.M.C.; Valent, L.J.M.; van Koppenhagen, C.F.; HandbikeBattle group; van der Woude, L.H.V.; Post, M.W.M. Changes in Quality of Life During Training for the HandbikeBattle and Associations With Cardiorespiratory Fitness. Arch. Phys. Med. Rehabil. 2020, 101, 1017–1024. [Google Scholar] [CrossRef]
- Treharne, G.J.; Kitas, G.D.; Lyons, A.C.; Booth, D.A. Well-being in rheumatoid arthritis: The effects of disease duration and psychosocial factors. J. Health Psychol. 2005, 10, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.; Taal, E.; van der Linden, S.; Boonen, A. A review of instruments to assess illness representations in patients with rheumatic diseases. Ann. Rheum. Dis. 2009, 68, 305–309. [Google Scholar] [CrossRef]
- Sawyer, A.T.; Harris, S.L.; Koenig, H.G. Illness perception and high readmission health outcomes. Health Psychol. Open 2019, 6, 2055102919844504. [Google Scholar] [CrossRef]
- De Raaij, E.J.; Ostelo, R.W.; Maissan, F.; Mollema, J.; Wittink, H. The association of illness perception and prognosis for pain and physical function in patients with noncancer musculoskeletal pain: A systematic literature review. J. Orthop. Sports Phys. Ther. 2018, 48, 789–800. [Google Scholar] [CrossRef]
- Arat, S.; de Cock, D.; Moons, P.; Vandenberghe, J.; Westhovens, R. Modifiable correlates of illness perceptions in adults with chronic somatic conditions: A systematic review. Res. Nurs. Health 2018, 41, 173–184. [Google Scholar] [CrossRef]
- Han, J.; Liu, J.E.; Qiu, H.; Nie, Z.H.; Su, Y.L. Illness cognitions and the associated socio-demographic and clinical factors in Chinese women with breast cancer. Eur. J. Oncol. Nurs. 2018, 32, 33–39. [Google Scholar] [CrossRef]
- Chao, H.L.; Tsai, T.Y.; Livneh, H.; Lee, H.C.; Hsieh, P.C. Patients with colorectal cancer: Relationship between demographic and disease characteristics and acceptance of disability. J. Adv. Nurs. 2010, 66, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Lauwerier, E.; Crombez, G.; van Damme, S.; Goubert, L.; Vogelaers, D.; Evers, A.W.M. The construct validity of the Illness Cognition Questionnaire: The robustness of the three-factor structure across patients with chronic pain and chronic fatigue. Int. J. Behav. Med. 2010, 17, 90–96. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaiamnuay, S.; Bertoli, A.M.; Fernandez, M.; Apte, M.; Vila, L.M.; Reveille, J.D.; Alarcon, G.S.; LUMINA study group. The impact of increased body mass index on systemic lupus erythematosus: Data from LUMINA, a multiethnic cohort. J. Clin. Rheumatol. 2007, 13, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Tığlı, A.; Soy, E.H.A.; Aytar, A.; Moray, G.; Haberal, M. Relationship between exercise perception with physical activity level, body awareness, and illness cognition in renal transplant patients: A pilot study. Exp. Clin. Transplant. 2019, 17, 270–276. [Google Scholar] [CrossRef]
- Lupash, E.; Ehrman, J.J. (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Voerman, G.E.; Erren-Wolters, C.V.; Fleuren, J.F.; Hermens, H.J.; Geurts, A.C. Perceived spasticity in chronic spinal cord injured patients: Associations with psychological factors. Disabil. Rehabil. 2010, 32, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Hoeymans, N.; Garssen, A.A.; Westert, G.P.; Verhaak, P.F.M. Measuring mental health of the Dutch population: A comparison of the GHQ-12 and the MHI-5. Health Qual. Life Outcomes 2004, 36, 1–6. [Google Scholar]
- Van Leeuwen, C.M.C.; van der Woude, L.H.V.; Post, M.W.M. Validity of the mental health subscale of the SF-36 in persons with spinal cord injury. Spinal Cord 2012, 50, 707–710. [Google Scholar] [CrossRef]
- Bosscher, R.J.; Smit, J.H. Confirmatory factor analysis of the general self-efficacy scale. Behav. Res. Ther. 1998, 36, 339–343. [Google Scholar] [CrossRef]
- Sherer, M.; Maddux, J.E.; Mercandante, B.; Prentice-Dunn, S.; Jacobs, B.; Rogers, R.W. The self-efficacy scale: Construction and validation. Psychol. Rep. 1982, 51, 663–671. [Google Scholar] [CrossRef]
- Van Drongelen, S.; De Groot, S.; Veeger, H.E.J.; Angenot, E.L.D.; Dallmeijer, A.J.; Post, M.W.M.; Van Der Woude, L.H.V. Upper extremity musculoskeletal pain during and after rehabilitation in wheelchair-using persons with a spinal cord injury. Spinal Cord 2006, 44, 152–159. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sorensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Rasbash, J.; Charlton, C.; Browne, W.J.; Healy, M.; Cameron, B. MLwiN Version 2.36. Centre for Multilevel Modelling, University of Bristol. 2005. Available online: https://www.bristol.ac.uk/cmm/software/mlwin/refs.html (accessed on 6 October 2023).
- Maas, C.J.M.; Snijders, T.A.B. The Multilevel Approach to Repeated Measures for Complete and Incomplete Data. Qual. Quant. 2003, 37, 71–89. [Google Scholar] [CrossRef]
- Van Mierlo, M.L.; van Heugten, C.M.; Post, M.W.M.; de Kort, P.L.M.; Visser-Meily, J.M.A. Life satisfaction post stroke: The role of illness cognitions. J. Psychosom. Res. 2015, 79, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Callahan, L.F.; Shreffler, J.H.; Altpeter, M.; Schoster, B.; Hootman, J.; Houenou, L.O.; Martin, K.R.; Schwartz, T.A. Evaluation of group and self-directed formats of the arthritis foundation’s Walk with Ease program. Arthritis Care Res. 2011, 63, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.N.; Deuster, P.A. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus 2014, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, B.N.; Fleshner, M. Exercise, learned helplessness, and the stress-resistant brain. Neuro Mol. Med. 2008, 10, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.L.; Martin, K.A.; Ditor, D.S.; Latimer, A.E.; Craven, C.; Bugaresti, J.; McCartney, N. Long-term exercise training in persons with spinal cord injury: Effects on strength, arm ergometry performance and psychological well-being. Spinal Cord 2003, 41, 34–43. [Google Scholar] [CrossRef]
- Motl, R.W.; McAuley, E. Pathways Between Physical Activity and Quality of Life in Adults with Multiple Sclerosis. Health Psychol. 2009, 28, 682–689. [Google Scholar] [CrossRef]
- Sweet, S.N.; Martin Ginis, K.A.; Tomasone, J.R. Investigating Intermediary Variables in the Physical Activity and Quality of Life Relationship in Persons With Spinal Cord Injury. Health Psychol. 2013, 32, 877–885. [Google Scholar] [CrossRef]
- Haisma, J.A.; Post, M.W.M.; van der Woude, L.H.V.; Stam, H.J.; Bergen, M.P.; Sluis, T.A.; van den Berg-Emons, H.J.; Bussmann, J.B. Functional independence and health-related functional status following spinal cord injury: A prospective study of the association with pfysical capacity. J. Rehabil. Med. 2008, 40, 812–818. [Google Scholar] [CrossRef]
- Martin Ginis, K.A.; Jetha, A.; Mack, D.E.; Hetz, S. Physical activity and subjective well-being among people with spinal cord injury: A meta-analysis. Spinal Cord 2010, 48, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Aaby, A.; Lykkegaard Ravn, S.; Kasch, H.; Andersen, T.E. The associations of acceptance with quality of life and mental health following spinal cord injury: A systematic review. Spinal Cord 2020, 58, 130–148. [Google Scholar] [CrossRef] [PubMed]
- Moyano, S.; Scolnik, M.; Vergara, F.; Garcia, M.V.; Sabelli, M.R.; Rosa, J.E.; Catoggio, L.J.; Soriano, E.R. Evaluation of Learned Helplessness, Perceived Self-efficacy, and Functional Capacity in Patients with Fibromyalgia and Rheumatoid Arthritis. J. Clin. Rheumatol. 2019, 25, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Calderon, J.; Meeus, M.; Struyf, F.; Luque-Suarez, A. The role of self-efficacy in pain intensity, function, psychological factors, health behaviors, and quality of life in people with rheumatoid arthritis: A systematic review. Physiother. Theory Pract. 2020, 36, 21–37. [Google Scholar] [CrossRef]
- La Touche, R.; Grande-Alonso, M.; Arnés-Prieto, P.; Paris-Alemany, A. How does self-efficacy influence pain perception, postural stability and range of motion in individuals with chronic low back pain? Pain Physician 2019, 22, E1–E13. [Google Scholar] [CrossRef]
- Schulz, U.; Mohamed, N.E. Turning the tide: Benefit finding after cancer surgery. Soc. Sci. Med. 2004, 59, 653–662. [Google Scholar] [CrossRef]
- Ames, H.; Wilson, C.; Barnett, S.D.; Njoh, E.; Ottomanelli, L. Does functional motor incomplete (AIS D) spinal cord injury confer unanticipated challenges? Rehabil. Psychol. 2017, 62, 401–406. [Google Scholar] [CrossRef]
- Prince, S.A.; Adamo, K.B.; Hamel, M.; Hardt, J.; Connor Gorber, S.; Tremblay, M. A comparison of direct versus self-report measures for assessing physical activity in adults: A systematic review. Int. J. Behav. Nutr. Phys. Act. 2008, 5, 56. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).