Tomographic Findings in the Retina of Unvaccinated Patients with COVID Pneumonia: Prospective Longitudinal Study
Abstract
1. Introduction
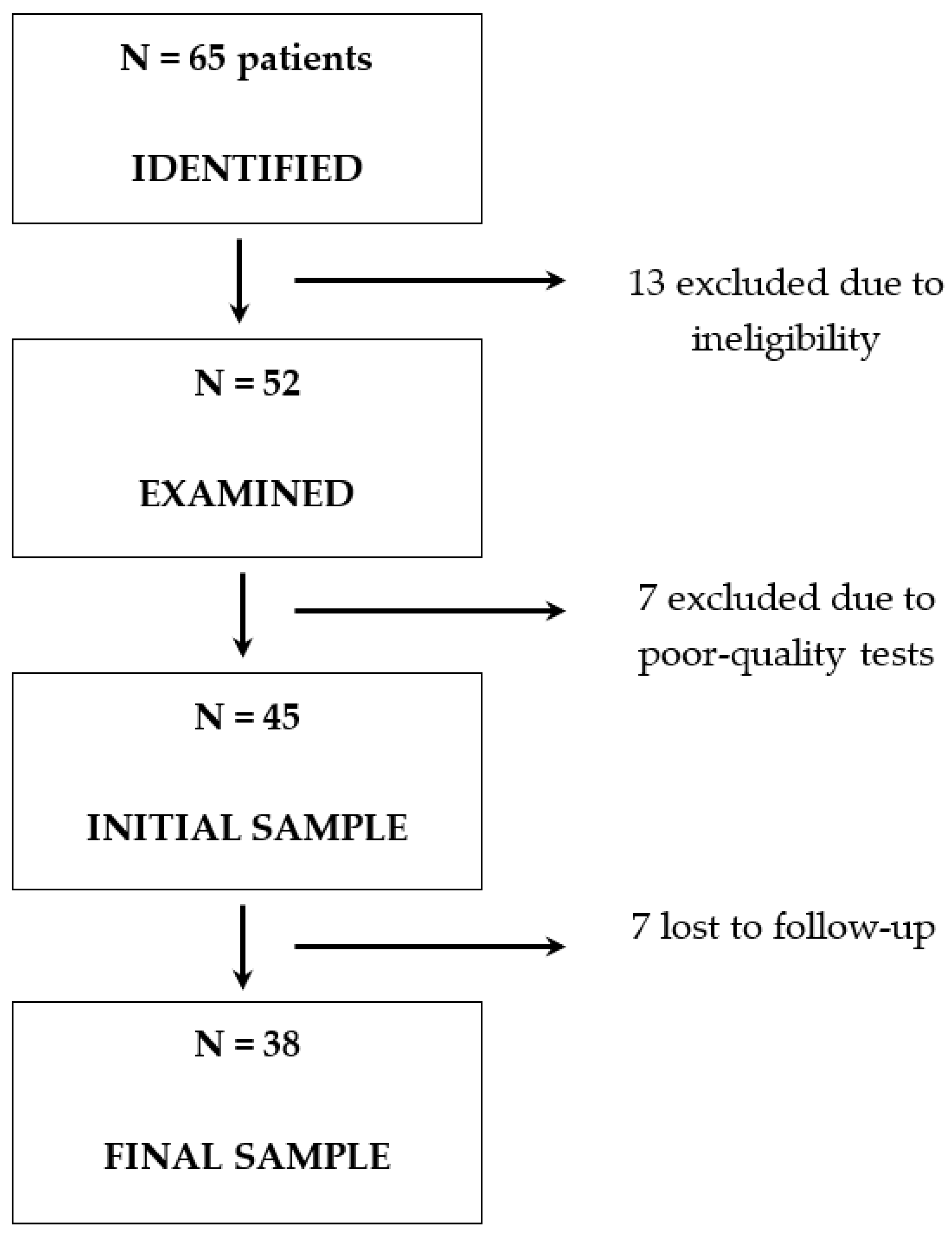
2. Materials and Methods
2.1. Study Population
2.2. Data Collection and Measurement
2.3. Study Variables
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef] [PubMed]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenck, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Ribes, A.; Vardon-Bounes, F.; Mémier, V.; Poette, M.; Au-Duong, J.; Garcia, C.; Minville, V.; Sié, P.; Bura-Rivière, A.; Voisin, S.; et al. Thromboembolic events and COVID-19. Adv. Biol. Regul. 2020, 77, 100735. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.W.; Yuen, T.T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.Y.; Tsang, J.O.L.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef]
- Dahiya, D.S.; Kichloo, A.; Albosta, M.; Pagad, S.; Wani, F. Gastrointestinal implications in COVID-19. J. Investig. Med. Off. Publ. Am. Fed. Clin. Res. 2020, 68, 1397–1401. [Google Scholar] [CrossRef] [PubMed]
- Orsini, A.; Corsi, M.; Santangelo, A.; Riva, A.; Peroni, D.; Foiadelli, T.; Savasta, S.; Striano, P. Challenges and management of neurological and psychiatric manifestations in SARS-CoV-2 (COVID-19) patients. Neurol. Sci. Off. J. Ital. Neurol. Soc. Ital. Soc. Clin. Neurophysiol. 2020, 41, 2353–2366. [Google Scholar] [CrossRef]
- Wollina, U.; Karadağ, A.S.; Rowland-Payne, C.; Chiriac, A.; Lotti, T. Cutaneous signs in COVID-19 patients: A review. Dermatol. Ther. 2020, 33, e13549. [Google Scholar] [CrossRef]
- Douglas, K.A.A.; Douglas, V.P.; Moschos, M.M. Ocular Manifestations of COVID-19 (SARS-CoV-2): A Critical Review of Current Literature. In Vivo 2020, 34 (Suppl. 3), 1619–1628. [Google Scholar] [CrossRef]
- Sen, S.; Kannan, N.B.; Kumar, J.; Rajan, R.P.; Kumar, K.; Baliga, G.; Reddy, H.; Upadhyay, A.; Ramasamy, K. Retinal manifestations in patients with SARS-CoV-2 infection and pathogenetic implications: A systematic review. Int. Ophthalmol. 2022, 42, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Teo, K.Y.; Invernizzi, A.; Staurenghi, G.; Cheung, C.M.G. COVID-19-Related Retinal Micro-vasculopathy—A Review of Current Evidence. Am. J. Ophthalmol. 2022, 235, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.M.G.; Lee, W.K.; Koizumi, H.; Dansingani, K.; Lai, T.Y.Y.; Freund, K.B. Pachychoroid disease. Eye 2019, 33, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Mazzeo, T.J.M.M.; Leber, H.M.; da Silva, A.G.; Freire, R.C.M.; Barbosa, G.C.S.; Criado, G.G.; Jacob, G.A.V.; Machado, C.G.; Gomes, A.M.V. Pachychoroid disease spectrum: Review article. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 723–735. [Google Scholar] [CrossRef]
- Naderi Beni, A.; Dehghani, A.; Kianersi, F.; Ghanbari, H.; Habibidastenae, Z.; Memarzadeh, S.E.; Naderi Beni, Z. Retinal findings of COVID-19 patients using ocular coherence tomography angiography two to three months after infection: Ocular appearance recovered COVID-19 patient. Photodiagn. Photodyn. Ther. 2022, 38, 102726. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, A.M.; Gunduz, G.U.; Yalcinbayir, O.; Ozturk, N.A.A.; Avci, R.; Coskun, F. SD-OCT assessment of macular and optic nerve alterations in patients recovered from COVID-19. Can. J. Ophthalmol. 2022, 57, 75–81. [Google Scholar] [CrossRef]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Vidal-Villegas, B.; Garcia-Feijoo, J.; Donate-Lopez, J.; Martin-Sanchez, F.J.; Gonzalez-Armengol, J.J.; Mendez-Hernandez, C.D. Optic Nerve Head Vessel Density Assessment in Recovered COVID-19 Patients: A Prospective Study Using Optical Coherence Tomography Angiography. J. Glaucoma 2021, 30, 711–717. [Google Scholar] [CrossRef]
- Dag Seker, E.; Erbahceci Timur, I.E. COVID-19: More than a respiratory virus, an optical coherence tomography study. Int. Ophthalmol. 2021, 41, 3815–3824. [Google Scholar] [CrossRef] [PubMed]
- Szkodny, D.; Wylęgała, E.; Sujka-Franczak, P.; Chlasta-Twardzik, E.; Fiolka, R.; Tomczyk, T.; Wylęgała, A. Retinal OCT Findings in Patients after COVID Infection. J. Clin. Med. 2021, 10, 3233. [Google Scholar] [CrossRef]
- Bayram, N.; Gundogan, M.; Ozsaygılı, C.; Adelman, R.A. Posterior ocular structural and vascular alterations in severe COVID-19 patients. Graefe’s Arch. Clin. Exp. Ophthalmol. 2022, 260, 993–1004. [Google Scholar] [CrossRef]
- Oren, B.; Aksoy Aydemır, G.; Aydemır, E.; Atesoglu, H.I.; Goker, Y.S.; Kızıltoprak, H.; Ozcelık, K.C. Quantitative assessment of retinal changes in COVID-19 patients. Clin. Exp. Optom. 2021, 104, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Marinho, P.M.; Marcos, A.A.A.; Romano, A.C.; Nascimento, H.; Belfort, R. Retinal findings in patients with COVID-19. Lancet 2020, 395, 1610. [Google Scholar] [CrossRef] [PubMed]
- Landecho, M.F.; Yuste, J.R.; Gándara, E.; Sunsundegui, P.; Quiroga, J.; Alcaide, A.B.; García-Layana, A. COVID-19 retinal microangiopathy as an in vivo biomarker of systemic vascular disease? J. Intern. Med. 2021, 289, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Lopez, J.J.; Felix Espinar, B.; Ye-Zhu, C. Symptomatic Retinal Microangiophaty in a Patient with Coronavirus Disease 2019 (COVID-19): Single Case Report. Ocul. Immunol. Inflamm. 2020, 29, 642–644. [Google Scholar] [CrossRef]
- Savastano, M.C.; Gambini, G.; Cozzupoli, G.M.; Crincoli, E.; Savastano, A.; De Vico, U.; Culiersi, C.; Falsini, B.; Martelli, F.; Minnella, A.M.; et al. Retinal capillary involvement in early post-COVID-19 patients: A healthy controlled study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 2157–2165. [Google Scholar] [CrossRef] [PubMed]
- Invernizzi, A.; Torre, A.; Parrulli, S.; Zicarelli, F.; Schiuma, M.; Colombo, V.; Giacomelli, A.; Cigada, M.; Milazzo, L.; Ridolfo, A.; et al. Retinal findings in patients with COVID-19: Results from the SERPICO-19 study. EClinicalMedicine 2020, 27, 100550. [Google Scholar] [CrossRef]
- Patel, N.S.; Moon, J.Y.; Katz, R.; Wai, K.M.; Sobrin, L.; Vavvas, D.G.; Miller, J.B. Retrospective Analysis of Retinal Imaging in COVID-19 Positive Patients at a Tertiary Eye Care Center. Clin. Ophthalmol. 2021, 15, 3727–3731. [Google Scholar] [CrossRef]
- Lin, C.Y.; Wang, I.; Lin, C.J.; Lai, C.T.; Bair, H.; Chen, W.L.; Lin, J.M.; Tien, P.T.; Hsia, N.Y.; Tsai, Y.Y. Short-term anatomic response of the choroid to tropicamide in myopic patients. Medicine 2022, 101, e30481. [Google Scholar] [CrossRef]
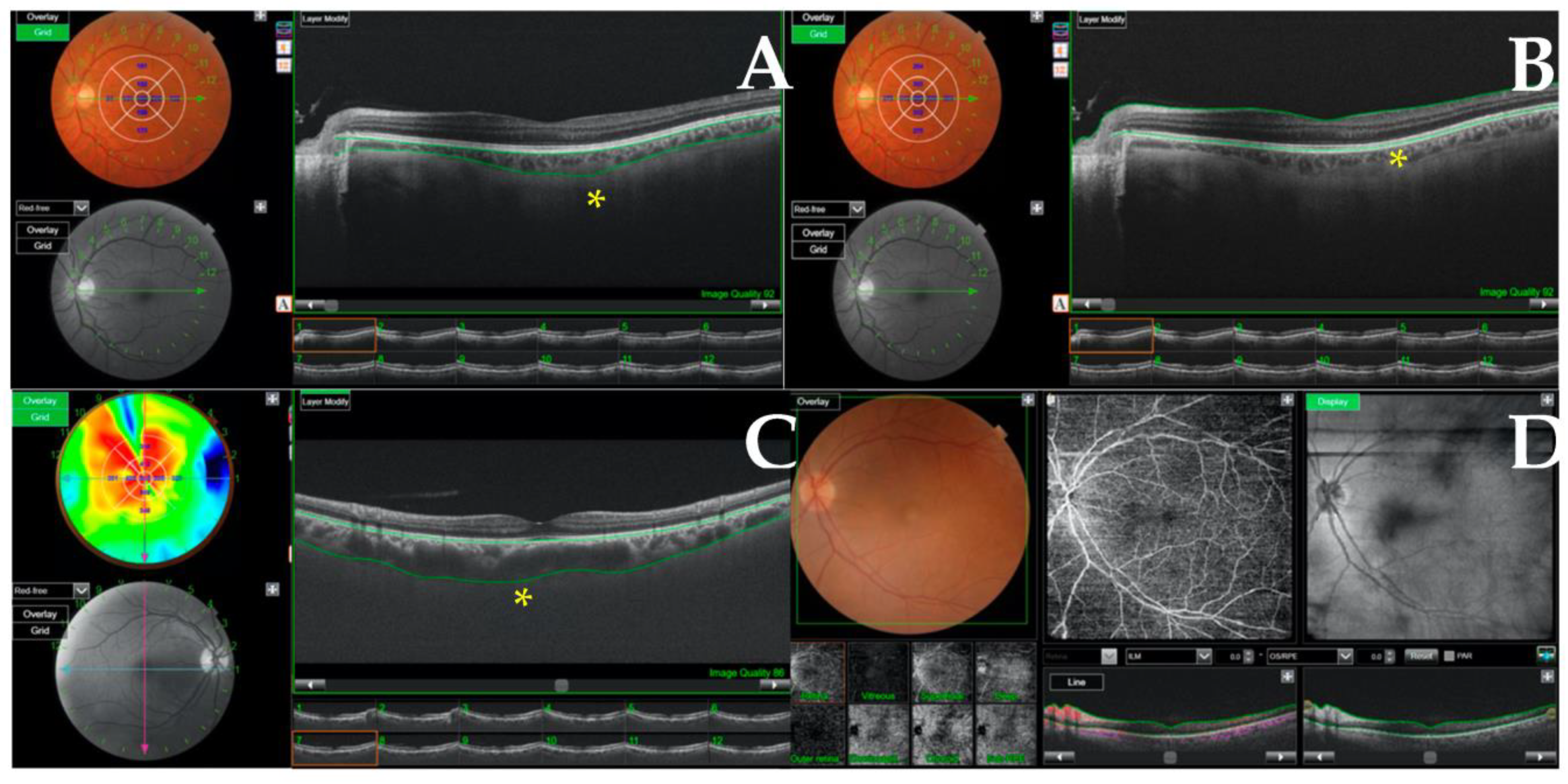
| Baseline Variables | COVID-19 Group (n = 38) | Control Group (n = 30) |
|---|---|---|
| Age in years, mean (SD) | 57.3 (13.6) | 63.3 (15.7) |
| Women, n (%) | 19 (50.0) | 21 (70.0) |
| Tobacco use, n (%) | 0 (0) | 5 (16.7%) |
| Hypertension, n (%) | 10 (26.3) | 8 (26.7%) |
| Diabetes mellitus, n (%) | 3 (7.9) | 6 (20%) |
| Fibrinogen (mg/dL), mean (SD) | 781 (224.6) | |
| D-dimers(μg/mL), mean (SD) | 2.1 (3.5) | |
| IL-6 (pg/mL), mean (SD) | 349 (496.2) | |
| Days from onset of symptoms to admission, mean (SD) | 7.5 (4.1) | |
| Days from diagnosis to admission, mean (SD) | 8.3 (4.1) | |
| Days from hospitalization to completion of eye exams, mean (SD) | 3.1 (2.0) | |
| Posterior segment findings, n (%) | 0 (0) |
| Variables | Acute COVID-19 (n = 38) | Control Group (n = 30) | p Value |
|---|---|---|---|
| Age in years, mean (SD) | 57.3 (13.6) | 63.3 (15.7) | 0.096 |
| Women, n (%) | 19 (50.0) | 21 (70.0) | 0.096 |
| Eye laterality, right, n (%) | 19 (50.0) | 14 (46.7) | 0.79 |
| Central retinal thickness, µm, mean (SD) | 264.5 (34.0) | 246.6 (20.7) | 0.006 * |
| Central choroidal thickness, µm, mean (SD) | 237.0 (62.7) | 227.1 (67.9) | 0.54 |
| Retinal nerve fiber layer, µm, median (IQR) | 9.0 (7.0, 14.0) | 7.0 (5.0, 12.0) | 0.17 |
| Ganglion cell layer, µm, median (IQR) | 62.5 (52.0, 73.0) | 57.0 (50.0, 66.0) | 0.076 |
| Outcome Measure | Acute Phase | 12-Week Follow-Up | p Value |
|---|---|---|---|
| Central retinal thickness, µm, mean (SD) | 264.5 (34.0) | 268.2 (34.3) | 0.056 1 |
| Central choroidal thickness, µm, mean (SD) | 237.0 (62.7) | 240.6 (71.0) | 0.99 1 |
| Retinal nerve fiber layer, µm, median (IQR) | 9.0 (7.0, 14.0) | 11.0 (8.0, 16.0) | 0.21 2 |
| Ganglion cell layer, µm, median (IQR) | 62.5 (52.0, 73.0) | 66.0 (56.0, 76.0) | 0.32 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monera Lucas, C.E.; Baeza Diaz, M.V.; Quesada, J.A.; Lopez-Pineda, A.; Fernandez Martinez, C.; Martinez Toldos, J.J.; Gil-Guillén, V.F. Tomographic Findings in the Retina of Unvaccinated Patients with COVID Pneumonia: Prospective Longitudinal Study. Int. J. Environ. Res. Public Health 2023, 20, 5659. https://doi.org/10.3390/ijerph20095659
Monera Lucas CE, Baeza Diaz MV, Quesada JA, Lopez-Pineda A, Fernandez Martinez C, Martinez Toldos JJ, Gil-Guillén VF. Tomographic Findings in the Retina of Unvaccinated Patients with COVID Pneumonia: Prospective Longitudinal Study. International Journal of Environmental Research and Public Health. 2023; 20(9):5659. https://doi.org/10.3390/ijerph20095659
Chicago/Turabian StyleMonera Lucas, Carlos Enrique, Manuel Vicente Baeza Diaz, Jose A. Quesada, Adriana Lopez-Pineda, Cristian Fernandez Martinez, Jose Juan Martinez Toldos, and Vicente F. Gil-Guillén. 2023. "Tomographic Findings in the Retina of Unvaccinated Patients with COVID Pneumonia: Prospective Longitudinal Study" International Journal of Environmental Research and Public Health 20, no. 9: 5659. https://doi.org/10.3390/ijerph20095659
APA StyleMonera Lucas, C. E., Baeza Diaz, M. V., Quesada, J. A., Lopez-Pineda, A., Fernandez Martinez, C., Martinez Toldos, J. J., & Gil-Guillén, V. F. (2023). Tomographic Findings in the Retina of Unvaccinated Patients with COVID Pneumonia: Prospective Longitudinal Study. International Journal of Environmental Research and Public Health, 20(9), 5659. https://doi.org/10.3390/ijerph20095659







