First Steps towards a near Real-Time Modelling System of Vibrio vulnificus in the Baltic Sea
Abstract
1. Introduction
2. Materials and Methods
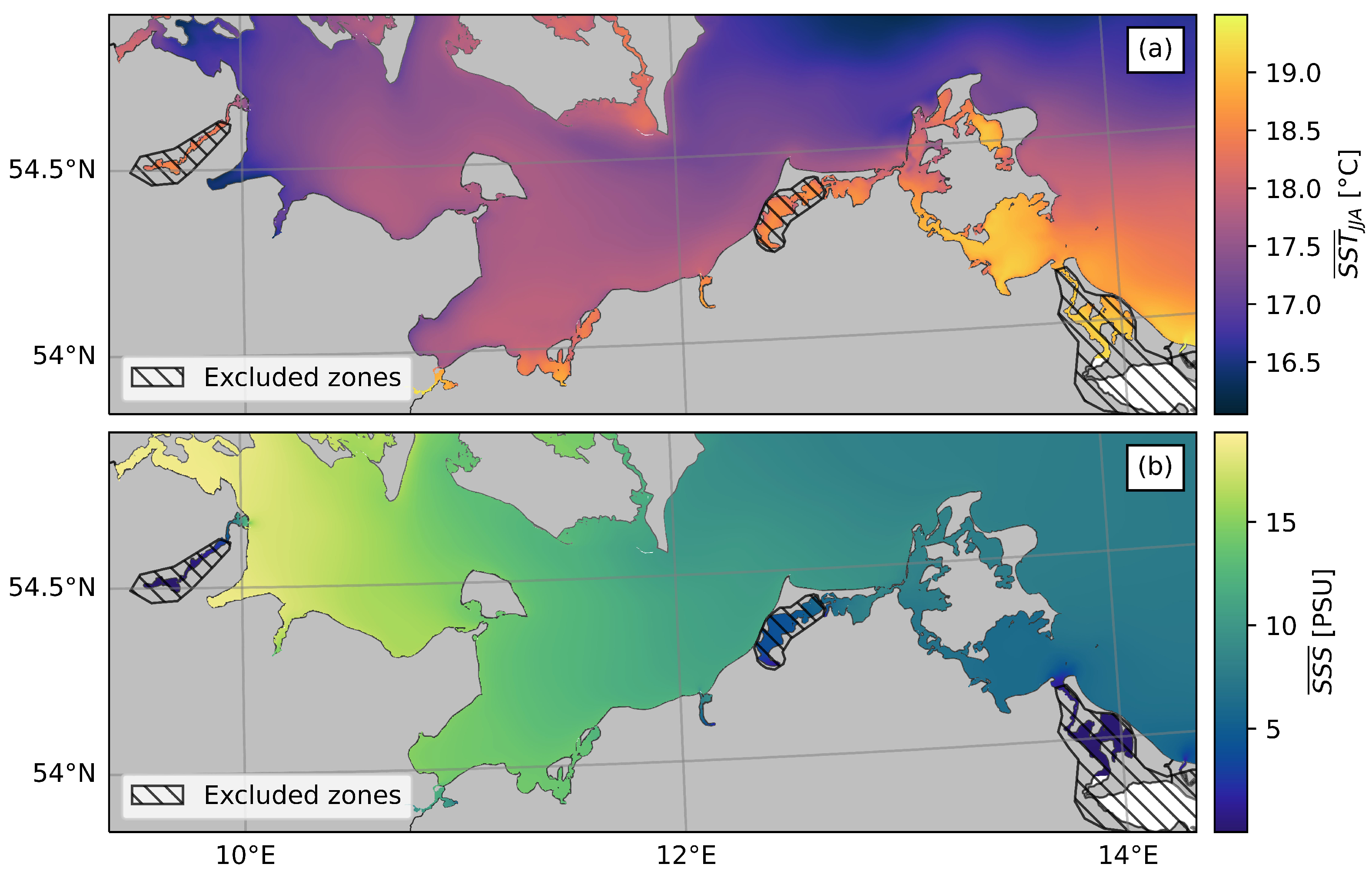
2.1. Study Area
2.2. V. vulnificus Quantification
2.3. Environmental Data
2.3.1. High-Resolution Hydrodynamic Model
2.3.2. Biogeochemical Data
2.3.3. Meteorological Data
2.3.4. Aggregation of Environmental Data and Identification of Lead Time
2.4. Statistical Analysis
3. Results
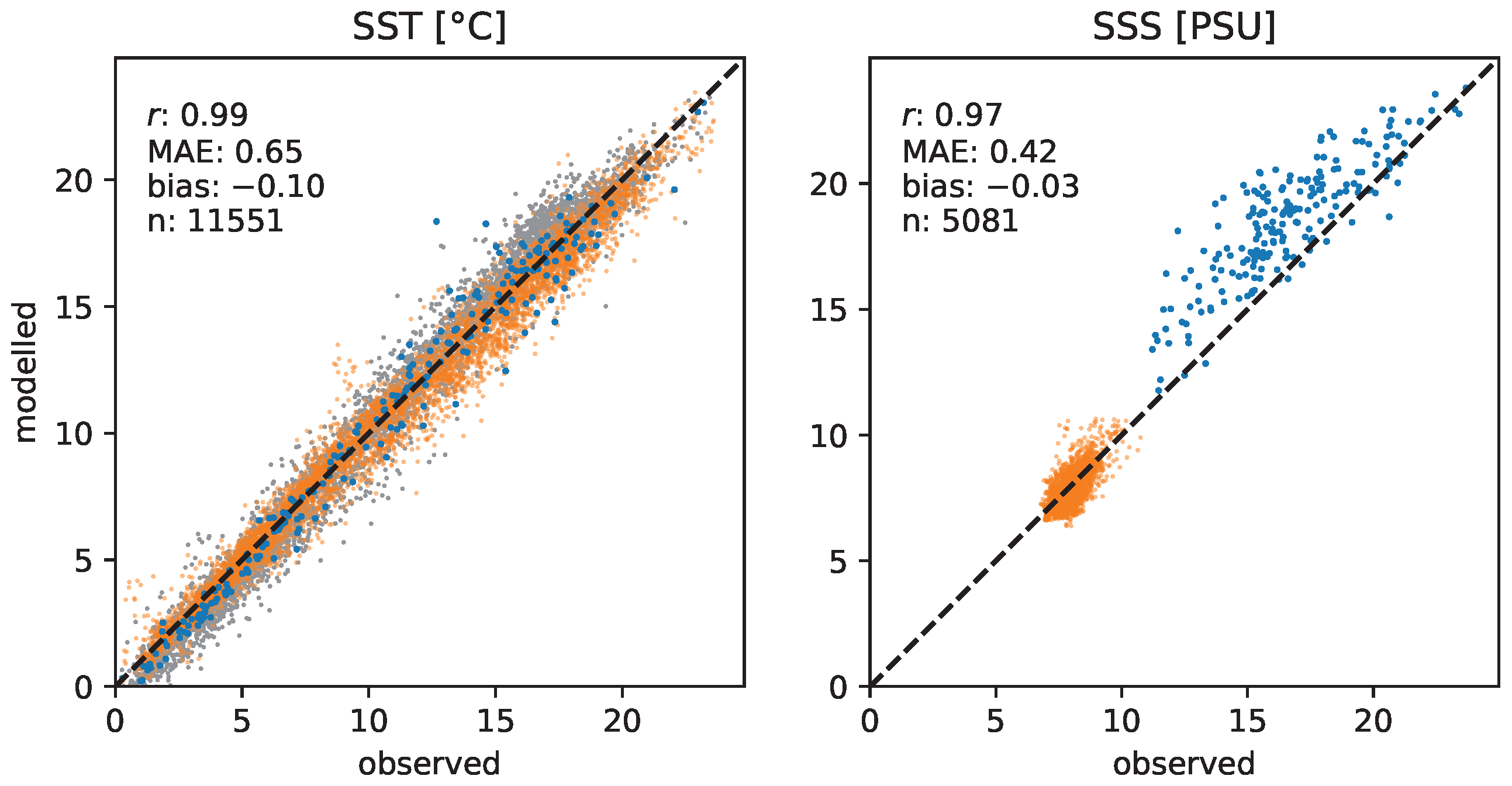
3.1. Validation of the Hydrodynamic Model
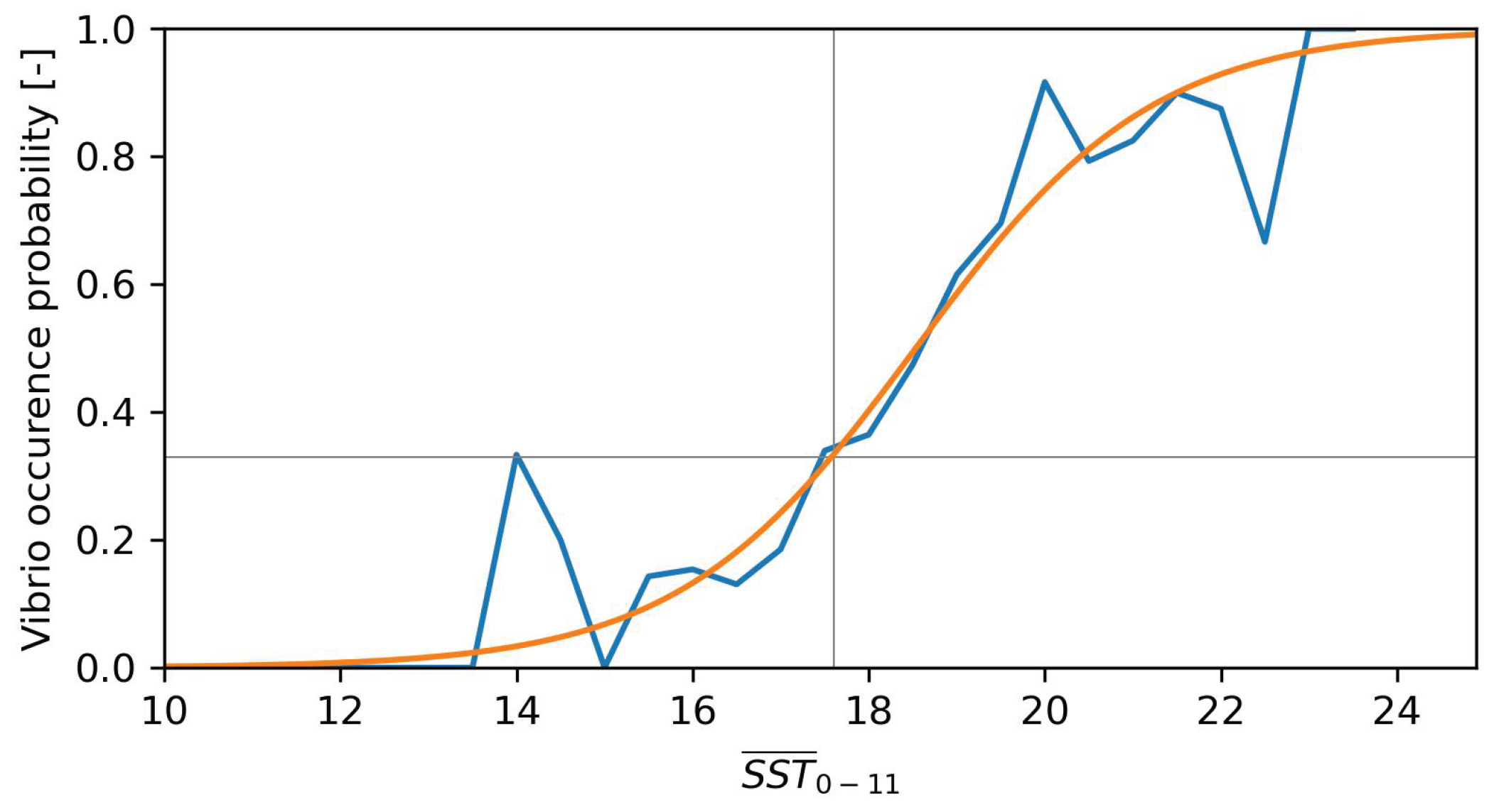
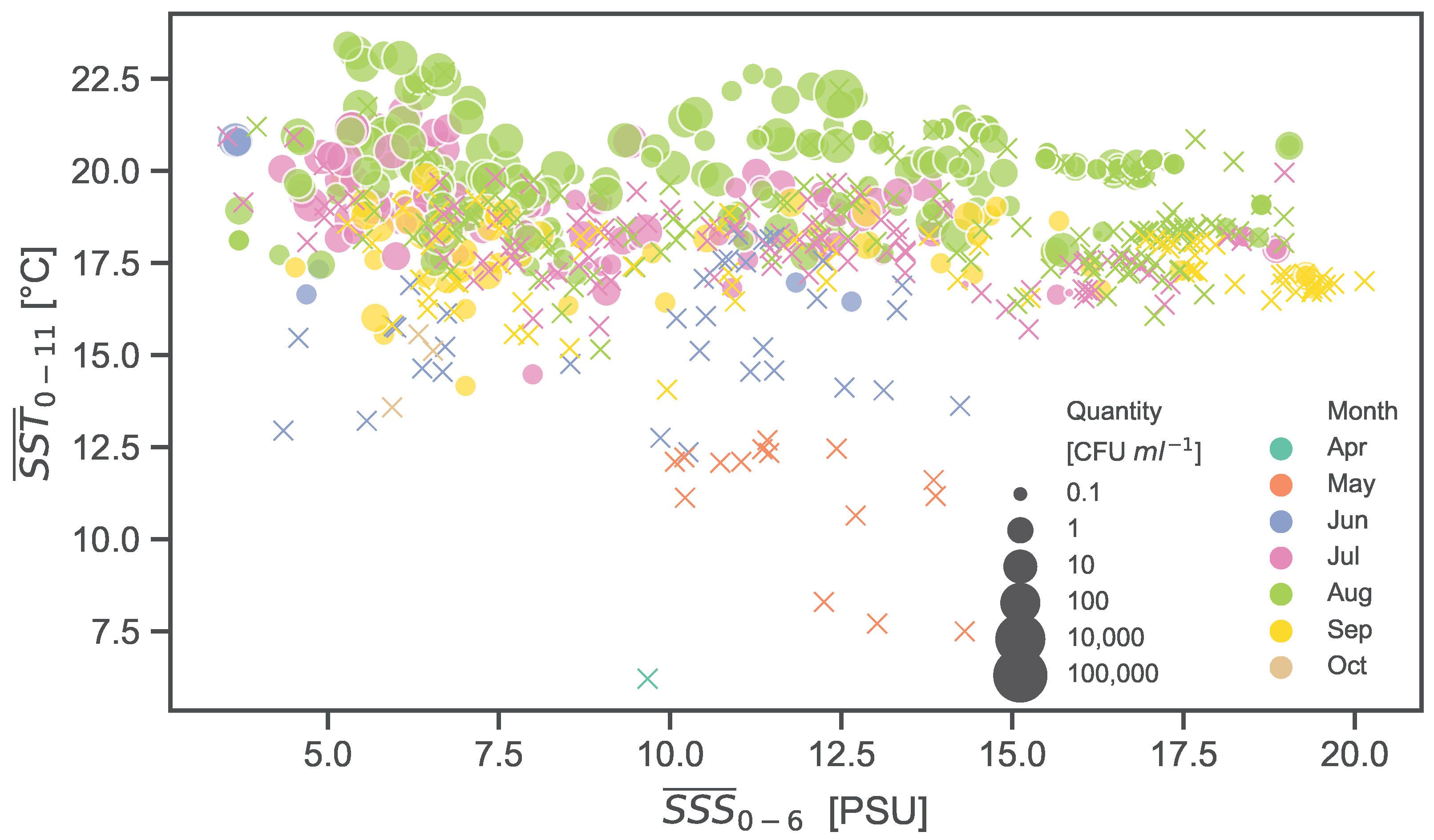
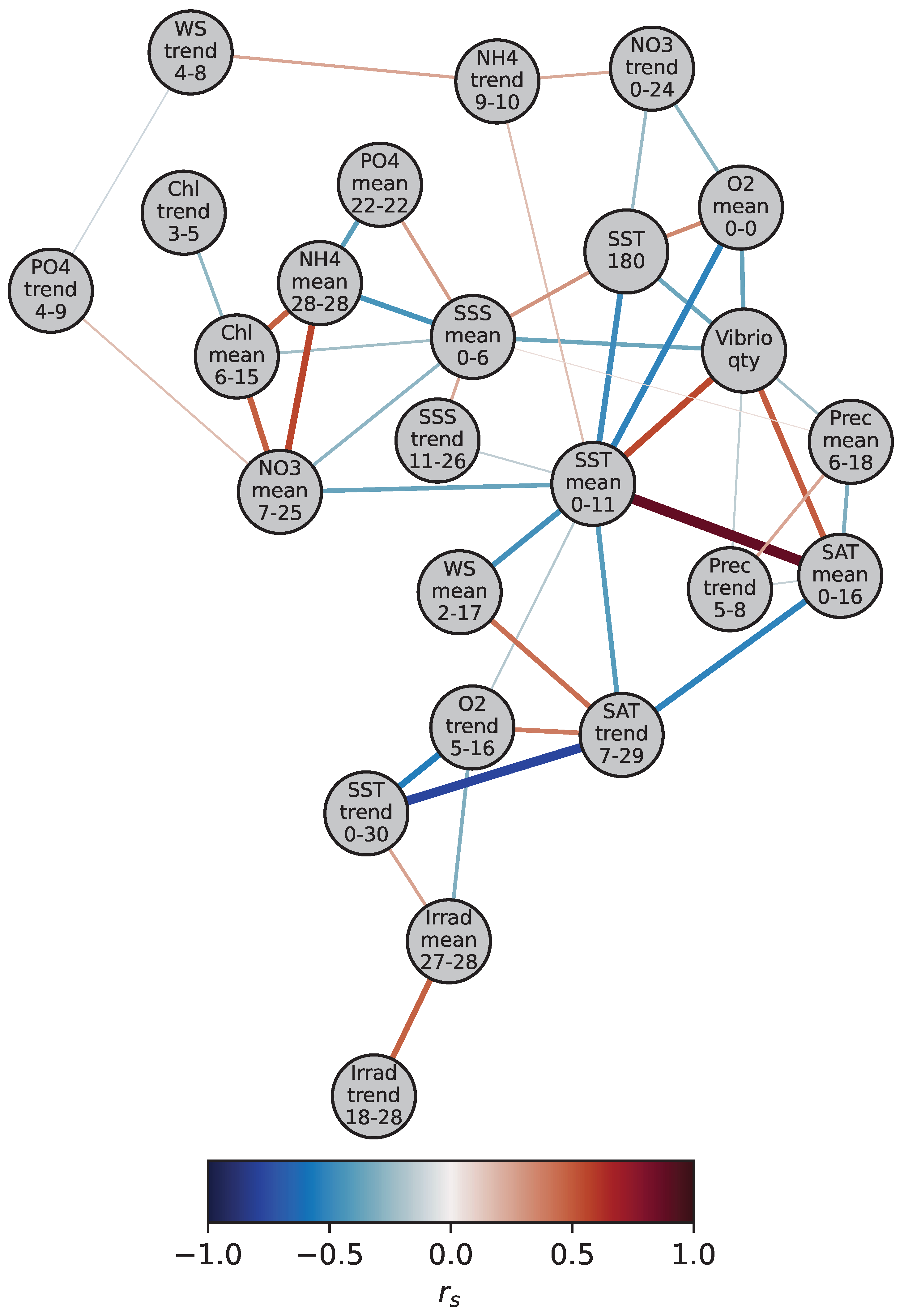
3.2. Analysis of Lag Windows and SNHA
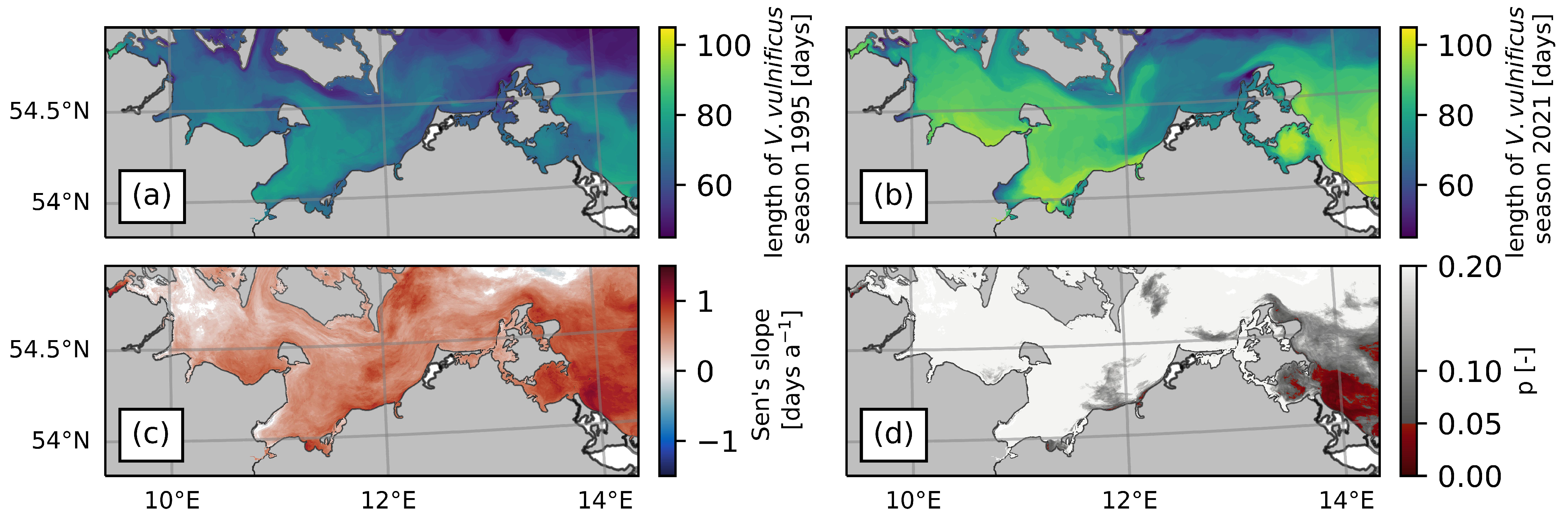
3.3. Trends of the V. vulnificus Season Length
4. Discussion
4.1. Ecological Characteristics of V. vulnificus in the South-Western Baltic Sea
4.2. Perspectives for NRT Monitoring
4.3. Climate Change
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFU | colony forming units |
| Chl | Chlorophyll a |
| CMEMS | Copernicus Marine Environment Monitoring Service |
| COSMO | Consortium for Small-scale Modelling |
| DOC | Dissolved Organic Carbon |
| DWD | German Weather Service |
| GETM | General Estuarine Transport Model |
| Irrad | Solar Irradiance |
| LAGuS | State Agency for Health and Social Affairs Mecklenburg-Western Pomerania |
| MAE | Mean Absolute Error |
| MV | Mecklenburg-Western Pommerania |
| NEMO | Nucleus for European Modelling of the Ocean |
| NH4 | Ammonium |
| NO3 | Nitrate |
| NRT | Near real-time |
| O2 | Oxygen |
| PO4 | Phosphate |
| Prec | Precipitation |
| PSU | Pratcital Salinity Unit |
| r | Pearson correlation coefficient |
| Spearman’s rank correlation coefficient | |
| SAT | Surface Air Temperature |
| SH | Schleswig-Holstein |
| SNHA | Saint Nicolas House Analysis |
| SoZMI | Ministry of Social Affairs Youth, Family, Senior Citizens, Integration and Equality |
| Schleswig-Holstein | |
| SSS | sea surface salinity |
| SST | sea surface temperature |
| V. | Vibrio |
| VBNC | Viable but non-culturable state |
| WS | Windspeed |
Appendix A
| Parameter | Aggregation | Lag Window | Max | ||
|---|---|---|---|---|---|
| Start | End | ||||
| SST | mean | 0 | 11 | 0.55 | yes |
| trend | 0 | 30 | 0.11 | SSTtrend24–25 (0.14) | |
| max | 180 | 180 | −0.35 | yes | |
| SSS | mean | 0 | 6 | −0.35 | yes |
| trend | 11 | 26 | −0.12 | yes | |
| NH | mean | 28 | 28 | 0.09 | yes |
| trend | 9 | 10 | 0.11 | yes | |
| NO | mean | 7 | 25 | −0.14 | yes |
| trend | 0 | 24 | 0.22 | yes | |
| PO | mean | 22 | 22 | −0.18 | yes |
| trend | 4 | 9 | −0.11 | PO4trend20–21 (−0.13) | |
| O | mean | 0 | 0 | −0.37 | yes |
| trend | 5 | 16 | −0.12 | yes | |
| Chl | mean | 6 | 15 | −0.07 | Chlmean15–15 (−0.09) |
| trend | 3 | 5 | −0.12 | yes | |
| SAT | mean | 0 | 16 | 0.49 | yes |
| trend | 7 | 29 | −0.17 | yes | |
| WS | mean | 2 | 17 | −0.28 | yes |
| trend | 4 | 8 | 0.11 | yes | |
| Prec | mean | 6 | 18 | −0.21 | yes |
| trend | 5 | 8 | −0.16 | yes | |
| Irrad | mean | 27 | 28 | 0.15 | yes |
| trend | 18 | 28 | 0.11 | yes | |

References
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Takemura, A.F.; Chien, D.M.; Polz, M.F. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.R.; Polz, M.F. Dynamics of Vibrio Populations and Their Role in Environmental Nutrient Cycling. In The Biology of Vibrios; Thompson, F.L., Austin, B., Swings, J., Eds.; ASM Press: Washington, DC, USA, 2006; pp. 190–203. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, H.; Wang, X.; Austin, B. Significance of Vibrio species in the marine organic carbon cycle—A review. Sci. China Earth Sci. 2018, 61, 1357–1368. [Google Scholar] [CrossRef]
- Stabb, E.V. The Vibrio fischeri-Euprymna scolopes Light Organ Symbiosis. In The Biology of Vibrios; Thompson, F.L., Austin, B., Swings, J., Eds.; ASM Press: Washington, DC, USA, 2006; pp. 204–218. [Google Scholar] [CrossRef]
- Janda, J.M.; Newton, A.E.; Bopp, C.A. Vibriosis. Clin. Lab. Med. 2015, 35, 273–288. [Google Scholar] [CrossRef]
- Sampaio, A.; Silva, V.; Poeta, P.; Aonofriesei, F. Vibrio spp.: Life Strategies, Ecology, and Risks in a Changing Environment. Diversity 2022, 14, 97. [Google Scholar] [CrossRef]
- Jones, M.K.; Oliver, J.D. Vibrio vulnificus: Disease and pathogenesis. Infect. Immun. 2009, 77, 1723–1733. [Google Scholar] [CrossRef]
- Alter, T.; Appel, B.; Bartelt, E.; Dieckmann, R.; Eichhorn, C.; Erler, R.; Frank, C.; Gerdts, G.; Gunzer, F.; Hühn, S.; et al. Vibrio-Infektionen durch Lebensmittel und Meerwasser. Das Netzwerk “VibrioNet” stellt sich vor. Bundesgesundheitsblatt-Gesundheitsforschung-Gesundheitsschutz 2011, 54, 1235–1240. [Google Scholar] [CrossRef]
- Le Roux, F.; Wegner, K.M.; Baker-Austin, C.; Vezzulli, L.; Osorio, C.R.; Amaro, C.; Ritchie, J.M.; Defoirdt, T.; Destoumieux-Garzón, D.; Blokesch, M.; et al. The emergence of Vibrio pathogens in Europe: Ecology, evolution, and pathogenesis (Paris, 11–12 March 2015). Front. Microbiol. 2015, 6, 830. [Google Scholar] [CrossRef]
- Brehm, T.T.; Berneking, L.; Sena Martins, M.; Dupke, S.; Jacob, D.; Drechsel, O.; Bohnert, J.; Becker, K.; Kramer, A.; Christner, M.; et al. Heatwave-associated Vibrio infections in Germany, 2018 and 2019. Euro Surveill. 2021, 26, 17–27. [Google Scholar] [CrossRef]
- Amato, E.; Riess, M.; Thomas-Lopez, D.; Linkevicius, M.; Pitkänen, T.; Wołkowicz, T.; Rjabinina, J.; Jernberg, C.; Hjertqvist, M.; MacDonald, E.; et al. Epidemiological and microbiological investigation of a large increase in vibriosis, northern Europe, 2018. Euro Surveill. 2022, 27, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Trinanes, J.A.; Taylor, N.G.H.; Hartnell, R.; Siitonen, A.; Martinez-Urtaza, J. Emerging Vibrio risk at high latitudes in response to ocean warming. Nat. Clim. Change 2013, 3, 73–77. [Google Scholar] [CrossRef]
- Metelmann, C.; Metelmann, B.; Gründling, M.; Hahnenkamp, K.; Hauk, G.; Scheer, C. Vibrio vulnificus, eine zunehmende Sepsisgefahr in Deutschland? Der Anaesthesist 2020, 69, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Semenza, J.C.; Trinanes, J.; Lohr, W.; Sudre, B.; Löfdahl, M.; Martinez-Urtaza, J.; Nichols, G.L.; Rocklöv, J. Environmental Suitability of Vibrio Infections in a Warming Climate: An Early Warning System. Environ. Health Perspect. 2017, 125, 107004. [Google Scholar] [CrossRef]
- Brumfield, K.D.; Usmani, M.; Chen, K.M.; Gangwar, M.; Jutla, A.S.; Huq, A.; Colwell, R.R. Environmental parameters associated with incidence and transmission of pathogenic Vibrio spp. Environ. Microbiol. 2021, 23, 7314–7340. [Google Scholar] [CrossRef]
- Gilbert, J.A.; Steele, J.A.; Caporaso, J.G.; Steinbrück, L.; Reeder, J.; Temperton, B.; Huse, S.; McHardy, A.C.; Knight, R.; Joint, I.; et al. Defining seasonal marine microbial community dynamics. ISME J. 2012, 6, 298–308. [Google Scholar] [CrossRef]
- Thorstenson, C.A.; Ullrich, M.S. Ecological Fitness of Vibrio cholerae, Vibrio parahaemolyticus, and Vibrio vulnificus in a Small-Scale Population Dynamics Study. Front. Mar. Sci. 2021, 8, 623988. [Google Scholar] [CrossRef]
- Randa, M.A.; Polz, M.F.; Lim, E. Effects of temperature and salinity on Vibrio vulnificus population dynamics as assessed by quantitative PCR. Appl. Environ. Microbiol. 2004, 70, 5469–5476. [Google Scholar] [CrossRef]
- Böer, S.I.; Heinemeyer, E.A.; Luden, K.; Erler, R.; Gerdts, G.; Janssen, F.; Brennholt, N. Temporal and spatial distribution patterns of potentially pathogenic Vibrio spp. at recreational beaches of the German north sea. Microb. Ecol. 2013, 65, 1052–1067. [Google Scholar] [CrossRef]
- Fleischmann, S.; Herrig, I.; Wesp, J.; Stiedl, J.; Reifferscheid, G.; Strauch, E.; Alter, T.; Brennholt, N. Prevalence and Distribution of Potentially Human Pathogenic Vibrio spp. on German North and Baltic Sea Coasts. Front. Cell. Infect. Microbiol. 2022, 12, 846819. [Google Scholar] [CrossRef]
- Esteves, K.; Hervio-Heath, D.; Mosser, T.; Rodier, C.; Tournoud, M.G.; Jumas-Bilak, E.; Colwell, R.R.; Monfort, P. Rapid proliferation of Vibrio parahaemolyticus, Vibrio vulnificus, and Vibrio cholerae during freshwater flash floods in French Mediterranean coastal lagoons. Appl. Environ. Microbiol. 2015, 81, 7600–7609. [Google Scholar] [CrossRef] [PubMed]
- Belkin, I.M. Rapid warming of Large Marine Ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Hauk, G.; (State Agency for Health and Social Affairs Mecklenburg-Western Pomerania, Rostock, Germany). Personal communication, 2022.
- Hippelein, M.; (University Medical Center Schleswig Holstein, Kiel, Germany). Personal communication, 2022.
- Baker-Austin, C.; Trinanes, J.; Martinez-Urtaza, J. The new tools revolutionizing Vibrio science. Environ. Microbiol. 2020, 22, 4096–4100. [Google Scholar] [CrossRef] [PubMed]
- Eiler, A.; Johansson, M.; Bertilsson, S. Environmental influences on Vibrio populations in northern temperate and boreal coastal waters (Baltic and Skagerrak Seas). Appl. Environ. Microbiol. 2006, 72, 6004–6011. [Google Scholar] [CrossRef]
- Hackbusch, S.; Wichels, A.; Gimenez, L.; Döpke, H.; Gerdts, G. Potentially human pathogenic Vibrio spp. in a coastal transect: Occurrence and multiple virulence factors. Sci. Total Environ. 2020, 707, 136113. [Google Scholar] [CrossRef]
- Bier, N.; Jäckel, C.; Dieckmann, R.; Brennholt, N.; Böer, S.I.; Strauch, E. Virulence Profiles of Vibrio vulnificus in German Coastal Waters, a Comparison of North Sea and Baltic Sea Isolates. Int. J. Environ. Res. Public Health 2015, 12, 15943–15959. [Google Scholar] [CrossRef]
- Groth, D.; Scheffler, C.; Hermanussen, M. Body height in stunted Indonesian children depends directly on parental education and not via a nutrition mediated pathway—Evidence from tracing association chains by St. Nicolas House Analysis. Anthropol. Anz. 2019, 76, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Lange, X.; Klingbeil, K.; Burchard, H. Inversions of Estuarine Circulation Are Frequent in a Weakly Tidal Estuary With Variable Wind Forcing and Seaward Salinity Fluctuations. J. Geophys. Res. Ocean 2020, 125, e2019JC015789. [Google Scholar] [CrossRef]
- Lehmann, A.; Myrberg, K.; Post, P.; Chubarenko, I.; Dailidiene, I.; Hinrichsen, H.H.; Hüssy, K.; Liblik, T.; Meier, H.E.M.; Lips, U.; et al. Salinity dynamics of the Baltic Sea. Earth Syst. Dyn. 2022, 13, 373–392. [Google Scholar] [CrossRef]
- Aichner, B.; Rittweg, T.; Schumann, R.; Dahlke, S.; Duggen, S.; Dubbert, D. Spatial and temporal dynamics of water isotopes in the riverine–marine mixing zone along the German Baltic Sea coast. Hydrol. Process. 2022, 36, e14686. [Google Scholar] [CrossRef]
- Andersen, J.H.; Carstensen, J.; Conley, D.J.; Dromph, K.; Fleming-Lehtinen, V.; Gustafsson, B.G.; Josefson, A.B.; Norkko, A.; Villnäs, A.; Murray, C. Long-term temporal and spatial trends in eutrophication status of the Baltic Sea. Biol. Rev. Camb. Philos. Soc. 2017, 92, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Ministerium für Inneres, ländliche Räume und Integration des Landes Schleswig-Holstein. Landesverordnung über die Qualität und die Bewirtschaftung der Badegewässer (Badegewässerverordnung—BadegewVO) vom 10. September 2018. Gesetz- und Verordnungsblatt für Schleswig-Holstein. 2018, 14, 462–472. [Google Scholar]
- Teichert-Möller, K.; (University Medical Center Schleswig Holstein, Lübeck, Germany); Hippelein, M.; (University Medical Center Schleswig Holstein, Kiel, Germany). Unpublished work. 2015.
- Brennholt, N.; Böer, S.; Heinemeyer, E.A.; Luden, K.; Hauk, G.; Duty, O.; Baumgarten, A.L.; Potau Nú nez, R.; Rösch, T.; Wehrmann, A.; et al. KLIWAS-38/2014; Klimabedingte Änderungen der Gewässerhygiene und Auswirkungen auf das Baggergutmanagement in den Küstengewässern: Schlussbericht KLIWAS-Projekt 3.04; Bundesanstalt für Gewässerkunde: Koblenz, Germany, 2014. [Google Scholar] [CrossRef]
- European Union-Copernicus Marine Service. Baltic Sea Biogeochemistry Reanalysis. Available online: https://www.copernicus.eu/en/access-data/copernicus-services-catalogue/baltic-sea-biogeochemistry-reanalysis (accessed on 14 November 2022).
- Bollmeyer, C.; Keller, J.D.; Ohlwein, C.; Wahl, S.; Crewell, S.; Friederichs, P.; Hense, A.; Keune, J.; Kneifel, S.; Pscheidt, I.; et al. Towards a high–resolution regional reanalysis for the European CORDEX domain. Q. J. R. Meteorol. Soc. 2015, 141, 1–15. [Google Scholar] [CrossRef]
- Burchard, H.; Bolding, K. GETM: A General Estuarine Transport Model— Scientific Documentation; Joint Research Centre: Ispra, Italy, 2002. [Google Scholar]
- Hofmeister, R.; Burchard, H.; Beckers, J.M. Non-uniform adaptive vertical grids for 3D numerical ocean models. Ocean Model. 2010, 33, 70–86. [Google Scholar] [CrossRef]
- Gräwe, U.; Holtermann, P.; Klingbeil, K.; Burchard, H. Advantages of vertically adaptive coordinates in numerical models of stratified shelf seas. Ocean Model. 2015, 92, 56–68. [Google Scholar] [CrossRef]
- Burchard, H.; Bolding, K.; Villarreal, M.R. GOTM, a General Ocean Turbulence Model: Theory, Implementation and Test Cases; Report EUR 18745; European Comission: Brussels, Belgium, 1999; p. 103.
- Umlauf, L.; Burchard, H. Second-order turbulence closure models for geophysical boundary layers. A review of recent work. Cont. Shelf Res. 2005, 25, 795–827. [Google Scholar] [CrossRef]
- Lennartz, S.T.; Lehmann, A.; Herrford, J.; Malien, F.; Hansen, H.P.; Biester, H.; Bange, H.W. Long-term trends at the Boknis Eck time series station (Baltic Sea), 1957–2013: Does climate change counteract the decline in eutrophication? Biogeosciences 2014, 11, 6323–6339. [Google Scholar] [CrossRef]
- BSH. Marine Environmental Monitoring Network. Available online: https://www.bsh.de/EN/DATA/Climate-and-Sea/Marine_environment_monitoring_network/marine_environment_monitoring_network_node.html (accessed on 31 January 2023).
- Venrick, E.; Mogowan, J.; Cayan, D.; Hayward, T. Climate and Chlorophyll a: Long-Term Trends in the Central North Pacific Ocean. Science 1987, 238, 70–72. [Google Scholar] [CrossRef]
- Hordoir, R.; Axell, L.; Löptien, U.; Dietze, H.; Kuznetsov, I. Influence of sea level rise on the dynamics of salt inflows in the Baltic Sea. J. Geophys. Res. Ocean. 2015, 120, 6653–6668. [Google Scholar] [CrossRef]
- Pemberton, P.; Löptien, U.; Hordoir, R.; Höglund, A.; Schimanke, S.; Axell, L.; Haapala, J. Sea-ice evaluation of NEMO-Nordic 1.0: A NEMO–LIM3.6-based ocean–sea-ice model setup for the North Sea and Baltic Sea. Geosci. Model Dev. 2017, 10, 3105–3123. [Google Scholar] [CrossRef]
- Eilola, K.; Meier, H.M.; Almroth, E. On the dynamics of oxygen, phosphorus and cyanobacteria in the Baltic Sea; A model study. J. Mar. Syst. 2009, 75, 163–184. [Google Scholar] [CrossRef]
- Almroth-Rosell, E.; Eilola, K.; Kuznetsov, I.; Hall, P.O.; Meier, H.M. A new approach to model oxygen dependent benthic phosphate fluxes in the Baltic Sea. J. Mar. Syst. 2015, 144, 127–141. [Google Scholar] [CrossRef]
- Axell, L.; Liu, Y.; Jandt, S.; Lorkowski, I.; Lindenthal, A.; Verjovkina, S.; Schwichtenberg, F. QUID for BAL MFC Products—BALTICSEAREANALYSIS BIO 003012—Issue 2.5. Available online: https://catalogue.marine.copernicus.eu/documents/QUID/CMEMS-BAL-QUID-003-012.pdf (accessed on 16 November 2022).
- Bullington, J.A.; Golder, A.R.; Steward, G.F.; McManus, M.A.; Neuheimer, A.B.; Glazer, B.T.; Nigro, O.D.; Nelson, C.E. Refining real-time predictions of Vibrio vulnificus concentrations in a tropical urban estuary by incorporating dissolved organic matter dynamics. Sci. Total Environ. 2022, 829, 154075. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.W.; Harwood, V.J. Sewage Promotes Vibrio vulnificus Growth and Alters Gene Transcription in Vibrio vulnificus CMCP6. Microbiol. Spectr. 2022, 10, e0191321. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.D.; Hite, F.; McDougald, D.; Andon, N.L.; Simpson, L.M. Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl. Environ. Microbiol. 1995, 61, 2624–2630. [Google Scholar] [CrossRef] [PubMed]
- Hermanussen, M.; Aßmann, C.; Groth, D. Chain Reversion for Detecting Associations in Interacting Variables-St. Nicolas House Analysis. Int. J. Environ. Res. Public Health 2021, 18, 1741. [Google Scholar] [CrossRef]
- Hake, T.; Bodenberger, B.; Groth, D. In Python available: St. Nicolas House Algorithm (SNHA) with bootstrap support for improved performance in dense networks. Human Biol. Public Health, 2023; in press. [Google Scholar]
- Hussain, M.; Mahmud, I. pyMannKendall: A python package for non parametric Mann Kendall family of trend tests. J. Open Source Softw. 2019, 4, 1556. [Google Scholar] [CrossRef]
- Myrberg, K.; Andrejev, O. Main upwelling regions in the Baltic Sea—A statistical analysis based on three-dimensional modelling. Boreal Environ. Res. 2003, 8, 97–112. [Google Scholar]
- Oberbeckmann, S.; Fuchs, B.M.; Meiners, M.; Wichels, A.; Wiltshire, K.H.; Gerdts, G. Seasonal dynamics and modeling of a Vibrio community in coastal waters of the North Sea. Microb. Ecol. 2012, 63, 543–551. [Google Scholar] [CrossRef]
- Gyraite, G.; Katarzyte, M.; Schernewski, G. First findings of potentially human pathogenic bacteria Vibrio in the south-eastern Baltic Sea coastal and transitional bathing waters. Mar. Pollut. Bull. 2019, 149, 110546. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Rhodes, M.; Brown, C.W.; Hood, R.R.; Leight, A.; Long, W.; Wood, R. Modeling and forecasting the distribution of Vibrio vulnificus in Chesapeake Bay. J. Appl. Microbiol. 2014, 117, 1312–1327. [Google Scholar] [CrossRef]
- Kniebusch, M.; Meier, H.M.; Neumann, T.; Börgel, F. Temperature Variability of the Baltic Sea Since 1850 and Attribution to Atmospheric Forcing Variables. J. Geophys. Res. Ocean. 2019, 124, 4168–4187. [Google Scholar] [CrossRef]
- Pfeffer, C.S.; Hite, M.F.; Oliver, J.D. Ecology of Vibrio vulnificus in estuarine waters of eastern North Carolina. Appl. Environ. Microbiol. 2003, 69, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Blackwell, K.D.; Oliver, J.D. The ecology of Vibrio vulnificus, Vibrio cholerae, and Vibrio parahaemolyticus in North Carolina estuaries. J. Microbiol. 2008, 46, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Siboni, N.; Balaraju, V.; Carney, R.; Labbate, M.; Seymour, J.R. Spatiotemporal Dynamics of Vibrio spp. within the Sydney Harbour Estuary. Front. Microbiol. 2016, 7, 460. [Google Scholar] [CrossRef] [PubMed]
- Heinz, V.; Jäckel, W.; Kaltwasser, S.; Cutugno, L.; Bedrunka, P.; Graf, A.; Reder, A.; Michalik, S.; Dhople, V.M.; Madej, M.G.; et al. The Vibrio vulnificus stressosome is an oxygen-sensor involved in regulating iron metabolism. Commun. Biol. 2022, 5, 622. [Google Scholar] [CrossRef]
- Wasmund, N.; Nausch, G.; Matthäus, W. Phytoplankton spring blooms in the southern Baltic Sea—Spatio-temporal development and long-term trends. J. Plankton Res. 1998, 20, 1099–1117. [Google Scholar] [CrossRef]
- Wetz, J.J.; Blackwood, A.D.; Fries, J.S.; Williams, Z.F.; Noble, R.T. Trends in total Vibrio spp. and Vibrio vulnificus concentrations in the eutrophic Neuse River Estuary, North Carolina, during storm events. Aquat. Microb. Ecol. 2008, 53, 141–149. [Google Scholar] [CrossRef]
- DeLuca, N.M.; Zaitchik, B.F.; Guikema, S.D.; Jacobs, J.M.; Davis, B.J.; Curriero, F.C. Evaluation of remotely sensed prediction and forecast models for Vibrio parahaemolyticus in the Chesapeake Bay. Remote Sens. Environ. 2020, 250, 112016. [Google Scholar] [CrossRef]
- European Union-Copernicus Marine Service. Baltic Sea Biogeochemistry Analysis and Forecast. Available online: https://www.doi.org/10.48670/moi-00009 (accessed on 6 December 2022).
- Reinert, D.; Prill, F.; Frank, H.; Denhard, M.; Baldauf, M.; Schraff, C.; Gebhardt, C.; Marsigli, C.; Zängl, G. DWD Database Reference for the Global and Regional ICON and ICON-EPS Forecasting System; DWD. 2023. Available online: https://www.dwd.de/DWD/forschung/nwv/fepub/icon_database_main.pdf (accessed on 27 January 2023).
- Hecht, J.; Borowiak, M.; Fortmeier, B.; Dikou, S.; Gierer, W.; Klempien, I.; Nekat, J.; Schaefer, S.; Strauch, E. Case Report: Vibrio fluvialis isolated from a wound infection after a piercing trauma in the Baltic Sea. Access Microbiol. 2022, 4, 000312. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, C.; Meier, H.E.M.; Gröger, M.; Börgel, F. Understanding past and future sea surface temperature trends in the Baltic Sea. Clim. Dyn. 2022, 58, 3021–3039. [Google Scholar] [CrossRef]
- Löptien, U.; Meier, H.M. The influence of increasing water turbidity on the sea surface temperature in the Baltic Sea: A model sensitivity study. J. Mar. Syst. 2011, 88, 323–331. [Google Scholar] [CrossRef]
- Stramska, M.; Białogrodzka, J. Spatial and temporal variability of sea surface temperature in the Baltic Sea based on 32-years (1982–2013) of satellite data. Oceanologia 2015, 57, 223–235. [Google Scholar] [CrossRef]
- Wang, F.; Shao, W.; Yu, H.; Kan, G.; He, X.; Zhang, D.; Ren, M.; Wang, G. Re-evaluation of the Power of the Mann-Kendall Test for Detecting Monotonic Trends in Hydrometeorological Time Series. Front. Earth Sci. 2020, 8, 14. [Google Scholar] [CrossRef]
- Sanches-Fernandes, G.M.M.; Sá-Correia, I.; Costa, R. Vibriosis Outbreaks in Aquaculture: Addressing Environmental and Public Health Concerns and Preventative Therapies Using Gilthead Seabream Farming as a Model System. Front. Microbiol. 2022, 13, 904815. [Google Scholar] [CrossRef]
- Amaro, C.; Sanjuán, E.; Fouz, B.; Pajuelo, D.; Lee, C.T.; Hor, L.I.; Barrera, R. The Fish Pathogen Vibrio vulnificus Biotype 2: Epidemiology, Phylogeny, and Virulence Factors Involved in Warm-Water Vibriosis. Microbiol. Spectr. 2015, 3, 3.3.03:1–3.3.03:23. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Urtaza, J.; Simental, L.; Velasco, D.; DePaola, A.; Ishibashi, M.; Nakaguchi, Y.; Nishibuchi, M.; Carrera-Flores, D.; Rey-Alvarez, C.; Pousa, A. Pandemic Vibrio parahaemolyticus O3:K6, Europe. Emerg. Infect. Dis. 2005, 11, 1319–1320. [Google Scholar] [CrossRef] [PubMed]
- Citil, B.E.; Derin, S.; Sankur, F.; Sahan, M.; Citil, M.U. Vibrio alginolyticus Associated Chronic Myringitis Acquired in Mediterranean Waters of Turkey. Case Rep. Infect. Dis. 2015, 2015, 187212. [Google Scholar] [CrossRef]


| Dataset | Parameter | Abbreviation | Spatial Resolution | Temporal Coverage |
|---|---|---|---|---|
| GETM-IOW | Sea Surface Temperature | SST | 0.2 × 0.2 km | 1995–2021 |
| Sea Surface Salinity | SSS | |||
| CMEMS Baltic Sea Biogeochemistry Reanalysis [39] | Ammonium | NH | 4 × 4 km | 1993–2021 |
| Nitrate | NO | |||
| Phosphate | PO | |||
| Oxygen | O | |||
| Chlorophyll a | Chl | |||
| COSMO-REA6 [40] | Surface Air Temperature | SAT | 6 × 6 km | 1995–August 2019 |
| Wind speed | WS | |||
| Precipitation | Prec | |||
| Solar Irradiation | Irrad |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schütt, E.M.; Hundsdörfer, M.A.J.; von Hoyningen-Huene, A.J.E.; Lange, X.; Koschmider, A.; Oppelt, N. First Steps towards a near Real-Time Modelling System of Vibrio vulnificus in the Baltic Sea. Int. J. Environ. Res. Public Health 2023, 20, 5543. https://doi.org/10.3390/ijerph20085543
Schütt EM, Hundsdörfer MAJ, von Hoyningen-Huene AJE, Lange X, Koschmider A, Oppelt N. First Steps towards a near Real-Time Modelling System of Vibrio vulnificus in the Baltic Sea. International Journal of Environmental Research and Public Health. 2023; 20(8):5543. https://doi.org/10.3390/ijerph20085543
Chicago/Turabian StyleSchütt, Eike M., Marie A. J. Hundsdörfer, Avril J. E. von Hoyningen-Huene, Xaver Lange, Agnes Koschmider, and Natascha Oppelt. 2023. "First Steps towards a near Real-Time Modelling System of Vibrio vulnificus in the Baltic Sea" International Journal of Environmental Research and Public Health 20, no. 8: 5543. https://doi.org/10.3390/ijerph20085543
APA StyleSchütt, E. M., Hundsdörfer, M. A. J., von Hoyningen-Huene, A. J. E., Lange, X., Koschmider, A., & Oppelt, N. (2023). First Steps towards a near Real-Time Modelling System of Vibrio vulnificus in the Baltic Sea. International Journal of Environmental Research and Public Health, 20(8), 5543. https://doi.org/10.3390/ijerph20085543






