Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil
Abstract
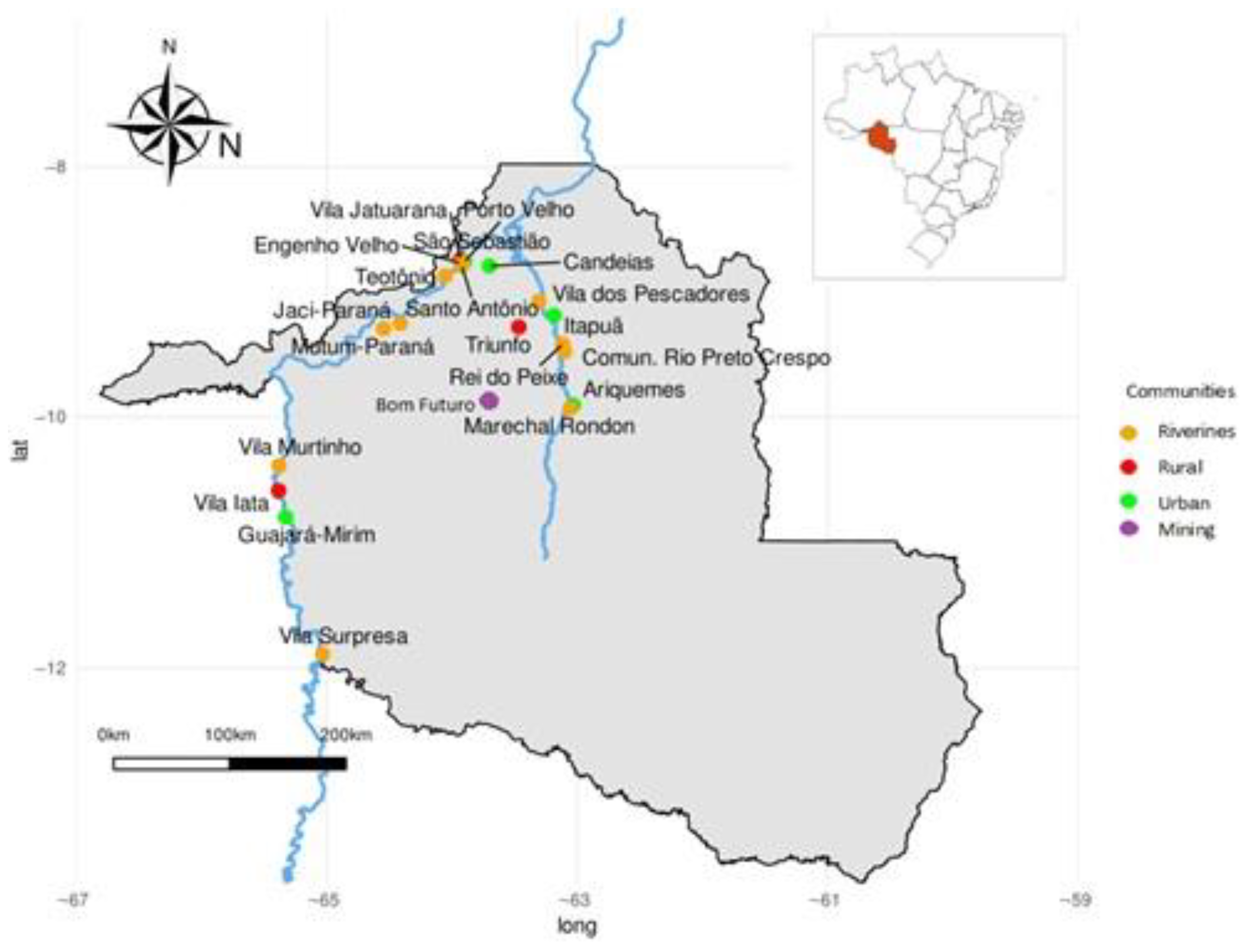
1. Introduction
2. Materials and Methods
2.1. Determination of Total Hg Concentrations
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). Environmental Health Criteria 101—Methylmercury; WHO: Geneva, Switzerland, 1990; 99p. [Google Scholar]
- von Rein, K.; Hylander, L.D. Experiences from phasing out the use of mercury in Sweden. Reg. Environ. Chang. 2000, 1, 126–134. [Google Scholar] [CrossRef]
- Legrand, M.; Feeley, M.; Tikhonov, C.; Schoen, D.; Li-Muller, A. Methylmercury blood guidance values for Canada. Can. J. Public Health 2010, 101, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Shi, P.; Morris, J.S.; Spiegelman, D.; Grandjean, P.; Siscovick, D.S.; Willett, W.C.; Rimm, E.B. Mercury exposure and risk of cardiovascular disease in two U.S. cohorts. N. Engl. J. Med. 2011, 364, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Bakir, F.; Damluji, S.F.; Amin-Zaki, L.; Murtadha, M.; Khalidi, A.; Al-Rawi, N.Y.; Tikriti, S.; Dahahir, H.I.; Clarkson, T.W.; Smith, J.C.; et al. Methylmercury poisoning in Iraq. Science 1973, 181, 230–241. [Google Scholar] [CrossRef]
- Malm, O. Gold mining as a source of mercury exposure in the Brazilian Amazon. Environ. Res. 1998, 77, 73–78. [Google Scholar] [CrossRef]
- Wang, X.; Sun, X.; Zhang, Y.; Chen, M.; Villanger, G.D.; Aase, H.; Xia, Y. Identifying a critical window of maternal metal exposure for maternal and neonatal thyroid function in China: A cohort study. Environ. Int. 2020, 139, 105696. [Google Scholar] [CrossRef]
- Cleary, D. Anatomy of the Amazon Gold Rush, 1st ed.; University of Iowa Press: Iowa City, IA, USA, 1990; p. 287. [Google Scholar]
- Roulet, M.; Saint-Aubin, M.; Tran, S.; Rhéault, I.; Farella, N.; da Silva, E.J.; Dezencourt, J.; Passos, C.J.S.; Soares, G.S.; Guimarães, J.R.; et al. The geochemistry of mercury in the central Amazonian soils developed on the Alter do Chao formation of the lower Tapajós River Valley, Pará State, Brazil. Sci. Total Environ. 1998, 223, 1–24. [Google Scholar] [CrossRef]
- Akagi, H.; Naganuma, A. Human exposure to mercury and the accumulation of methylmercury that is associated with gold mining in the Amazon Basin, Brazil. J. Health Sci. 2000, 46, 323–328. [Google Scholar] [CrossRef]
- Roulet, M.; Lucotte, M.; Guimarães, J.R.; Rheault, I. Methylmercury in water, seston, and epiphyton of an Amazonian river and its floodplain, Tapajós River, Brazil. Sci. Total Environ. 2000, 261, 43–59. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Minamata Convention on Mercury; United Nations: Geneva, Switzerland, 2019; 72p, Available online: http://www.mercuryconvention.org/Portals/11/documents/Booklets/COP3-version/Minamata-Convention-booklet-Sep2019-EN.pdf (accessed on 6 February 2023).
- Camara Dos Deputados. Decreto nº 9.470, de 14 de Agosto de 2018; Diário Oficial da República Federativa do Brasil; Poder Executivo: Brasília, Brasil, 2018; Seção 1; p. 65. [Google Scholar]
- European Food Safety Authority (EFSA). Statement on the benefits of fish/seafood consumption compared to the risks of methylmercury in fish/seafood. EFSA J. 2015, 13, 3982. [Google Scholar] [CrossRef]
- Henriques, M.C.; Loureiro, S.; Fardilha, M.; Herdeiro, M.T. Exposure to mercury and human reproductive health: A systematic review. Reprod. Toxicol. 2019, 85, 93–103. [Google Scholar] [CrossRef]
- Ruggieri, F.; Majorani, C.; Domanico, F.; Alimonti, A. Mercury in Children: Current state on exposure through human biomonitoring studies. Int. J. Environ. Res. Public Health 2017, 14, 519. [Google Scholar] [CrossRef]
- Schoeman, K.; Bend, J.R.; Hill, J.; Nash, K.; Koren, G. Defining a lowest observable adverse effect hair concentration of mercury for neurodevelopmental effects of Prenatal Methylmercury exposure through maternal fish consumption: A systematic review. Ther. Drug Monit. 2009, 31, 670–682. [Google Scholar] [CrossRef]
- Kirk, L.E.; Jørgensen, J.S.; Nielsen, F.; Grandjean, P. Public health benefits of hair-mercury analysis and dietary advice in lowering methylmercury exposure in pregnant women. Scand. J. Public Health 2017, 45, 444–451. [Google Scholar] [CrossRef]
- Schoeman, K.; Tanaka, T.; Bend, J.R.; Koren, G. Hair mercury levels of women of reproductive age in Ontario, Canada: Implications to fetal safety and fish consumption. J. Pediatr. 2010, 157, 127–131. [Google Scholar] [CrossRef]
- Dórea, J.G.; Marques, R.C. Mercury levels and human health in the Amazon Basin. Ann. Hum. Biol. 2016, 43, 349–359. [Google Scholar] [CrossRef]
- Lee, B.E.; Hong, Y.C.; Park, H.; Ha, M.; Koo, B.S.; Chang, N.; Roh, Y.M.; Kim, B.N.; Kim, Y.J.; Kim, B.M.; et al. Interaction between GSTM1/GSTT1 polymorphism and blood mercury on birth weight. Environ. Health Perspect. 2010, 118, 437–443. [Google Scholar] [CrossRef]
- Xue, F.; Holzman, C.; Rahbar, M.H.; Trosko, K.; Fisher, L. Fischer, Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ. Health Perspect. 2007, 115, 42–47. [Google Scholar] [CrossRef]
- Dallaire, R.; Dewailly, E.; Ayotte, P.; Forget-Dubois, N.; Jacobson, S.W.; Jacobson, J.; Muckle, G. Exposure to organochlorines and mercury through fish and marine mammal consumption: Associations with growth and duration of gestation among Inuit newborns. Environ. Int. 2013, 54, 85–91. [Google Scholar] [CrossRef]
- Castro, N.S.S.; Lima, M.O. Hair as a Biomarker of Long Term Mercury Exposure in Brazilian Amazon: A Systematic Review. Int. J. Environ. Res. Public Health 2018, 15, 500. [Google Scholar] [CrossRef]
- Airey, D. Mercury in human hair due to environment and diet: A review. Environ. Health Perspect. 1983, 52, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Bernardi, J.V.E.; Dórea, J.G.; Brandão, K.G.; Bueno, L.; Leão, R.S.; Malm, O. Fish consumption during pregnancy, mercury transfer, and birth weight along the Madeira River Basin in Amazonia. Int. J. Environ. Res. Public Health 2013, 10, 2150–2163. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Bastos, W.R.; Rebelo, M.F.; Fonseca, M.F.; Malm, O. Maternal mercury exposure and neuro-motor development in breastfed infants from Porto Velho (Amazon), Brazil. Int. J. Hyg. Environ. Health 2007, 210, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Bernardi, J.V.E.; Bastos, W.R.; Malm, O. Data relating neurodevelopment of exclusively breastfed children of urban mothers and pre- and post-natal mercury exposure. Data Brief 2019, 25, 104283. [Google Scholar] [CrossRef] [PubMed]
- Marques, R.C.; Dórea, J.G.; Cunha, M.P.L.; Bello, T.C.S.; Bernardi, J.V.E.; Malm, O. Data relating to maternal fish consumption, methylmercury exposure, and early child neurodevelopment in the traditional living of Western Amazonians. Data Brief 2019, 1, 104153. [Google Scholar] [CrossRef]
- Corvelo, T.C.O.; Oliveira, E.A.F.; de Parijós, A.M.; de Oliveira, C.S.B.; de Loiola, R.S.P.; de Araújo, A.A.; da Costa, C.A.; Silveira, L.C.L.; Pinheiro, M.C.N. Monitoring mercury exposure in reproductive aged women inhabiting the Tapajós River Basin, Amazon. Bull. Environ. Contam. Toxicol. 2014, 93, 42–46. [Google Scholar] [CrossRef]
- Vieira, S.M.; de Almeida, R.; Holanda, I.B.B.; Mussy, M.H.; Galvão, R.C.F.; Crispim, P.T.B.; Dórea, J.G.; Bastos, W.R. Total and methyl-mercury in hair and milk of mothers living in the city of Porto Velho and in villages along the Rio Madeira, Amazon, Brazil. Int. J. Hyg. Environ. Health 2013, 216, 682–689. [Google Scholar] [CrossRef]
- Dórea, J.G.; Barbosa, A.C.; Ferrari, I.; de Souza, J.R. Mercury in hair and in fish consumed by Riparian women of the Rio Negro, Amazon, Brazil. Int. J. Environ. Health Res. 2003, 13, 239–248. [Google Scholar] [CrossRef]
- Malm, O.; Pfeiffer, W.C.; Souza, C.M.M. Utilização do acessório de geração de vapor frio para análise de mercúrio em investigações ambientais por espectrofotometria de absorção atômica. Cien. Cult. 1989, 41, 88–92. [Google Scholar]
- Textor, J.; van der Zander, B.; Gilthorpe, M.S.; Liskiewicz, M.; Ellison, G.T. Robust causal inference using directed acyclic graphs: The R package ‘dagitty’. Int. J. Epidemiol. 2016, 45, 1887–1894. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Boischio, A.A.; East, G.A.; Ferrari, I.; Gonçalves, A.; Silva, P.R.M.; da Cruz, T.M.E. Mercury contamination in the Brazilian Amazon. Environmental and occupational aspects. Water Air Soil Pollut. 1995, 80, 109–121. [Google Scholar] [CrossRef]
- Hacon, S.S.; Yokoo, E.; Valente, J.; Campos, R.C.; da Silva, V.A.; de Menezes, A.C.; de Moraes, L.P.; Ignotti, E. Exposure to mercury in pregnant women from Alta Floresta- Amazon basin, Brazil. Environ. Res. 2000, 84, 204–210. [Google Scholar] [CrossRef]
- Marques, R.C.; Bernardi, J.V.E.; Dórea, J.G.; Leão, R.S.; Malm, O. Mercury transfer during pregnancy and breastfeeding: Hair mercury concentrations as biomarker. Biol. Trace Elem. Res. 2013, 154, 326–332. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Silva, S.R.; Dórea, J.G. Concentration of mercury in hair of indigenous mothers and infants from the Amazon basin. Arch. Environ. Contam. Toxicol. 1998, 34, 100–105. [Google Scholar] [CrossRef]
- Oliveira, R.C.; Dórea, J.G.; Bernardi, J.V.; Bastos, W.R.; Almeida, R.; Manzatto, A.G. Fish consumption by traditional subsistence villagers of the Rio Madeira (Amazon): Impact on hair mercury. Ann. Hum. Biol. 2010, 37, 629–642. [Google Scholar] [CrossRef]
- Faial, K.; Deus, R.; Deus, S.; Neves, R.; Jesus, I.; Santos, E.; Alves, C.N.; Brasil, D. Mercury levels assessment in hair of riverside inhabitants of the Tapajós River, Pará State, Amazon, Brazil: Fish consumption as a possible route of exposure. J. Trace Elem. Med. Biol. 2015, 30, 66–76. [Google Scholar] [CrossRef]
- Vega, C.; Orellana, J.D.Y.; Oliveira, M.W.; Hacon, S.S.; Basta, P.C. Human mercury exposure in Yanomami indigenous villages from the Brazilian Amazon. Int. J. Environ. Res. Public Health 2018, 15, 1051. [Google Scholar] [CrossRef]
- Peplow, D.; Augustine, S. Community-led assessment of risk from exposure to mercury by native Amerindian Wayana in Southeast Suriname. J. Environ. Public Health 2011, 2012, 674596. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Olivero-Verbel, J. Mercury exposure assessment in indigenous communities from Tarapaca village, Cotuhe and Putumayo Rivers, Colombian Amazon. Environ. Sci. Pollut. Res. Int. 2019, 26, 36458–36467. [Google Scholar] [CrossRef]
- Valdelamar-Villegas, J.; Olivero-Verbel, J. High Mercury Levels in the Indigenous Population of the Yaigojé Apaporis National Natural Park, Colombian Amazon. Biol. Trace Elem. Res. 2020, 194, 3–12. [Google Scholar] [CrossRef]
- Díaz, S.M.; Palma, R.M.; Muñoz, M.N.; Becerra-Arias, C.; Niño, J.A.F. Factors Associated with High Mercury Levels in Women and Girls from The Mojana Region, Colombia, 2013–2015. Int. J. Environ. Res. Public Health 2020, 17, 1827. [Google Scholar] [CrossRef] [PubMed]
- Baldewsingh, G.K.; Hindori-Mohangoo, A.D.; van Eer, E.D.; Covert, H.H.; Shankar, A.; Wickliffe, J.K.; Shi, L.; Lichtveld, M.Y.; Zijlmans, W.C.W.R. Association of Mercury Exposure and Maternal Sociodemographics on Birth Outcomes of Indigenous and Tribal Women in Suriname. Int. J. Environ. Res. Public Health 2021, 18, 6370. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.F.A.; Fraiji, N.A.; Barbosa, A.C.; de Lima, D.S.N.; Souza, J.R.; Dórea, J.G.; Cordeiro, G.W.O. Fish consumption, mercury exposure and serum antinuclear antibody in Amazonians. Int. J. Environ. Health Res. 2006, 16, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Drouillet-Pinard, P.; Huel, G.; Slama, R.; Forhan, A.; Sahuquillo, J.; Goua, V.; Thiébaugeorges, O.; Foliguet, B.; Magnin, G.; Kaminski, M.; et al. Prenatal mercury contamination: Relationship with maternal seafood consumption during pregnancy and fetal growth in the ‘EDEN mother–child’ cohort. Br. J. Nutr. 2010, 104, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, M.; Kubota, M.; Murata, K.; Nakai, K.; Sonoda, I.; Satoh, H. Changes in Mercury concentrations of segmental maternal hair during gestation and their correlations with other biomarkers of fetal exposure to methylmercury in the japanese population. Environ. Res. 2008, 106, 270–276. [Google Scholar] [CrossRef]
- Vejrup, K.; Brantsæter, A.L.; Knutsen, H.K.; Magnus, P.; Alexander, J.; Kvalem, H.E.; Meltzer, H.M.; Hougen, M. Prenatal Mercury exposure and infant birth weight in the Norwegian mother and child cohort study. Public Health Nutr. 2014, 17, 2071–2080. [Google Scholar] [CrossRef]
- Adlard, B.; Lemire, M.; Bonefeld-Jørgensen, E.C.; Long, M.; Ólafsdíttir, K.; Odland, J.O.; Rautio, A.; Myllynen, P.; Sandanger, T.M.; Dudarev, A.A.; et al. MercuNorth—Monitoring mercury in pregnant women from the Arctic as a baseline to assess the effectiveness of the Minamata Convention. Int. J. Public Health 2021, 80, 1881345. [Google Scholar] [CrossRef]
- Fearnside, P.M. Brazil’s Samuel dam: Lessons for hydroelectric development policy and the environment in Amazonia. Environ. Manag. 2005, 35, 1–19. [Google Scholar] [CrossRef]
- Fearnside, P.M.; Laurance, W.F.; Cochrane, M.A.; Bergen, S.; Sampaio, P.D.; Barber, C.; D’Angelo, S.; Fernandes, T. O futuro da Amazônia: Modelos para prever as consequências da infraestrutura futura nos planos plurianuais. Novos Cad. NAEA (Online) 2012, 15, 25–52. [Google Scholar] [CrossRef]
- Food and Agriculture Organization; World Health Organization (FAO/WHO). Report of the Joint FAO/WHO Expert Consultation on the Risks and Benefits of Fish Consumption; FAO: Rome, Italy; WHO: Geneva, Switzerland, 2011; 50pp, Available online: http://www.fao.org/3/ba0136e/ba0136e00.pdf (accessed on 20 October 2022).
- Bramante, C.T.; Spiller, P.; Landa, M. Fish consumption during pregnancy: An opportunity, not a risk. JAMA Pediatr. 2018, 172, 801–802. [Google Scholar] [CrossRef]
- Taylor, C.M.; Emmett, P.M.; Emond, A.M.; Golding, J. A review of guidance on fish consumption in pregnancy: Is it for purpose? Public Health Nutr. 2018, 21, 2149–2159. [Google Scholar] [CrossRef]
- Dorea, J.G. Cassava cyanogens and fish mercury are high but safely consumed in the diet of native Amazonians. Ecotoxicol. Environ. Saf. 2004, 57, 248–256. [Google Scholar] [CrossRef]
- Cunha, M.P.L.; Marques, R.C.; Dórea, J.G. Influence of Maternal Fish Intake on the Anthropometric Indices of Children in the Western Amazon. Nutrients 2018, 10, 1146. [Google Scholar] [CrossRef]
- Grandjean, P.; Jørgensen, P.J.; Weihe, P. Human milk as a source of methylmercury exposure in infants. Environ. Health Perspect. 1994, 102, 74–77. [Google Scholar] [CrossRef]
- Greenwood, M.R.; Clarkson, T.W.; Doherty, R.A.; Amin-Zaki, L.; Elhassani, S.; Majeed, M.A. Blood clearance half-times in lactating and nonlactating members of a population exposed to methymercury. Environ. Res. 1978, 16, 48–54. [Google Scholar] [CrossRef]
- Barbosa, A.C.; Dórea, J.G. Indices of mercury contamination during breast feeding in Amazon Basin. Environ. Toxicol. Pharmacol. 1998, 6, 71–79. [Google Scholar] [CrossRef]
- Matos, M.L.; Arruda, L.C. O uso da memória para investigação de ritos no parto e “resguardo” em Santarém (PA). Percursos 2016, 2, 10–22. [Google Scholar] [CrossRef]

| Variables | Median (Min–Max) | P25–P75% |
|---|---|---|
| Age (in years) | 21 (13–43) | 18–26 |
| Number of child (at enrolment) | 2 (0–12) | 1–3 |
| Number of child (at 2 y follow-up) | 3 (1–13) | 2–4 |
| Number of child (at 5 y follow-up) | 4 (1–13) | 3–5 |
| Years of education | 5 (0–17) | 4–8 |
| Family income (BRL) | 560 (50–4500) | 400–800 |
| Number of cohabitants | 6 (2–16) | 4–8 |
| Type of residence | n | % |
| Wood | 912 | 63.64 |
| Brick | 263 | 18.35 |
| Mixed | 230 | 16.05 |
| Straw | 28 | 1.95 |
| Household situation | ||
| Owned | 782 | 54.57 |
| Rented | 207 | 14.45 |
| From relatives | 280 | 19.53 |
| Borrowed | 164 | 11.44 |
| Water supply | ||
| Piped | 273 | 19.06 |
| Well | 700 | 48.88 |
| River | 293 | 20.46 |
| Piped/well | 146 | 10.19 |
| Public tap | 14 | 0.97 |
| Piped/river | 6 | 0.42 |
| Energy supply | ||
| Yes | 1021 | 71.24 |
| No | 412 | 28.76 |
| Fish consumption | ||
| Yes | 1342 | 93.64 |
| No | 91 | 6.36 |
| Variables | Median (Min–Max) | P25–P75% |
|---|---|---|
| Gestation period (in weeks) | 39 (32–43) | 38–40 |
| Breastfeeding duration (in months) | 6 (0–24) | 3–9 |
| Place of delivery | n | % |
| Hospital | 1072 | 74.80 |
| Residence | 361 | 25.19 |
| Type of delivery | ||
| Normal | 838 | 58.47 |
| Cesarean | 595 | 41.53 |
| Newborn sex | ||
| Female | 724 | 50.52 |
| Male | 709 | 49.48 |
| Fetal maturity | ||
| Preterm | 84 | 5.86 |
| Term | 1291 | 90.09 |
| Post-term | 58 | 4.04 |
| Variables | Tin Mining (n = 294) | Riverine (n = 396) | Rural (n = 67) | Urban (n = 676) | ||||
|---|---|---|---|---|---|---|---|---|
| Median (Min–Max) | P25–P75% | Median (Min–Max) | P25–P75% | Median (Min–Max) | P25–P75% | Median (Min–Max) | P25–P75% | |
| Number of children | ||||||||
| Prenatal | 2 (0–5) | 1–2 | 2 (0–12) | 1–3 | 2 (0–10) | 1–3 | 2 (0–8) | 1–3 |
| After 2 y | 3 (1–7) | 2–4 | 3 (1–13) | 2–4 | 3 (1–11) | 2–4 | 3 (1–9) | 2–4 |
| After 5 y | 3 (1–7) | 3–4 | 4 (2–13) | 3–5 | 4 (2–11) | 3–5 | 4 (1–10) | 3–5 |
| Breastfeeding duration (in months) | 5 (1–24) | 3–7 | 6 (0–24) | 5–11 | 6 (1–24) | 4–10 | 5 (0–24) | 2–10 |
| Hg (µg·g−1) | ||||||||
| Prenatal | 4.45 (1.53–11.94) | 3.4–5.51 | 12.11 (1.02–130.72) | 7.22–18.09 | 7.82 (2.56–41.10) | 6.23–9.89 | 5.36 (0.22–24.14) | 3.84–6.84 |
| After 6 months | 3.07 (0.88–9.66) | 2.33–4.12 | 11.33 (0.70–125.21) | 6.83–17.33 | 7.09 (2.64–41.75) | 5.91–9.15 | 4.66 (0.50–19.59) | 3.47–6.11 |
| After 2 y | 3.92 (1.04–9.14) | 3.20–4.94 | 11.50 (0.87–129.15) | 6.49–17.76 | 7.73 (1.91–42.34) | 5.70–9.46 | 5.28 (0.49–29.72) | 3.85–6.94 |
| After 5 y | 3.75 (0.68–9.88) | 3.13–4.95 | 12.22 (0.56–146.87) | 8.10–18.98 | 7.34 (1.53–44.86) | 5.14–9.88 | 5.36 (0.55–15.84) | 3.76–7.50 |
| Fish consumption (days per week) | 1 (0–2) | 1–2 | 5 (0–7) | 3–7 | 3 (1–7) | 2–5 | 2 (0–7) | 1–3 |
| Variables | Mining (n = 294) | Riverine (n = 396) | Rural (n = 67) | Urban (n = 676) |
|---|---|---|---|---|
| (tau) p-Value | (tau) p-Value | (tau) p-Value | (tau) p-Value | |
| ** Number of children and Hg levels prenatal | (0.041) 0.337 | (0.046) 0.202 | (−0.020) 0.821 | (0.004) 0.864 |
| Number of children and Hg levels at 2 y | (−0.028) 0.513 | (0.056) 0.121 | (−0.052) 0.561 | (−0.020) 0.476 |
| Number of children and Hg levels at 5 y | (−0.063) 0.142 | (0.060) 0.099 | (−0.007) 0.933 | (0.002) 0.941 |
| Breastfeeding duration and Hg levels prenatal | (0.045) 0.268 | (0.077) 0.028 * | (0.152) 0.081 | (0.042) 0.109 |
| Breastfeeding duration and Hg levels at 6 m | (−0.007) 0.848 | (0.073) 0.037 * | (0.080) 0.325 | (−0.002) 0.927 |
| Breastfeeding duration and Hg levels at 2 y | (0.009) 0.812 | (0.021) 0.548 | (0.133) 0.127 | (0.028) 0.281 |
| Breastfeeding duration and Hg levels at 5 y | (−0.005) 0.891 | (−0.013) 0.713 | (0.064) 0.460 | (−0.002) 0.940 |
| Fish consumption and Hg levels prenatal | (0.024) 0.596 | (0.663) <0.001 * | (0.418) <0.001 * | (0.541) <0.001 * |
| Fish consumption and Hg levels at 6 months | (0.025) 0.590 | (0.676) <0.001 * | (0.455) <0.001 * | (0.495) <0.001 * |
| Fish consumption and Hg levels at 2 y | (0.002) 0.960 | (0.687) <0.001 * | (0.393) <0.001 * | (0.263) <0.001 * |
| Fish consumption and Hg levels at 5 y | (−0.001) 0.982 | (0.686) <0.001 * | (0.470) <0.001 * | (0.426) <0.001 * |
| Coefficient * | 95% Confidence Interval | p-Value | ||
|---|---|---|---|---|
| 6 months | ||||
| Breastfeeding duration # | −0.0531607 | −0.0981262 | −0.0081952 | 0.02 ** |
| 2 years | ||||
| Breastfeeding duration # | −0.0372642 | −0.0658661 | −0.0086623 | 0.01 ** |
| Number of children ψ | −0.3023673 | −0.7058452 | 0.1011105 | 0.14 |
| 5 years | ||||
| Breastfeeding duration # | −0.0535429 | −0.0896773 | −0.0174084 | 0.004 ** |
| Number of children ψ | −0.1050237 | −0.3999480 | 0.1899007 | 0.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bello, T.C.S.; Buralli, R.J.; Cunha, M.P.L.; Dórea, J.G.; Diaz-Quijano, F.A.; Guimarães, J.R.D.; Marques, R.C. Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. Int. J. Environ. Res. Public Health 2023, 20, 5225. https://doi.org/10.3390/ijerph20065225
Bello TCS, Buralli RJ, Cunha MPL, Dórea JG, Diaz-Quijano FA, Guimarães JRD, Marques RC. Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. International Journal of Environmental Research and Public Health. 2023; 20(6):5225. https://doi.org/10.3390/ijerph20065225
Chicago/Turabian StyleBello, Thayssa C. S., Rafael J. Buralli, Mônica P. L. Cunha, José G. Dórea, Fredi A. Diaz-Quijano, Jean R. D. Guimarães, and Rejane C. Marques. 2023. "Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil" International Journal of Environmental Research and Public Health 20, no. 6: 5225. https://doi.org/10.3390/ijerph20065225
APA StyleBello, T. C. S., Buralli, R. J., Cunha, M. P. L., Dórea, J. G., Diaz-Quijano, F. A., Guimarães, J. R. D., & Marques, R. C. (2023). Mercury Exposure in Women of Reproductive Age in Rondônia State, Amazon Region, Brazil. International Journal of Environmental Research and Public Health, 20(6), 5225. https://doi.org/10.3390/ijerph20065225






