Electrochemotherapy as an Effective Alternative in the Treatment of Local Advanced Oral Squamous Cell Carcinoma: A Retrospective Analysis of Treated Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
2.2. Endpoints
2.3. Sample and Setting
2.4. Inclusion and Exclusion Criteria
2.5. Procedure
2.6. Response to Treatment
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.M.G.; García-Pola, M.J.; Varela-Centelles, P.; Seoane-Romero, J.M. On the role of physicians in oral cancer diagnosis. Oral Oncol. 2020, 108, 104843. [Google Scholar] [CrossRef]
- Sorrentino, A.; Ferragina, F.; Barca, I.; Arrotta, A.; Cristofaro, M.G. Extra-Nodal Lymphomas of the Head and Neck and Oral Cavity: A Retrospective Study. Curr. Oncol. 2022, 29, 7189–7197. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Mir, L.M.; Orlowski, S.; Belehradek, J.; Paoletti, C. Electrochemotherapy potentiation of antitumour effect of bleomycin by local electric pulses. Eur. J. Cancer 1991, 27, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, S.; Belehradek, J., Jr.; Paoletti, C.; Mir, L.M. Transient electropermeabilization of cells in culture: Increase of the cytotoxicity of anticancer drugs. Biochem. Pharmacol. 1988, 37, 4727–4733. [Google Scholar] [CrossRef]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Soden, D.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- De Bree, R.; Wessel, I. Electrochemotherapy in the head and neck area: An addition to the treatment armamentarium. Curr. Opin. Otolaryngol Head Neck Surg. 2020, 28, 112–117. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; De Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Acad. Dermatol. Venereol. 2017, 32, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Bloom, D.; Goldfarb, P. The role of intratumour therapy with electroporation and bleomycin in the management of advanced squamous cell carcinoma of the head and neck. Eur. J. Surg. Oncol. (EJSO) 2005, 31, 1029–1035. [Google Scholar] [CrossRef]
- Mir, L.M.; Gehl, J.; Sersa, G.; Collins, C.; Garbay, J.R.; Billard, V.; Geertsen, P.F.; Rudolf, Z.; O’Sullivan, G.C.; Marty, M. Standard operating procedures of the electrochemotherapy: Instructions for the use of bleomycin or cisplatin administered either systemically or locally and electric pulses derived by Cliniporator ™ by means of invasive or non-invasive electrodes. Eur. J. Cancer Suppl. 2006, 4, 14–25. [Google Scholar] [CrossRef]
- Borgognoni, L.; Pescitelli, L.; Gerlini, G.; Brandani, P.; Gelli, R.; Giannotti, V.; Bellucci, F.; Sestini, S. Efficacy of electrochemotherapy in the treatment of cutaneous melanoma metastases and rare non-melanoma skincancer. Anticancer Res. 2020, 40, 6485–6492. [Google Scholar] [CrossRef] [PubMed]
- Burian, M.; Formanek, M.; Regele, H. Electroporation therapy in head and neck cancer. Acta Oto-Laryngol. 2003, 123, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, M.; Papa, A.; Capasso, P.; Moio, M.; Cubicciotti, E.; Parascandolo, S. Electrochemotherapy for non-melanoma head and neck cancers: Clinical outcomes in 25 patients. Ann. Surg. 2012, 255, 1158–1164. [Google Scholar] [CrossRef] [PubMed]
- Campana, L.G.; Edhemovic, I.; Soden, D.; Perrone, A.M.; Scarpa, M.; Campanacci, L.; Cemazar, M.; Valpione, S.; Miklavčič, D.; Mocellin, S.; et al. Electrochemotherapy—Emerging applications technical advances, new indications, combined approaches, and multi-institutional collaboration. Eur. J. Surg. Oncol. 2019, 45, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Haraldstad, K.; Wahl, A.; Andenæs, R.; Andersen, J.R.; Andersen, M.H.; Beisland, E.; Borge, C.R.; Engebretsen, E.; Eisemann, M.; Halvorsrud, L.; et al. A systematic review of quality of life research in medicine and health sciences. Qual. Life Res. 2019, 28, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Novembre, D.; Barca, I.; Cordaro, R.; Kallaverja, E.; Ferragina, F.; Cristofaro, M.G. Malignant transformation of oral lichen planus. A retrospective analysis from 2003-2014: Our experience. Ann Ital. Chir. 2020, 91, 445–450. [Google Scholar]
- Leoncini, E.; Vukovic, V.; Cadoni, G.; Pastorino, R.; Arzani, D.; Bosetti, C.; Canova, C.; Garavello, W.; La Vecchia, C.; Maule, M.; et al. Clinical features and prognostic factors in patients with head and neck cancer: Results from a multicentric study. Cancer Epidemiol. 2015, 39, 367–374. [Google Scholar] [CrossRef]
- Barca, I.; Mignogna, C.; Novembre, D.; Ferragina, F.; Cristofaro, M.G. Immunohistochemical Analysis of the Beclin-1 Expression Predicts the Progression of Oral Squamous Cell Carcinoma. Int. J. Environ. Res. Public Health 2021, 18, 11125. [Google Scholar] [CrossRef]
- Bertino, G.; Sersa, G.; De Terlizzi, F.; Occhini, A.; Plaschke, C.C.; Groselj, A.; Langdon, C.; Grau, J.J.; McCaul, J.A.; Heuveling, D.; et al. European Research on Electrochemotherapy in Head and Neck Cancer (EURECA) project: Results of the treatment of skin cancer. Eur. J. Cancer 2016, 63, 41–52. [Google Scholar] [CrossRef]
- Chen, C.; Smye, S.; Robinson, M.; Evans, J. Membrane electroporation theories: A review. Med. Biol. Eng. Comput. 2006, 44, 5–14. [Google Scholar] [CrossRef]
- Larkin, J.O.; Collins, C.G.; Aarons, S.; Tangney, M.; Whelan, M.; O’Reily, S.; Breathnach, O.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy: Aspects of preclinical development and early clinical experience. Ann. Surg. 2007, 245, 469–479. [Google Scholar] [CrossRef]
- Sadadcharam, M.; Soden, D.M.; O’Sullivan, G.C. Electrochemotherapy: An emerging cancer treatment. Int. J. Hyperth. 2008, 24, 263–273. [Google Scholar] [CrossRef]
- Quaglino, P.; Mortera, C.; Osella-Abate, S.; Barberis, M.; Illengo, M.; Rissone, M.; Savoia, P.; Bernengo, M.G. Electrochemotherapy with Intravenous Bleomycin in the Local Treatment of Skin Melanoma Metastases. Ann. Surg. Oncol. 2008, 15, 2215–2222. [Google Scholar] [CrossRef]
- Campana, L.G.; Valpione, S.; Mocellin, S.; Sundararajan, R.; Granziera, E.; Sartore, L.; Sileni, V.C.; Rossi, C.R. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br. J. Surg. 2012, 99, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Tarantino, L.; Busto, G.; Nasto, A.; Fristachi, R.; Cacace, L.; Talamo, M.; Accardo, C.; Bortone, S.; Gallo, P.; Tarantino, P.; et al. Percutaneous electrochemotherapy in the treatment of portal vein tumor thrombosis at hepatic hilum in patients with hepatocellular carcinoma in cirrhosis: A feasibility study. World J. Gastroenterol. 2017, 23, 906–918. [Google Scholar] [CrossRef]
- Mevio, N.; Bertino, G.; Occhini, A.; Scelsi, D.; Tagliabue, M.; Mura, F.; Benazzo, M. Electrochemotherapy for the Treatment of Recurrent Head and Neck Cancers: Preliminary Results. Tumori J. 2012, 98, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Perri, F.; Longo, F.; Fusco, R.; D’Alessio, V.; Aversa, C.; Pavone, E.; Pontone, M.; Marciano, M.L.; Villano, S.; Franco, P.; et al. Electrochemotherapy as a First Line Treatment in Recurrent Squamous Cell Carcinoma of the Oral Cavity and Oropharynx PDL-1 Negative and/or with Evident Contraindication to Immunotherapy: A Randomized Multicenter Controlled Trial. Cancers 2021, 13, 2210. [Google Scholar] [CrossRef] [PubMed]
- Strojan, P.; Grošelj, A.; Serša, G.; Plaschke, C.C.; Vermorken, J.B.; Nuyts, S.; de Bree, R.; Eisbruch, A.; Mendenhall, W.M.; Smee, R.; et al. Electrochemotherapy in Mucosal Cancer of the Head and Neck: A Systematic Review. Cancers 2021, 13, 1254. [Google Scholar] [CrossRef]
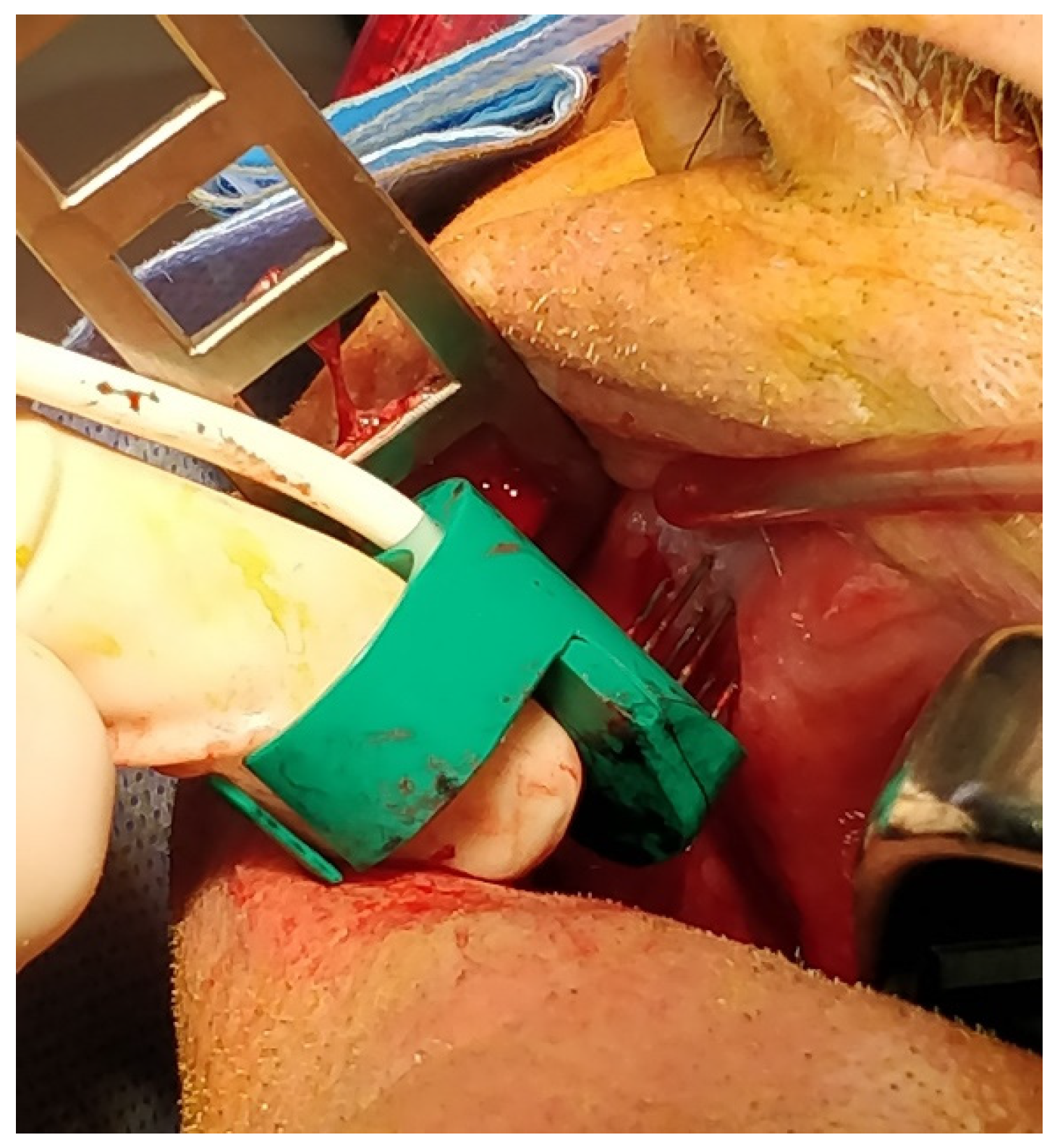
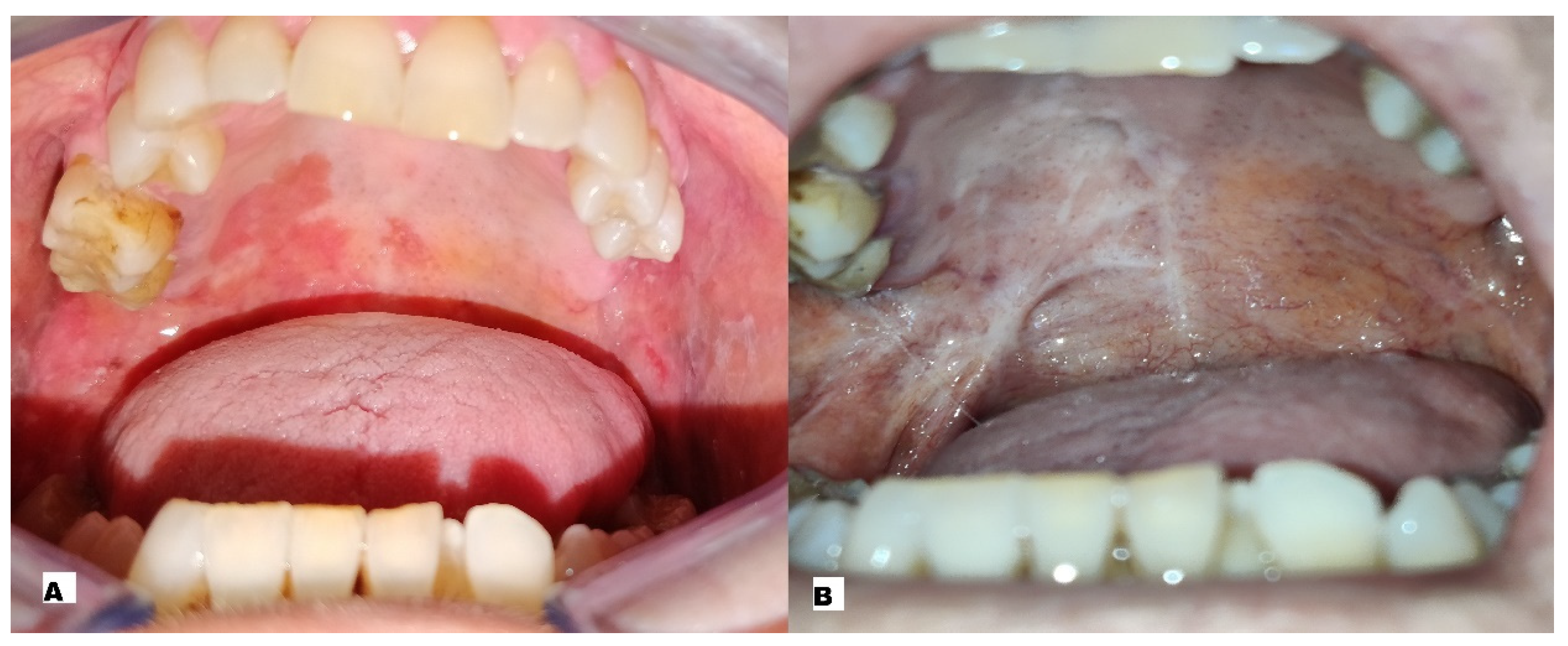
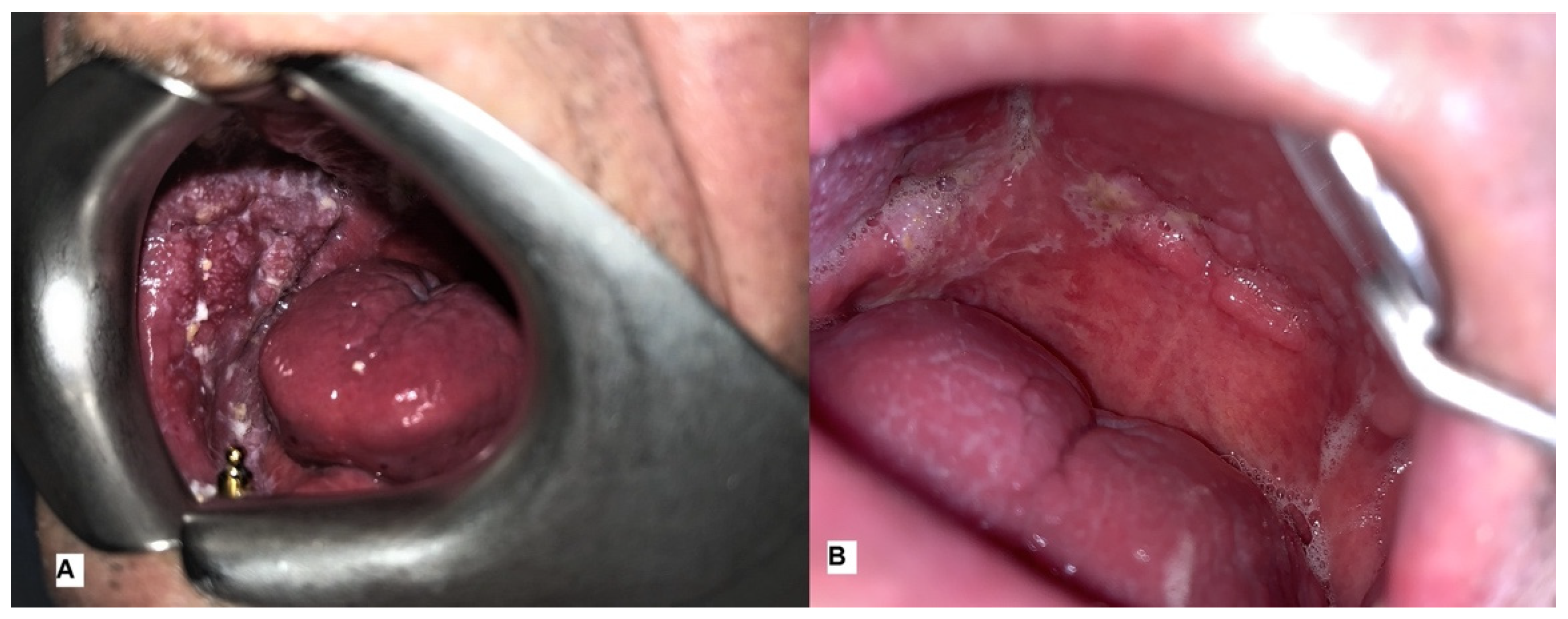
| Patient | Sex | Age | Smoker | Alcohol | Grading | Localisation | Stage | N° of Sessions of ECT |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 85 | Strong smokers | Moderate drinker | G2 | Palate | T3N0 | 1 ECT session |
| 2 | M | 68 | Strong smokers | Strong drinker | G2 | Palate | TEN0 | 2 ECT sessions |
| 3 | M | 71 | Strong smokers | Strong drinker | G2 | Buccal mucosa | T3N0 | 1 ECT session |
| 4 | F | 54 | Strong smokers | Non-drinker | G2 | Palate | T3N0 | 3 ECT sessions |
| 5 | F | 76 | Moderate smoker | Moderate drinker | G3 | Buccal Mucosa | T3N0 | 3 ECT sessions |
| 6 | F | 81 | Strong smokers | Moderate drinker | G3 | Oral Floor | T3N1 | 1 ECT session |
| VAS Score after 1 Day from ECT Treatment | VAS Score after 2 Days from ECT Treatment | VAS Score after 15 Days from ECT Treatment | VAS Score after 30 Days from ECT Treatment | |
|---|---|---|---|---|
| patient 1 | 9 | 9 | 5, 6 | 3, 6 |
| patient 2 | 8, 6 | 8, 2 | 6 | 2, 1 |
| patient 3 | 8, 6 | 7, 8 | 5, 3 | 2, 6 |
| patient 4 | 7, 9 | 7, 6 | 4, 9 | 0, 9 |
| patient 5 | 9, 3 | 8, 1 | 5, 6 | 1, 8 |
| patient 6 | 9, 1 | 8, 4 | 5 | 3, 2 |
| Average VAS Score | 8.75 | 8.18 | 5.4 | 2.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barca, I.; Ferragina, F.; Kallaverja, E.; Arrotta, A.; Cristofaro, M.G. Electrochemotherapy as an Effective Alternative in the Treatment of Local Advanced Oral Squamous Cell Carcinoma: A Retrospective Analysis of Treated Cases. Int. J. Environ. Res. Public Health 2023, 20, 5170. https://doi.org/10.3390/ijerph20065170
Barca I, Ferragina F, Kallaverja E, Arrotta A, Cristofaro MG. Electrochemotherapy as an Effective Alternative in the Treatment of Local Advanced Oral Squamous Cell Carcinoma: A Retrospective Analysis of Treated Cases. International Journal of Environmental Research and Public Health. 2023; 20(6):5170. https://doi.org/10.3390/ijerph20065170
Chicago/Turabian StyleBarca, Ida, Francesco Ferragina, Elvis Kallaverja, Antonella Arrotta, and Maria Giulia Cristofaro. 2023. "Electrochemotherapy as an Effective Alternative in the Treatment of Local Advanced Oral Squamous Cell Carcinoma: A Retrospective Analysis of Treated Cases" International Journal of Environmental Research and Public Health 20, no. 6: 5170. https://doi.org/10.3390/ijerph20065170
APA StyleBarca, I., Ferragina, F., Kallaverja, E., Arrotta, A., & Cristofaro, M. G. (2023). Electrochemotherapy as an Effective Alternative in the Treatment of Local Advanced Oral Squamous Cell Carcinoma: A Retrospective Analysis of Treated Cases. International Journal of Environmental Research and Public Health, 20(6), 5170. https://doi.org/10.3390/ijerph20065170







