Brain Injury Is Prevalent and Precedes Tobacco Use among Youth and Young Adults Experiencing Homelessness
Abstract
1. Background
2. Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic Characteristics
2.2.2. Blunt Force Head Trauma Exposure
2.2.3. Brain Oxygen Deprivation Exposure
2.2.4. Altered Consciousness/Brain Injury
2.2.5. Age at First Exposure to Events That Can Cause Brain Injury
2.2.6. Age at First Tobacco Use
2.3. Statistical Analyses
3. Results
3.1. Participant Characteristics
3.2. Co-Occurrence of Exposures That Can Lead to Brain Injury
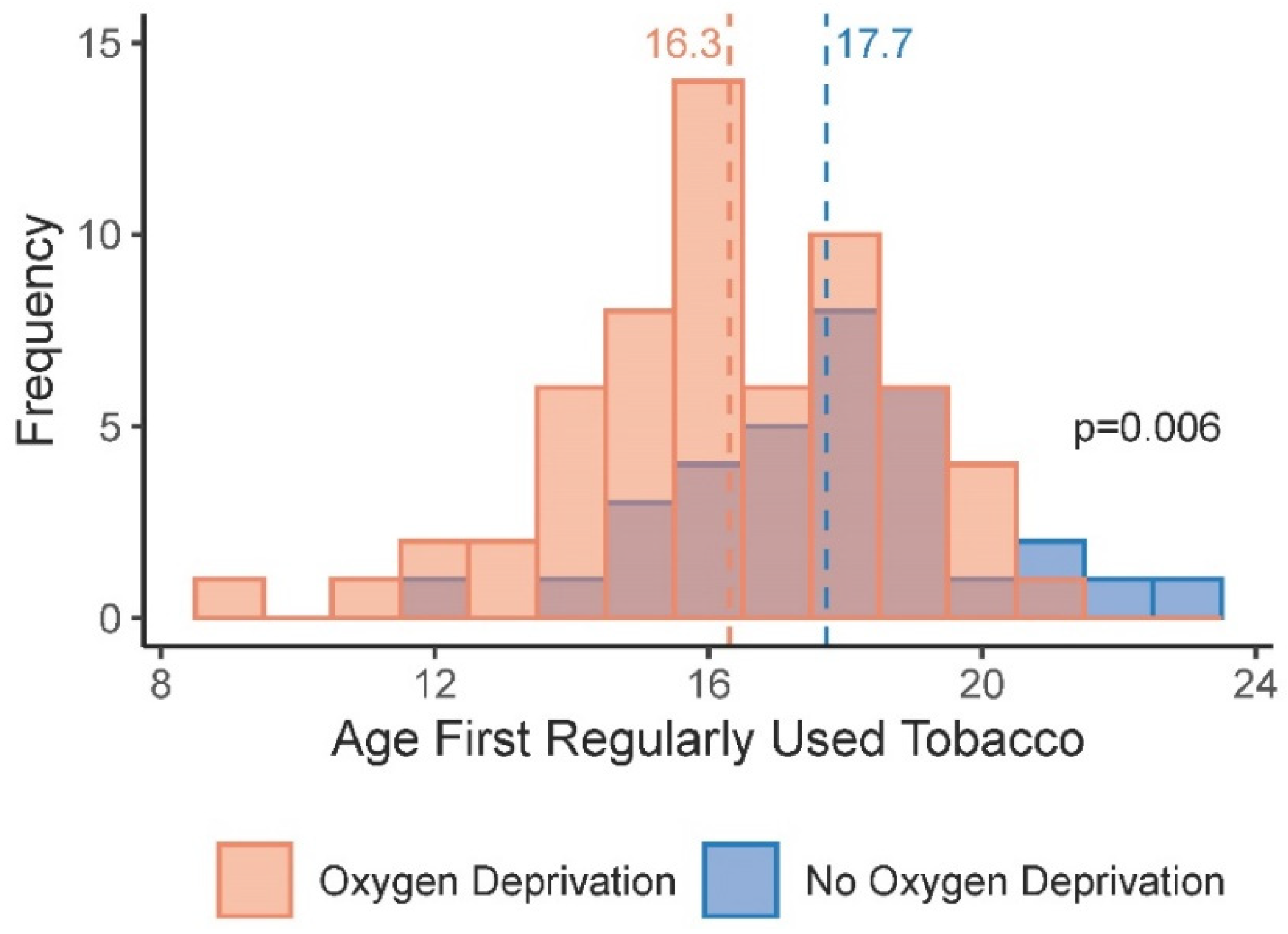
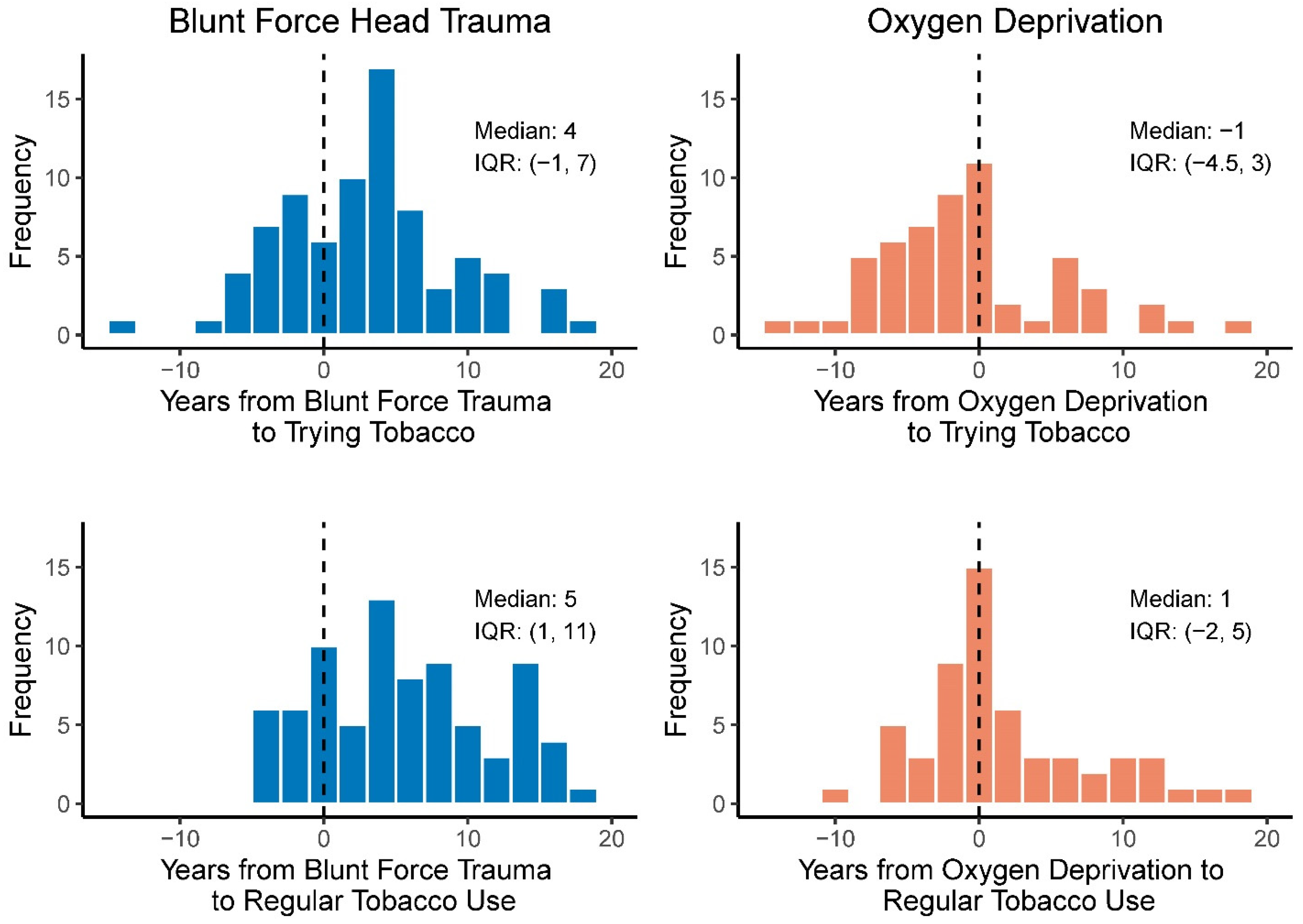
3.3. Age at Tobacco Initiation and Exposures That Can Lead to Brain Injury
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conroy, E.; Burns, L.; Wilson, S. Alcohol Use Disorder and Cognitive Impairment among Older Homeless Persons: Implications for Service Delivery; Foundation for Alcohol Research & Education: Sydney, NS, Australia, 2013. [Google Scholar]
- Hwang, S.W.; Chambers, C.; Chiu, S.; Katic, M.; Kiss, A.; Redelmeier, D.A.; Levinson, W. A comprehensive assessment of health care utilization among homeless adults under a system of universal health insurance. Am. J. Public Health 2013, 103 (Suppl. 2), S294–S301. [Google Scholar] [CrossRef]
- Ilie, G.; Mann, R.E.; Hamilton, H.; Adlaf, E.M.; Boak, A.; Asbridge, M.; Rehm, J.; Cusimano, M.D. Substance Use and Related Harms among Adolescents with and without Traumatic Brain Injury. J. Head Trauma Rehabil. 2015, 30, 293–301. [Google Scholar] [CrossRef]
- CARF International. CARF-CCAC Standards Manual 2015; CARF International: Tucson, AZ, USA, 2015. [Google Scholar]
- Mackelprang, J.L.; Harpin, S.B.; Grubenhoff, J.A.; Rivara, F.P. Adverse outcomes among homeless adolescents and young adults who report a history of traumatic brain injury. Am. J. Public Health 2014, 104, 1986–1992. [Google Scholar] [CrossRef]
- Adshead, C.D.; Norman, A.; Holloway, M. The inter-relationship between acquired brain injury, substance use and homelessness; the impact of adverse childhood experiences: An interpretative phenomenological analysis study. Disabil. Rehabil. 2019, 43, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.W.; Colantonio, A.; Chiu, S.; Tolomiczenko, G.; Kiss, A.; Cowan, L.; Redelmeier, D.A.; Levinson, W. The effect of traumatic brain injury on the health of homeless people. CMAJ 2008, 179, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, J.L.; Thornton, A.E.; Sevick, J.M.; Silverberg, n.D.; Barr, A.M.; Honer, W.G.; Panenka, W.J. Traumatic brain injury in homeless and marginally housed individuals: A systematic review and meta-analysis. Lancet Public Health 2020, 5, e19–e32. [Google Scholar] [CrossRef]
- Ilie, G.; Adlaf, E.M.; Mann, R.E.; Boak, A.; Hamilton, H.; Asbridge, M.; Colantonio, A.; Turner, n.E.; Rehm, J.; Cusimano, M.D. The moderating effects of sex and age on the association between traumatic brain injury and harmful psychological correlates among adolescents. PLoS ONE 2014, 9, e108167. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.; Heron, J.; Munafo, M. Substance use, criminal behaviour and psychiatric symptoms following childhood traumatic brain injury: Findings from the ALSPAC cohort. Eur. Child Adolesc. Psychiatry 2017, 26, 1197–1206. [Google Scholar] [CrossRef]
- Bogner, J.; Corrigan, J.D.; Yi, H.; Singichetti, B.; Manchester, K.; Huang, L.; Yang, J. Lifetime History of Traumatic Brain Injury and Behavioral Health Problems in a Population-Based Sample. J. Head Trauma Rehabil. 2020, 35, E43–E50. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.; Stewart, S.H.; Cusimano, M.; Asbridge, M. Examining the Relationship Between Traumatic Brain Injury and Substance Use Outcomes in the Canadian Population. Subst. Use Misuse 2016, 51, 1577–1586. [Google Scholar] [CrossRef]
- Barnes, A.J.; Gower, A.L.; Sajady, M.; Lingras, K.A. Health and adverse childhood experiences among homeless youth. BMC Pediatr. 2021, 21, 164. [Google Scholar] [CrossRef] [PubMed]
- Yeoman, K.; Safranek, T.; Buss, B.; Cadwell, B.L.; Mannino, D. Adverse childhood experiences and adult smoking, Nebraska, 2011. Prev. Chronic. Dis. 2013, 10, E159. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; McDonald, S.E.; Conley, D. Patterns of adverse childhood experiences and substance use among young adults: A latent class analysis. Addict. Behav. 2018, 78, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Moss, H.B.; Ge, S.; Trager, E.; Saavedra, M.; Yau, M.; Ijeaku, I.; Deas, D. Risk for Substance Use Disorders in young adulthood: Associations with developmental experiences of homelessness, foster care, and adverse childhood experiences. Compr. Psychiatry 2020, 100, 152175. [Google Scholar] [CrossRef] [PubMed]
- Mullins, L.; O’Hanlon, C.E.; Shadel, W.G.; Tucker, J.S. A qualitative study of smoking cessation experiences and perceptions among homeless young adults. J. Soc. Distress Homeless 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Arnsten, J.H.; Reid, K.; Bierer, M.; Rigotti, n. Smoking behavior and interest in quitting among homeless smokers. Addict. Behav. 2004, 29, 1155–1161. [Google Scholar] [CrossRef]
- Goldade, K.; Choi, K.; Bernat, D.H.; Klein, E.G.; Okuyemi, K.S.; Forster, J. Multilevel predictors of smoking initiation among adolescents: Findings from the Minnesota Adolescent Community Cohort (MACC) study. Prev. Med. 2012, 54, 242–246. [Google Scholar] [CrossRef]
- Mahabee-Gittens, E.M.; Xiao, Y.; Gordon, J.S.; Khoury, J.C. The dynamic role of parental influences in preventing adolescent smoking initiation. Addict. Behav. 2013, 38, 1905–1911. [Google Scholar] [CrossRef]
- O’Loughlin, J.; O’Loughlin, E.K.; Wellman, R.J.; Sylvestre, M.P.; Dugas, E.n.; Chagnon, M.; Dutczak, H.; Lague, J.; McGrath, J.J. Predictors of Cigarette Smoking Initiation in Early, Middle, and Late Adolescence. J. Adolesc. Health 2017, 61, 363–370. [Google Scholar] [CrossRef]
- Weiss, J.W.; Mouttapa, M.; Cen, S.; Johnson, C.A.; Unger, J. Longitudinal effects of hostility, depression, and bullying on adolescent smoking initiation. J. Adolesc. Health 2011, 48, 591–596. [Google Scholar] [CrossRef]
- Song, A.V.; Morrell, H.E.; Cornell, J.L.; Ramos, M.E.; Biehl, M.; Kropp, R.Y.; Halpern-Felsher, B.L. Perceptions of smoking-related risks and benefits as predictors of adolescent smoking initiation. Am. J. Public Health 2009, 99, 487–492. [Google Scholar] [CrossRef]
- Bernat, D.H.; Klein, E.G.; Forster, J.L. Smoking initiation during young adulthood: A longitudinal study of a population-based cohort. J. Adolesc. Health 2012, 51, 497–502. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System ACE Data. Available online: https://www.cdc.gov/violenceprevention/aces/ace-brfss.html (accessed on 12 February 2019).
- Valera, E.M.; Berenbaum, H. Brain injury in battered women. J. Consult. Clin. Psychol. 2003, 71, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Haarbauer-Krupa, J.; Lebrun-Harris, L.A.; Black, L.I.; Veliz, P.; Daugherty, J.; Desrocher, R.; Schulenberg, J.; Pilkey, D.; Breiding, M. Comparing prevalence estimates of concussion/head injury in US. children and adolescents in national surveys. Ann. Epidemiol. 2021, 54, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Jetelina, K.K.; Reingle Gonzalez, J.M.; Brown, C.V.R.; Foreman, M.L.; Field, C. Acute Alcohol Use, History of Homelessness, and Intent of Injury Among a Sample of Adult Emergency Department Patients. Violence Vict. 2017, 32, 658–670. [Google Scholar] [CrossRef]
- Petering, R.; Wenzel, S.L.; Winetrobe, H. Systematic Review of Current Intimate Partner Violence Prevention Programs and Applicability to Homeless Youth. J. Soc. Soc. Work Res. 2014, 5, 107–135. [Google Scholar] [CrossRef]
- Pavao, J.; Alvarez, J.; Baumrind, n.; Induni, M.; Kimerling, R. Intimate partner violence and housing instability. Am. J. Prev. Med. 2007, 32, 143–146. [Google Scholar] [CrossRef]
- Reitsma, M.B.; Flor, L.S.; Mullany, E.C.; Gupta, V.; Hay, S.I.; Gakidou, E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Public Health 2021, 6, e472–e481. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Lotfipour, S. Nicotine Gateway Effects on Adolescent Substance Use. West J. Emerg. Med. 2019, 20, 696–709. [Google Scholar] [CrossRef]
- Rao, R.K.; McConnell, D.D.; Litofsky, n.S. The impact of cigarette smoking and nicotine on traumatic brain injury: A review. Brain Inj. 2022, 36, 1–20. [Google Scholar] [CrossRef]
- Ganesalingam, K.; Sanson, A.; Anderson, V.; Yeates, K.O. Self-regulation as a mediator of the effects of childhood traumatic brain injury on social and behavioral functioning. J. Int. Neuropsychol. Soc. 2007, 13, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.M.; Viveiros, n.; Lynch, K.R.; Andersen, T.S.; Fisher, B. Adolescent reproductive and sexual coercion: Measurement invariance in a population-based sample of male and female high school students. J. Fam. Violence 2020, 35, 619–632. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.E.; Anderson, M.L.; Nemeth, J.; Rivara, F.P.; Buettner, C. History of dating violence and the association with late adolescent health. BMC Public Health 2013, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.M.; Mengo, C.; Kulow, E.; Brown, A.; Ramirez, R. Provider perceptions and domestic violence (dv) survivor experiences of traumatic and anoxic-hypoxic brain injury: Implications for dv advocacy service provision. J. Aggress. Maltreat. Trauma 2019, 28, 744–763. [Google Scholar] [CrossRef]
| All Participants (n = 96) | Participants with Brain Injury (n = 59) | |||
|---|---|---|---|---|
| Age (mean, SD) | 21.82 | 2.00 | 21.84 | 2.12 |
| Gender (n, %) | ||||
| Cisgender Male | 52 | 54.17 | 30 | 50.85 |
| Cisgender Female | 39 | 40.63 | 25 | 42.37 |
| Transgender Female | 2 | 2.08 | 2 | 3.39 |
| Transgender Male | 2 | 2.08 | 1 | 1.69 |
| Non-binary | 1 | 1.04 | 1 | 1.69 |
| Race (n, %) | ||||
| White | 15 | 15.63 | 12 | 20.34 |
| Black | 51 | 53.13 | 27 | 45.76 |
| Other | 30 | 31.25 | 20 | 33.90 |
| Ethnicity (n, %) | ||||
| Non-Hispanic | 88 | 91.67 | 54 | 91.53 |
| Hispanic | 8 | 8.33 | 5 | 8.47 |
| Sexual Orientation (n, %) | ||||
| Heterosexual/Straight | 71 | 73.96 | 42 | 71.19 |
| Bisexual | 19 | 19.79 | 13 | 22.03 |
| Other | 6 | 6.25 | 4 | 6.78 |
| Education (n, %) | ||||
| Less than High School | 31 | 32.29 | 22 | 37.29 |
| High School Diploma | 46 | 47.92 | 28 | 47.46 |
| GED | 4 | 4.17 | 1 | 1.69 |
| More than High School | 15 | 15.63 | 8 | 13.56 |
| Where Slept Most Nights (n, %) | ||||
| With family or friends/Own home | 31 | 32.29 | 19 | 32.20 |
| Shelter/Drop-in-center | 27 | 28.13 | 18 | 30.51 |
| Group home/Treatment facility/Detention facility | 13 | 13.54 | 7 | 11.86 |
| Outside/Car/Tent | 25 | 26.04 | 15 | 25.42 |
| BRFSS ACE (mean, SD) | 4.23 | 2.65 | 4.84 | 2.59 |
| Age First Tried Tobacco (mean, SD) | 14.15 | 3.69 | 13.81 | 3.57 |
| Age First Regularly Used Tobacco (mean, SD) | 16.77 | 2.46 | 16.39 | 2.38 |
| All Participants (n = 96) | Participants with Brain Injury (n = 59) | |||
|---|---|---|---|---|
| Brain Oxygen DeprivationExposure (n, %) | ||||
| None | 33 | 35.11 | 0 | 0.00 |
| Any | 61 | 64.89 | 59 | 100.00 |
| Intentional Oxygen Deprivation Exposure (Choking Games or Intentional Choking) (n, %) | ||||
| None | 46 | 48.42 | 12 | 20.34 |
| Any | 49 | 51.58 | 47 | 79.66 |
| Choking Games (n, %) | ||||
| Never | 84 | 87.50 | 48 | 81.36 |
| Once | 5 | 5.21 | 5 | 8.47 |
| A few times | 4 | 4.17 | 4 | 6.78 |
| Too many times to remember | 2 | 2.08 | 2 | 3.39 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| Intentional Choking (n, %) | ||||
| Never | 51 | 53.13 | 17 | 28.81 |
| Once | 12 | 12.50 | 11 | 18.64 |
| A few times | 25 | 26.04 | 24 | 40.68 |
| Too many times to remember | 7 | 7.29 | 7 | 11.86 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| Stopped Breathing on Accident (n, %) | ||||
| Never | 53 | 55.21 | 18 | 30.51 |
| Once | 18 | 18.75 | 18 | 30.51 |
| A few times | 17 | 17.71 | 17 | 28.81 |
| Too many times to remember | 4 | 4.17 | 4 | 6.78 |
| Don’t Know | 3 | 3.13 | 2 | 3.39 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| People Responsible for Intentional Oxygen Deprivation Exposure (n, %) a | ||||
| Parent/Guardian | 11 | 26.19 | 11 | 27.50 |
| Brother or Sister | 12 | 28.57 | 11 | 27.50 |
| Other Family Member | 8 | 19.05 | 8 | 20.00 |
| Romantic Partner | 25 | 59.52 | 25 | 62.50 |
| Peer | 12 | 29.27 | 12 | 30.00 |
| Other | 7 | 16.67 | 6 | 15.38 |
| Age of First Brain Oxygen Deprivation Exposure (mean, SD) | 14.11 | 5.63 | 13.90 | 5.59 |
| Most Recent Brain Oxygen Deprivation Exposure (n, %) | ||||
| Past 3 Days | 3 | 4.92 | 3 | 5.08 |
| Past Month | 7 | 11.48 | 7 | 11.86 |
| Past Year | 15 | 24.59 | 15 | 25.42 |
| Longer than a Year ago | 33 | 54.10 | 31 | 52.54 |
| Don’t Know | 3 | 4.92 | 3 | 5.08 |
| Brain Oxygen Deprivation Exposure Prior to First Trying Tobacco (n, %) | ||||
| No | 38 | 67.86 | 36 | 66.67 |
| Yes | 18 | 32.14 | 18 | 33.33 |
| Brain Oxygen Deprivation Exposure Prior to First Regularly Using Tobacco (n, %) | ||||
| No | 25 | 44.64 | 23 | 42.59 |
| Yes | 31 | 55.36 | 31 | 57.41 |
| Blunt Force Head TraumaExposure (n, %) | ||||
| None | 12 | 12.63 | 1 | 1.69 |
| Any | 83 | 87.37 | 58 | 98.31 |
| Intentional Blunt Force Head Trauma Exposure (Hit in Head of Shaken Violently) (n, %) | ||||
| None | 25 | 26.60 | 6 | 10.34 |
| Any | 69 | 73.40 | 52 | 89.66 |
| Hit in Head (n, %) | ||||
| Never | 29 | 30.21 | 9 | 15.25 |
| Once | 10 | 10.42 | 7 | 11.86 |
| A few times | 36 | 37.50 | 27 | 45.76 |
| Too many times to remember | 20 | 20.83 | 16 | 27.12 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| Shaken Violently (n, %) | ||||
| Never | 56 | 58.33 | 24 | 40.68 |
| Once | 6 | 6.25 | 6 | 10.17 |
| A few times | 19 | 19.79 | 15 | 25.42 |
| Too many times to remember | 13 | 13.54 | 13 | 22.03 |
| Don’t Know | 1 | 1.04 | 1 | 1.69 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| Accidentally Hurt (n, %) | ||||
| Never | 29 | 30.21 | 12 | 20.34 |
| Once | 20 | 20.83 | 14 | 23.73 |
| A few times | 32 | 33.33 | 23 | 38.98 |
| Too many times to remember | 14 | 14.58 | 10 | 16.95 |
| Refuse | 1 | 1.04 | 0 | 0.00 |
| People Responsible for Intentional Blunt Force Head Trauma Exposure (n, %) a | ||||
| Parent/Guardian | 22 | 33.33 | 20 | 40.00 |
| Brother or Sister | 22 | 32.84 | 20 | 40.00 |
| Other Family Member | 12 | 17.91 | 9 | 18.00 |
| Romantic Partner | 34 | 50.75 | 28 | 56.00 |
| Peer | 39 | 59.09 | 27 | 54.00 |
| Other | 14 | 20.90 | 9 | 18.37 |
| Age of First Blunt Force Head Trauma Exposure (mean, SD) | 10.65 | 5.76 | 10.78 | 5.51 |
| Most Recent Blunt Force Head Trauma Exposure (n, %) | ||||
| Past 3 Days | 4 | 4.82 | 2 | 3.45 |
| Past Month | 11 | 13.25 | 9 | 15.52 |
| Past Year | 28 | 33.73 | 22 | 37.93 |
| Longer than a Year ago | 39 | 46.99 | 24 | 41.38 |
| Don’t Know | 1 | 1.20 | 1 | 1.72 |
| Blunt Force Head Trauma Exposure Prior to First Trying Tobacco (n, %) | ||||
| No | 24 | 30.38 | 17 | 31.48 |
| Yes | 55 | 69.62 | 37 | 68.52 |
| Blunt Force Head Trauma Exposure Prior to First Regularly Using Tobacco (n, %) | ||||
| No | 17 | 21.52 | 12 | 22.22 |
| Yes | 62 | 78.48 | 42 | 77.78 |
| Type of Intentional Exposure (n, %) | ||||
| None | 23 | 24.47 | 4 | 6.90 |
| Brain Oxygen Deprivation Exposure Only | 2 | 2.13 | 2 | 3.45 |
| Blunt Force Trauma Exposure Only | 23 | 24.47 | 8 | 13.79 |
| Both Exposures | 46 | 48.94 | 44 | 75.86 |
| Exposure That Can Lead to Brain Injury Prior to First Trying Tobacco (n, %) | ||||
| Both Exposures | 16 | 18.82 | 14 | 24.14 |
| Brain Oxygen Deprivation Exposure Only | 4 | 4.71 | 4 | 6.90 |
| Blunt Force Trauma Exposure Only | 41 | 48.24 | 23 | 39.66 |
| Neither | 24 | 28.24 | 17 | 29.31 |
| Exposure That Can Lead to Brain Injury Prior to First Regularly Using Tobacco (n, %) | ||||
| Both Exposures | 25 | 30.12 | 25 | 43.10 |
| Brain Oxygen Deprivation Exposure Only | 6 | 7.23 | 6 | 10.34 |
| Blunt Force Trauma Exposure Only | 37 | 44.58 | 17 | 29.31 |
| Neither | 15 | 18.07 | 10 | 17.24 |
| Participants with Brain Injury (n = 59) | ||
|---|---|---|
| Brain Injury Severity Assessment (BISA) Item (n, %) | ||
| Black out or lose consciousness (n, %) | ||
| Never | 23 | 38.98 |
| Once | 11 | 18.64 |
| A few times | 20 | 33.90 |
| Too many times to remember | 4 | 6.78 |
| Don’t Know | 1 | 1.69 |
| Feel dazed or confused or disoriented (n, %) | ||
| Never | 8 | 13.56 |
| Once | 20 | 33.90 |
| A few times | 25 | 42.37 |
| Too many times to remember | 6 | 10.17 |
| Have memory loss about what happened (n, %) | ||
| Never | 31 | 52.54 |
| Once | 9 | 15.25 |
| A few times | 14 | 23.73 |
| Too many times to remember | 5 | 8.47 |
| See stars or spots (n, %) | ||
| Never | 19 | 32.20 |
| Once | 11 | 18.64 |
| A few times | 23 | 38.98 |
| Too many times to remember | 6 | 10.17 |
| Feel dizzy (n, %) | ||
| Never | 9 | 15.25 |
| Once | 15 | 25.42 |
| A few times | 24 | 40.68 |
| Too many times to remember | 11 | 18.64 |
| Age Tried Tobacco | n | % | p-Value |
|---|---|---|---|
| Brain Oxygen Deprivation Exposure (n = 54) | 0.014 | ||
| Tried Tobacco Before Exposure | 36 | 66.67 | |
| Exposure Before Tried Tobacco | 18 | 33.33 | |
| Blunt Force Head Trauma Exposure (n = 54) | 0.007 | ||
| Tried Tobacco Before Exposure | 17 | 31.48 | |
| Exposure Before Tried Tobacco | 37 | 68.52 | |
| Brain Oxygen Deprivation or Blunt Force Head Trauma Exposure (n = 58) | 0.002 | ||
| Tried Tobacco Before Exposure | 17 | 29.31 | |
| Exposure Before Tried Tobacco | 41 | 70.67 | |
| Age First Regularly Used Tobacco | |||
| Brain Oxygen Deprivation Exposure (n = 54) | 0.276 | ||
| Use Tobacco Before Exposure | 23 | 42.59 | |
| Exposure Before Use Tobacco | 31 | 57.41 | |
| Blunt Force Head Trauma Exposure (n = 54) | <0.001 | ||
| Use Tobacco Before Exposure | 12 | 22.22 | |
| Exposure Before Use Tobacco | 42 | 77.78 | |
| Brain Oxygen Deprivation or Blunt Force Head Trauma Exposure (n = 58) | <0.001 | ||
| Use Tobacco Before Exposure | 10 | 17.24 | |
| Exposure Before Use Tobacco | 48 | 82.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemeth, J.M.; Glasser, A.M.; Hinton, A.; Macisco, J.M.; Wermert, A.; Smith, R.; Kemble, H.; Sasser, G. Brain Injury Is Prevalent and Precedes Tobacco Use among Youth and Young Adults Experiencing Homelessness. Int. J. Environ. Res. Public Health 2023, 20, 5169. https://doi.org/10.3390/ijerph20065169
Nemeth JM, Glasser AM, Hinton A, Macisco JM, Wermert A, Smith R, Kemble H, Sasser G. Brain Injury Is Prevalent and Precedes Tobacco Use among Youth and Young Adults Experiencing Homelessness. International Journal of Environmental Research and Public Health. 2023; 20(6):5169. https://doi.org/10.3390/ijerph20065169
Chicago/Turabian StyleNemeth, Julianna M., Allison M. Glasser, Alice Hinton, Joseph M. Macisco, Amy Wermert, Raya Smith, Hannah Kemble, and Georgia Sasser. 2023. "Brain Injury Is Prevalent and Precedes Tobacco Use among Youth and Young Adults Experiencing Homelessness" International Journal of Environmental Research and Public Health 20, no. 6: 5169. https://doi.org/10.3390/ijerph20065169
APA StyleNemeth, J. M., Glasser, A. M., Hinton, A., Macisco, J. M., Wermert, A., Smith, R., Kemble, H., & Sasser, G. (2023). Brain Injury Is Prevalent and Precedes Tobacco Use among Youth and Young Adults Experiencing Homelessness. International Journal of Environmental Research and Public Health, 20(6), 5169. https://doi.org/10.3390/ijerph20065169






