The Effects of High-Altitude Mountaineering on Cognitive Function in Mountaineers: A Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Statistical Analysis
3. Results
3.1. TMB
3.2. DSF
3.3. DSB
3.4. FTL
3.5. FTR
3.6. WMSV
3.7. AST-Ver
3.8. AST-Vis
4. Discussion
4.1. Executive Function
4.2. Motor Speed
4.3. Memory Function
4.4. Verbal Function
4.5. Limitations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crust, L.; Swann, C.; Allen-Collinson, J. The Thin Line: A Phenomenological Study of Mental Toughness and Decision Making in Elite High-Altitude Mountaineers. J. Sport Exerc. Psychol. 2016, 38, 598–611. [Google Scholar] [CrossRef] [PubMed]
- Paralikar, S.J.; Paralikar, J.H. High-altitude medicine. Indian J. Occup. Environ. Med. 2010, 14, 6–12. [Google Scholar] [CrossRef]
- Du, J.Y.; Li, X.Y.; Zhuang, Y.; Wu, X.Y.; Wang, T. Effects of Acute Mild and Moderate Hypoxia on Human Short Memory. Space Med. Med. Eng. 1999, 12, 270–273. [Google Scholar]
- Li, X.; Wu, X.; Han, L.; Wei, Y.; Wang, T. The effects of acute moderate hypoxia on humanerformance of attention span and atiention shift. J. Fourth Mil. Med. Univ. 1999, 20, 71–73. [Google Scholar]
- Pelamatti, G.; Pascotto, M.; Semenza, C. Verbal free recall in high altitude: Proper names vs common names. Cortex 2003, 39, 97–103. [Google Scholar] [CrossRef]
- Bonnon, M.; Noel, M.C.; Therme, P. Effects of different stay durations on attentional performance during two mountain expeditions. Aviat. Space Environ. Med. 2000, 71, 678–684. [Google Scholar] [PubMed]
- Leifflen, D.; Poquin, D.; Savourey, G.; Barraud, P.A.; Raphel, C.; Bittel, J. Cognitive performance during short acclimation to severe hypoxia. Aviat. Space Environ. Med. 1997, 68, 993–997. [Google Scholar]
- Wang, J.; Ke, T.; Zhang, X.; Chen, Y.; Liu, M.; Chen, J.; Luo, W. Effects of acetazolamide on cognitive performance during high-altitude exposure. Neurotoxicol. Teratol. 2013, 35, 28. [Google Scholar] [CrossRef] [PubMed]
- De, A.L.V.; Antunes, H.K.; dos Santos, R.V.; Lira, F.S.; Tufik, S.; de Mello, M.T. High altitude exposure impairs sleep patterns, mood, and cognitive functions. Psychophysiology 2012, 49, 1298–1306. [Google Scholar] [CrossRef]
- Grocott, M.P.; Martin, D.S.; Wilson, M.H.; Mitchell, K.; Dhillon, S.; Mythen, M.G.; Montgomery, H.E.; Levett, D.Z. Caudwell xtreme everest expedition. High Alt. Med. Biol. 2010, 11, 133–137. [Google Scholar] [CrossRef]
- West, J.B. American medical research expedition to Everest. High Alt. Med. Biol. 2010, 11, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Hornbein, T.F.; Townes, B.D.; Schoene, R.B.; Sutton, J.R.; Houston, C.S. The cost to the central nervous system of climbing to extremely high altitude. N. Engl. J. Med. 1989, 321, 1714–1719. [Google Scholar] [CrossRef]
- Clark, C.F.; Heaton, R.K.; Wiens, A.N. Neuropsychological functioning after prolonged high altitude exposure in mountaineering. Aviat. Space Environ. Med. 1983, 54, 202–207. [Google Scholar]
- Jason, G.W.; Pajurkova, E.M.; Lee, R.G. High-altitude mountaineering and brain function: Neuropsychological testing of members of a mount everest expedition. Aviat. Space Environ. Med. 1989, 60, 170–173. [Google Scholar] [PubMed]
- Wu, Z.A.; Chen, N.R. Behavioral psychology test and analysis of hypoxic environmental stress. Chin. J. Behav. Med. Brain Sci. 1994, 3, 66–68. [Google Scholar]
- Lieberman, P.; Protopapas, A.; Kanki, B.G. Speech production and cognitive deficits on Mt. Everest. Aviat. Space Environ. Med. 1995, 66, 857–864. [Google Scholar]
- Petiet, C.A.; Townes, B.D.; Brooks, R.J.; Kramer, J.H. Neurobehavioral and psychosocial functioning of women exposed to high altitude in mountaineering. Arch. Clin. Neuropsychol. 1989, 4, 443–452. [Google Scholar] [CrossRef]
- Harris, G.A.; Cleland, J.; Collie, A.; Mccrory, P. Cognitive assessment of a trekking expedition to 5100 m: A comparison of computerized and written testing methods. Wilderness Environ. Med. 2009, 20, 261–268. [Google Scholar] [CrossRef]
- Malle, C.; Ginon, B.; Bourrilhon, C. Brief working memory and physiological monitoring during a high-altitude expedition. High Alt. Med. Biol. 2016, 17, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Hannah, R.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons, Ltd.: Oxford, UK, 2009; pp. 87–102. [Google Scholar]
- Cohen, J. The statistical power of abnormal-social psychological research: A review. J. Abnorm. Soc. Psychol. 1962, 65, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Hedges, L.V.; Olkin, I. Statistical Methods for Meta-Analysis; Academic Press: San Diego, CA, USA, 1986. [Google Scholar]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press, Inc.: New York, NY, USA, 2012. [Google Scholar]
- Di Paola, M.; Caltagirone, C.; Fadda, L.; Sabatini, U.; Serra, L.; Carlesimo, G.A. Hippocampal atrophy is the critical brain change in patients with hypoxic amnesia. Hippocampus 2008, 18, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Caine, D.; Watson, J.D. Neuropsychological and neuropathological sequelae of cerebral anoxia: A critical review. J. Int. Neuropsychol. Soc. 2000, 6, 86–99. [Google Scholar] [CrossRef]
- Malenka, R.C.; Nestler, E.J.; Hyman, S.E. Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin. In Molecular Neuropharmacology: A Foundation for Clinical Neuroscience, 2nd ed.; Sydor, A., Brown, R.Y., Eds.; McGraw-Hill Medical: New York, NY, USA, 2009; pp. 155–157. [Google Scholar]
- Arnett, J.A.; Labovitz, S.S. Effect of physical layout in performance of the trail making test. Psychol. Assess. 1995, 7, 220–221. [Google Scholar] [CrossRef]
- Chauhan, P.; Jethwa, K.; Rathawa, A.; Chauhan, G.; Mehra, S. The Anatomy of the Hippocampus. In Cerebral Ischemia; Pluta, R., Ed.; Exon Publications: Brisbane, Australia, 2021. [Google Scholar]
- Austin, D.; Jimison, H.; Hayes, T.; Mattek, N.; Kaye, J.; Pavel, M. Measuring motor speed through typing: A surrogate for the finger tapping test. Behav. Res. Methods 2011, 43, 903–909. [Google Scholar] [CrossRef]
- Gu, Y.Q. The Effect of the Altitude Acclimatization on Incidence of Acute Mountain Sickness, Mental State and Cognitive Function of the Soldiers Ascending Quickly to the High—Altitude. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2006. [Google Scholar]
- Sherwood, L. Human Physiology: From Cells to Systems; Cengage Learning: Boston, MA, USA, 2015; pp. 157–162. [Google Scholar]
- Hunsley, J.; Lee, C.M. Introduction to Clinical Psychology: An Evidence-Based Approach; John Wiley & Sons, Inc.: New York, NY, USA, 2010; print. [Google Scholar]
- Diekelmann, S.; Born, J. The memory function of sleep. Nat. Rev. Neurosci. 2010, 11, 114–127. [Google Scholar] [CrossRef]
- Bloch, K.E.; Buenzli, J.C.; Latshang, T.D.; Ulrich, S. Sleep at high altitude: Guesses and facts. J. Appl. Physiol. 2015, 119, 1466–1480. [Google Scholar] [CrossRef]
- Pagani, M.; Ravagnan, G.; Salmaso, D. Effect of acclimatisation to altitude on learning. Cortex 1998, 34, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, P. The cognitive functions of language. Behav. Brain Sci. 2002, 25, 674–725. [Google Scholar] [CrossRef]
- Holcomb, M.J.; Dean, R.S. Aphasia Screening Test; Springer US: New York, NY, USA, 2011; pp. 133–134. [Google Scholar]
- Baidu Baike. Available online: https://baike.baidu.com/item/%E5%A4%B1%E8%AF%AD%E7%97%87/2603042?fr=aladdin (accessed on 18 November 2022).
| Domains | Examinations |
|---|---|
| Executive function | Trail-Making Test Part B (TMB) |
| Motor speed | Finger Tapping Test-Left (FTL) Finger Tapping Test-Right (FTR) |
| Memory function | Digit Span Test Forward (DSF) Digit Span Test Backward (DSB) Wechsler Memory Scale Visual (WMSV) |
| Verbal function | Aphasia Screening Test-Visual Motor Errors (AST-Vis) Aphasia Screening Test-Verbal Items (AST-Ver) |
| Studies | Type of Study | Date of Issue | Country | Age | Group Size | above Sea Level (m) | Test Period | Used Supplemental Oxygen | Indicators |
|---|---|---|---|---|---|---|---|---|---|
| Hornbein | Between Subject Design | 1989 | USA and Canada | 24–45 | 41 | 5488 | 40 days | None | FTL, FTR, WMSV, AST-Vis, AST-Ver |
| Charles | Within-Subject Design | 1983 | USA | 31.1 ± 8.5 | 22 | 8848 | 20 weeks | Above 7315 m | TMB, DSF, AST-vis |
| Gregor | Within-Subject Design | 1989 | Canada | 26–49 | 9 | 8848 | 3–10 weeks | Above 7500 m | FTL, FTR |
| YouAn Wu | Within-Subject Design | 1994 | China | 18.7 | 58 | 3680 | 8–10 days | -- | DSF |
| Philip | Within-Subject Design | 1995 | USA | 35–52 | 5 | 8848 | 2 months | Above 8000 m | DSF, DSB |
| Carole | Within-Subject Design | 1988 | USA | 33.8 ± 3.8 | 8 | 7719 | 37 days | -- | TMB, FTL, FTR, DSF, WMSV, AST-Ver |
| Gregory | Within-Subject Design | 2009 | Australia | Male: 34.9, Female: 32.5 | 26 | 5100 | 18 days | -- | TMB, DSF, DSB |
| Carine | Within-Subject Design | 2016 | France | 29.2 ± 1.6 | 4 | 8043 | 6 weeks | None | DSF, DSB |
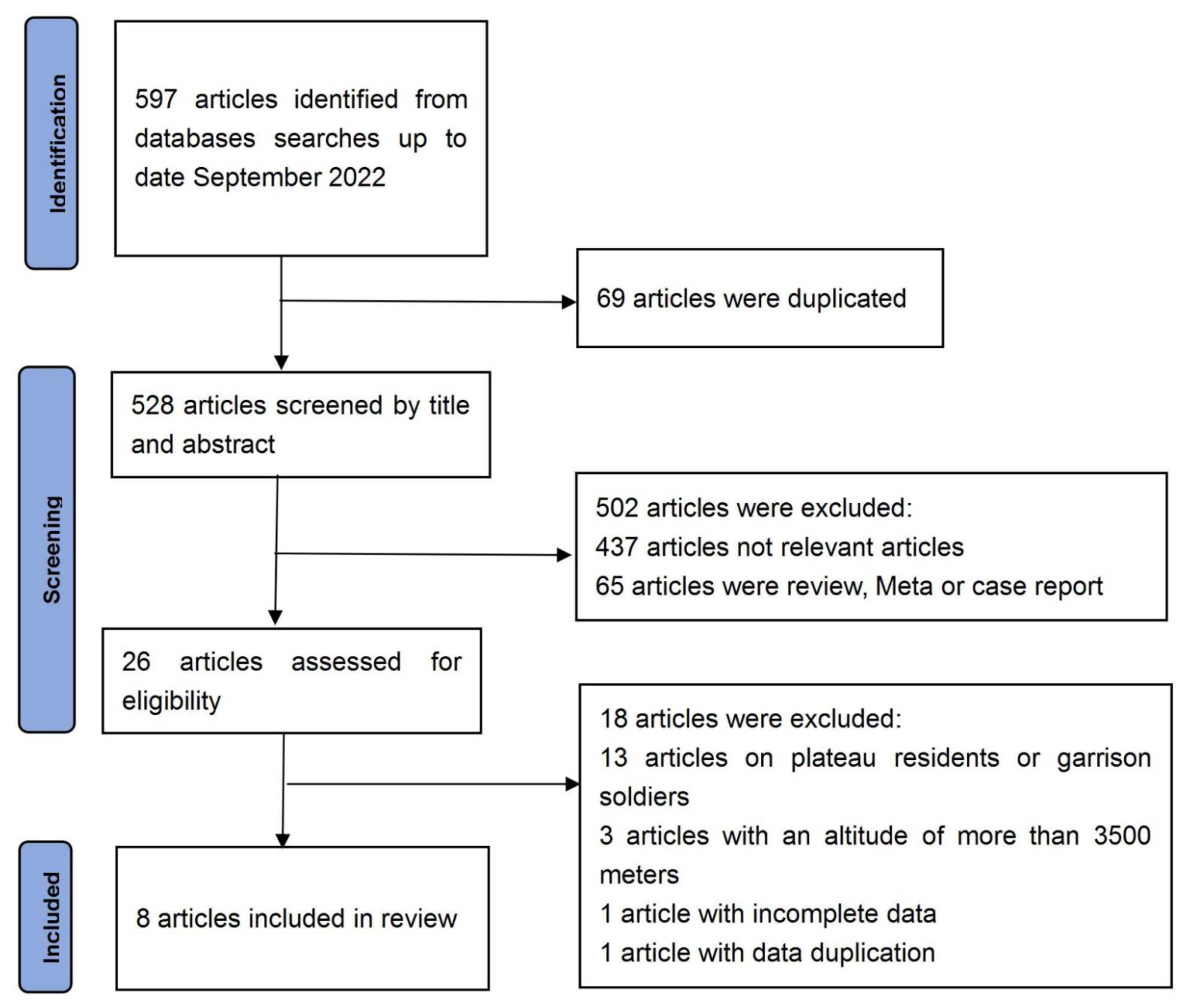
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Charles | 38.31% | 0.64 | 0.04 | 1.25 | 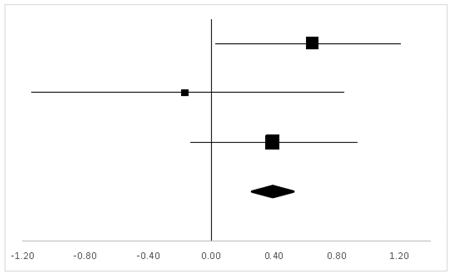 |
| Carole | 17.67% | −0.17 | −1.15 | 0.81 | |
| Gregory | 44.02% | 0.39 | −0.16 | 0.94 | |
| Total | 100% | 0.39 | −0.05 | 0.83 | Test for overall effect: Z = 1.72 (p > 0.05) |
| Heterogeneity: Q = 1.98, df = 2, C = 17.55, T2 = −0.001 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Charles | 22.2% | −0.19 | −0.79 | 0.40 | 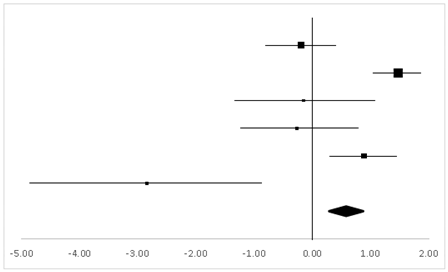 |
| Wu | 35.21% | 1.47 | 1.06 | 1.88 | |
| Philip | 6.52% | −0.15 | −1.39 | 1.09 | |
| Carole | 9.87% | −0.27 | −1.25 | 0.72 | |
| Gregory | 23.47% | 0.88 | 0.31 | 1.45 | |
| Carine | 2.74% | −2.85 | −4.81 | −0.88 | |
| Total | 100% | 0.57 | 0.24 | 0.90 | Test for overall effect: Z = 3.36 (p < 0.01) |
| Heterogeneity: Q = 42.10, df = 5, C = 40.30, T2 = 0.92 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Philip | 14.92% | −1.54 | −2.95 | −0.13 | 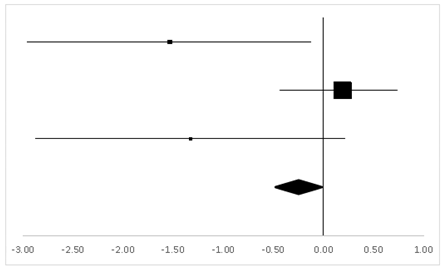 |
| Gregory | 72.28% | 0.19 | −0.36 | 0.73 | |
| Carine | 12.8% | −1.33 | −2.86 | 0.21 | |
| Total | 100% | −0.26 | −0.83 | 0.30 | Test for overall effect: Z = −0.91 (p > 0.05) |
| Heterogeneity: Q = 7.29, df = 2, C = 7.34, T2 = −0.72 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Gregor | 18.9% | 0.07 | −0.86 | 0.99 | 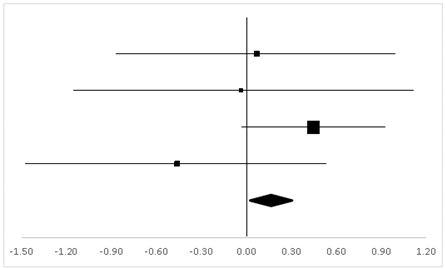 |
| Hornbein (Operation) | 13.24% | −0.04 | −1.17 | 1.09 | |
| Hornbein (Mountaineers) | 51.18% | 0.45 | −0.03 | 0.92 | |
| Carole | 16.69% | −0.46 | −1.46 | 0.53 | |
| Total | 100% | 0.16 | −0.27 | 0.59 | Test for overall effect: Z = 0.72 (p > 0.05) |
| Heterogeneity: Q = 3.07, df = 3, C = 18.39, T2 = 0.004 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Gregor | 19.4% | −0.26 | −1.19 | 0.67 | 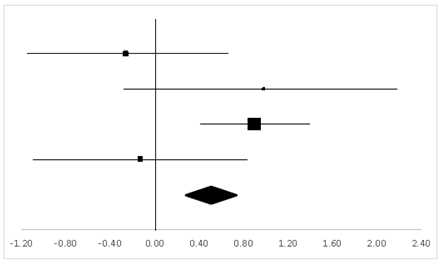 |
| Hornbein (Operation) | 12.35% | 0.98 | −0.22 | 2.18 | |
| Hornbein (Mountaineers) | 50.63% | 0.89 | 0.4 | 1.39 | |
| Carole | 17.62% | −0.13 | −1.11 | 0.85 | |
| Total | 100% | 0.50 | 0.06 | 0.94 | Test for overall effect: Z = −2.22 (p < 0.05) |
| Heterogeneity: Q = 7.38, df = 3, C = 17.73, T2 = −0.25 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Hornbein (Operation) | 14.51% | 1.18 | −0.04 | 2.41 |  |
| Hornbein (Mountaineers) | 64.69% | 0.51 | 0.03 | 0.98 | |
| Carole | 20.8% | 0.62 | −0.38 | 1.63 | |
| Total | 100% | 0.63 | 0.14 | 1.12 | Test for overall effect: Z = 2.52 (p < 0.05) |
| Heterogeneity: Q = 0.9, df = 2, C = 11.28, T2 = −0.1 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Hornbein (Operation) | 16.1% | −0.38 | −1.52 | 0.76 | 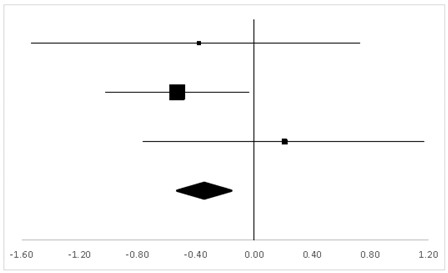 |
| Hornbein (Mountaineers) | 62.88% | −0.53 | −1.01 | −0.05 | |
| Carole | 21.01% | 0.21 | −0.77 | 1.19 | |
| Total | 100% | −0.35 | –0.83 | 0.13 | Test for overall effect: Z = −1.43 (p > 0.05) |
| Heterogeneity: Q = 1.84, df = 2, C = 12.07, T2 = −0.01 | |||||
| Effect Size | Forest Plot | ||||
|---|---|---|---|---|---|
| Studies | Weight | Random, 95% CI | Random, 95% CI | ||
| ES | Low | High | |||
| Charles | 37.22% | 0.27 | −0.33 | 0.86 | 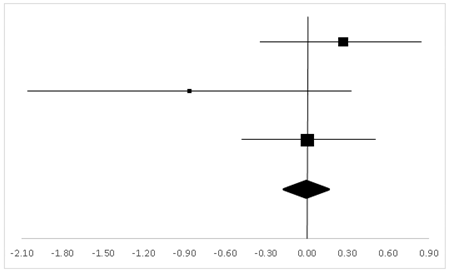 |
| Hornbein (Operation) | 11.94% | −0.87 | −2.05 | 0.32 | |
| Hornbein (Mountaineers) | 50.84% | 0 | −0.47 | 0.47 | |
| Total | 100% | 0 | −0.43 | 0.42 | Test for overall effect: Z = −0.02 (p > 0.05) |
| Heterogeneity: Q = 2.83, df = 2, C = 18.28, T2 = 0.05 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhou, Y.; Zou, S.; Wang, Y. The Effects of High-Altitude Mountaineering on Cognitive Function in Mountaineers: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 5101. https://doi.org/10.3390/ijerph20065101
Li L, Zhou Y, Zou S, Wang Y. The Effects of High-Altitude Mountaineering on Cognitive Function in Mountaineers: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(6):5101. https://doi.org/10.3390/ijerph20065101
Chicago/Turabian StyleLi, Lun, Yun Zhou, Shisi Zou, and Yongtai Wang. 2023. "The Effects of High-Altitude Mountaineering on Cognitive Function in Mountaineers: A Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 6: 5101. https://doi.org/10.3390/ijerph20065101
APA StyleLi, L., Zhou, Y., Zou, S., & Wang, Y. (2023). The Effects of High-Altitude Mountaineering on Cognitive Function in Mountaineers: A Meta-Analysis. International Journal of Environmental Research and Public Health, 20(6), 5101. https://doi.org/10.3390/ijerph20065101






