Effect of the N-hexanoyl-L-homoserine Lactone on the Carbon Fixation Capacity of the Algae–Bacteria System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Experimental Materials
2.3. Experimental Methods
2.3.1. Inoculation of Algae–Bacteria System
2.3.2. Detection of Growth Characteristics of Algae–Bacteria Systems
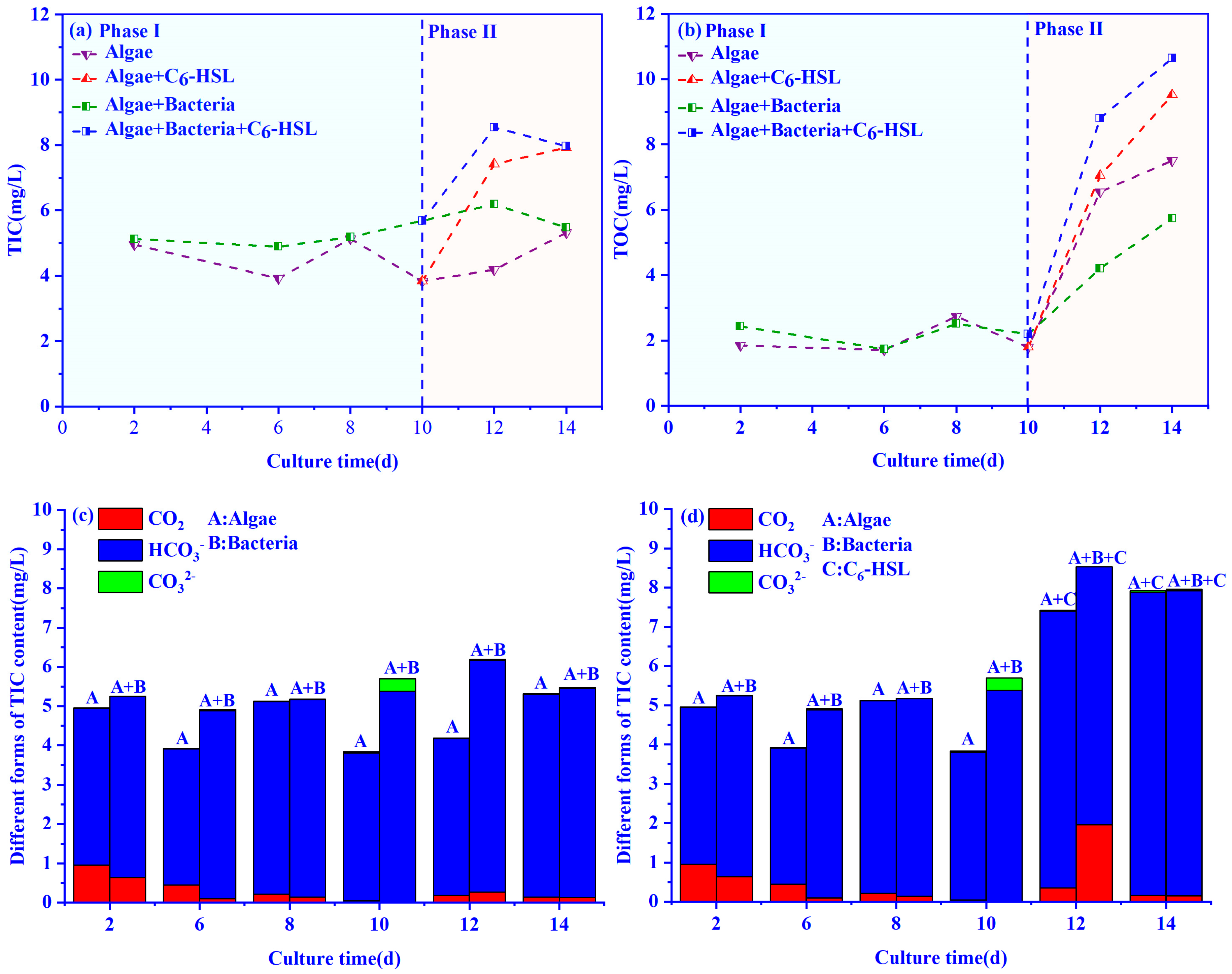
2.3.3. Carbon Concentration and Morphology Testing
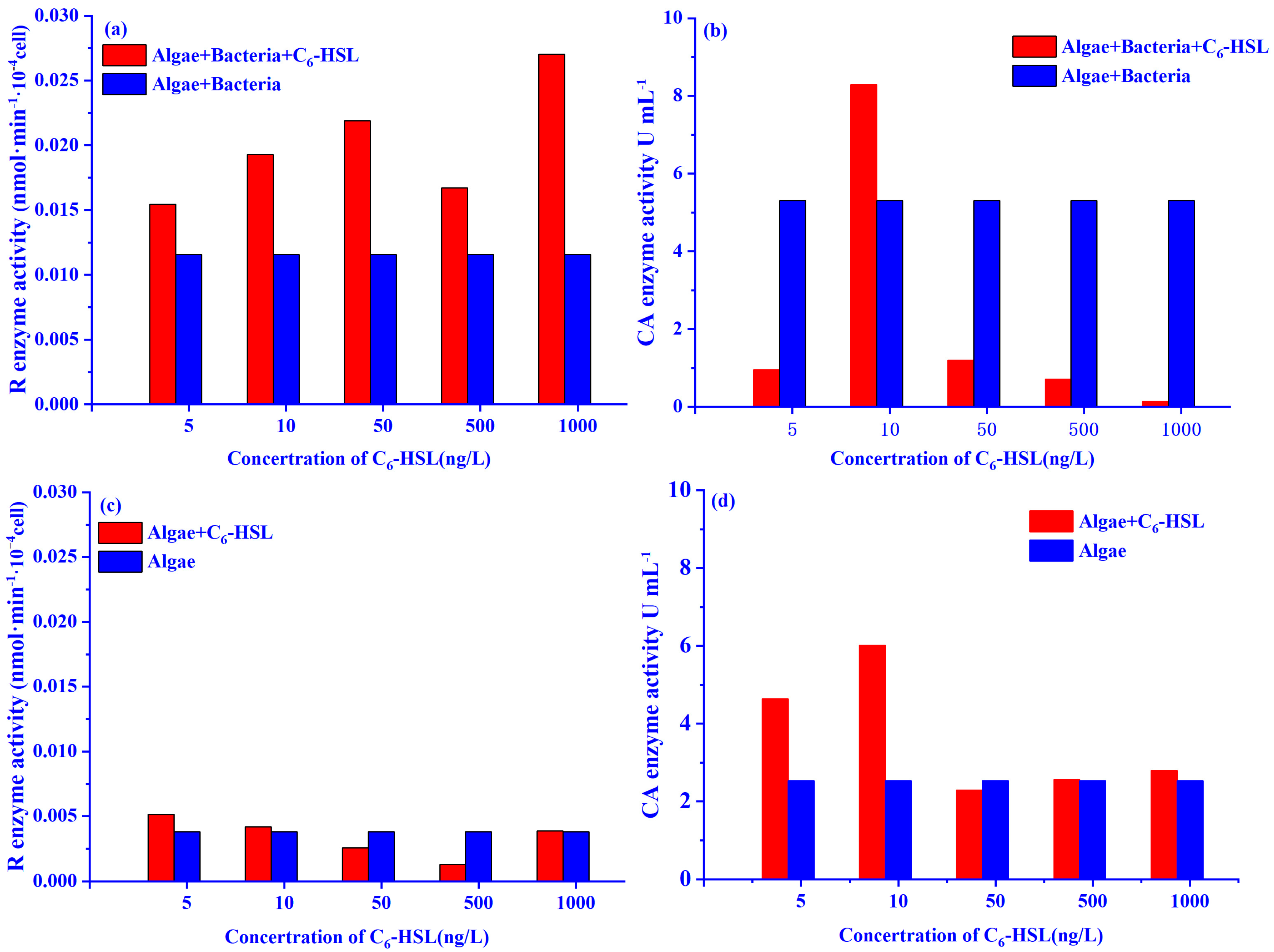
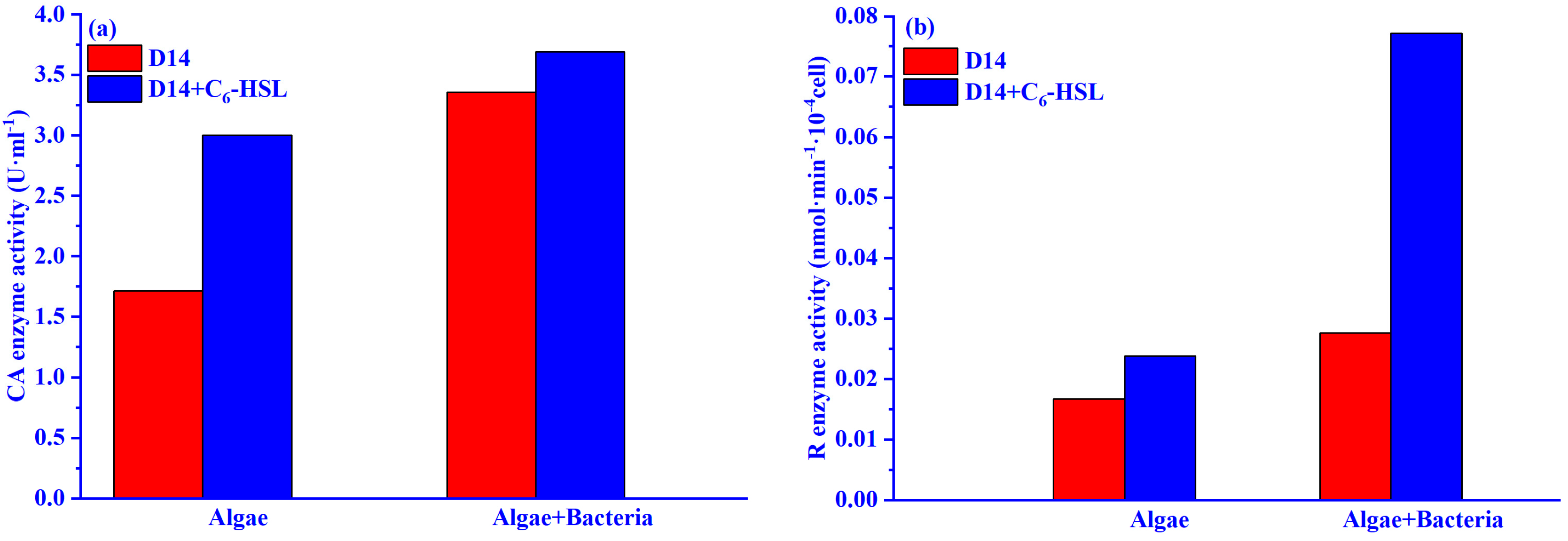
2.3.4. Carbonic Anhydrase and Rubisco Enzyme Activities
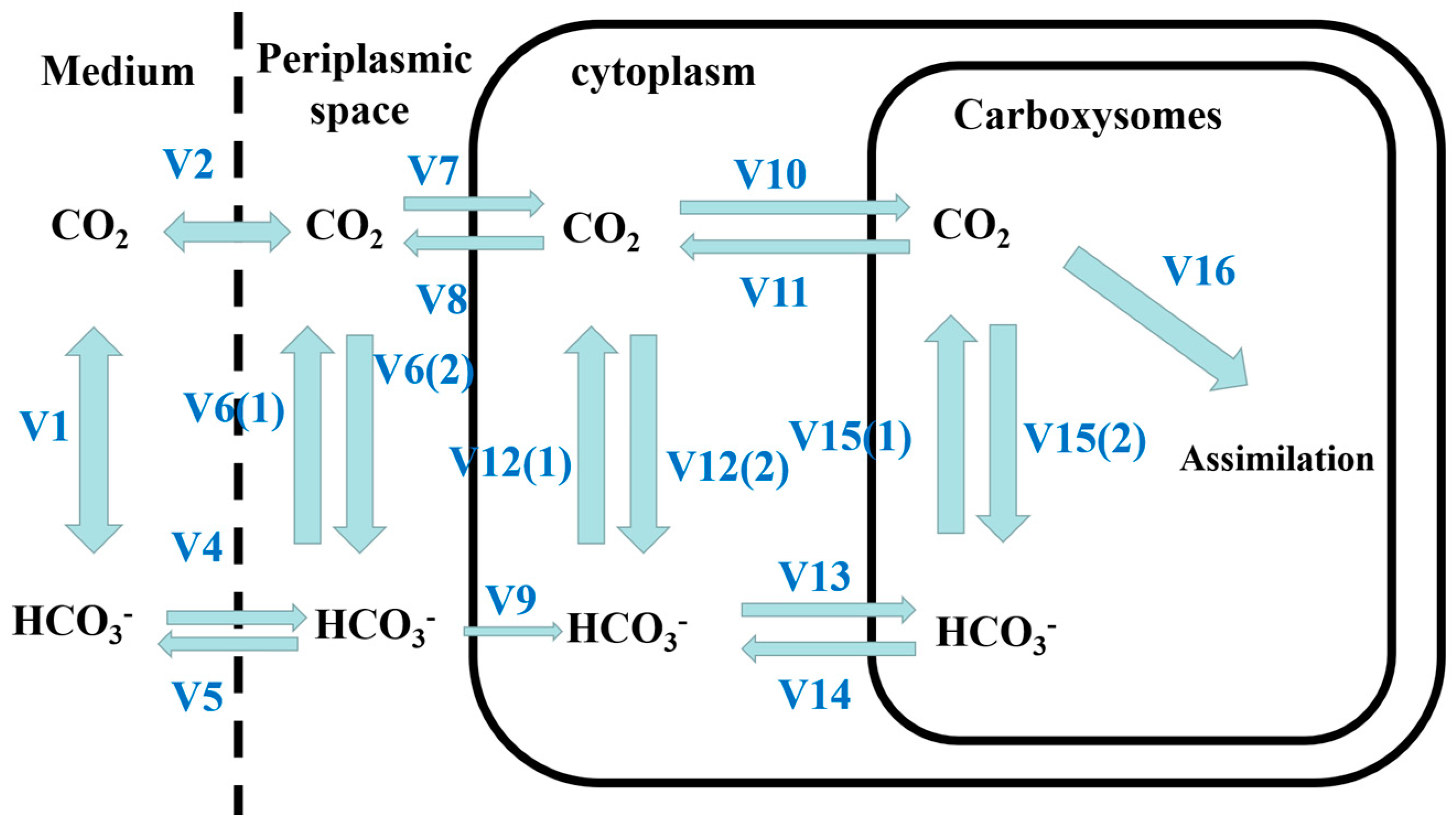
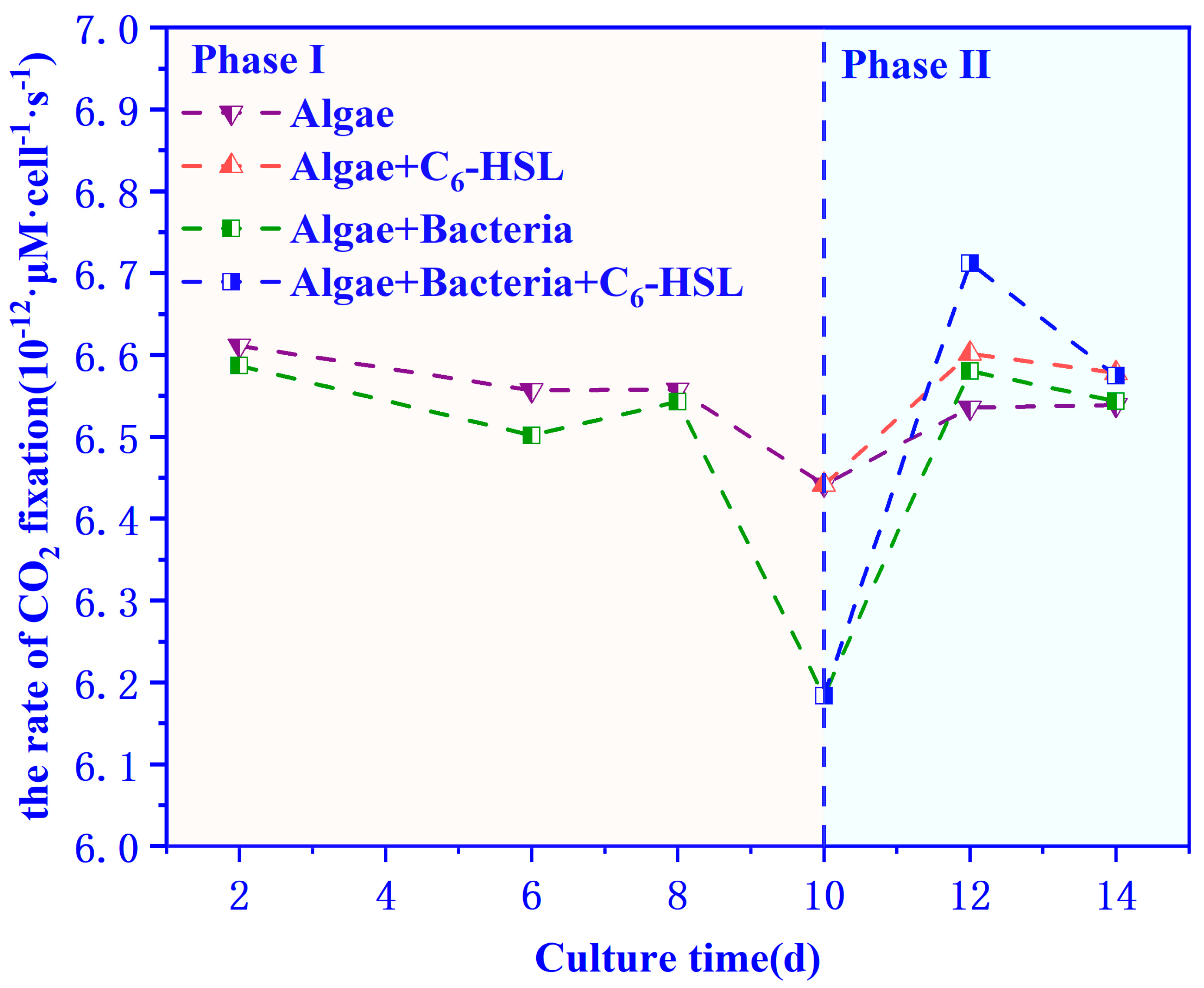
2.3.5. CCM Model
2.3.6. Statistical Analysis
3. Results
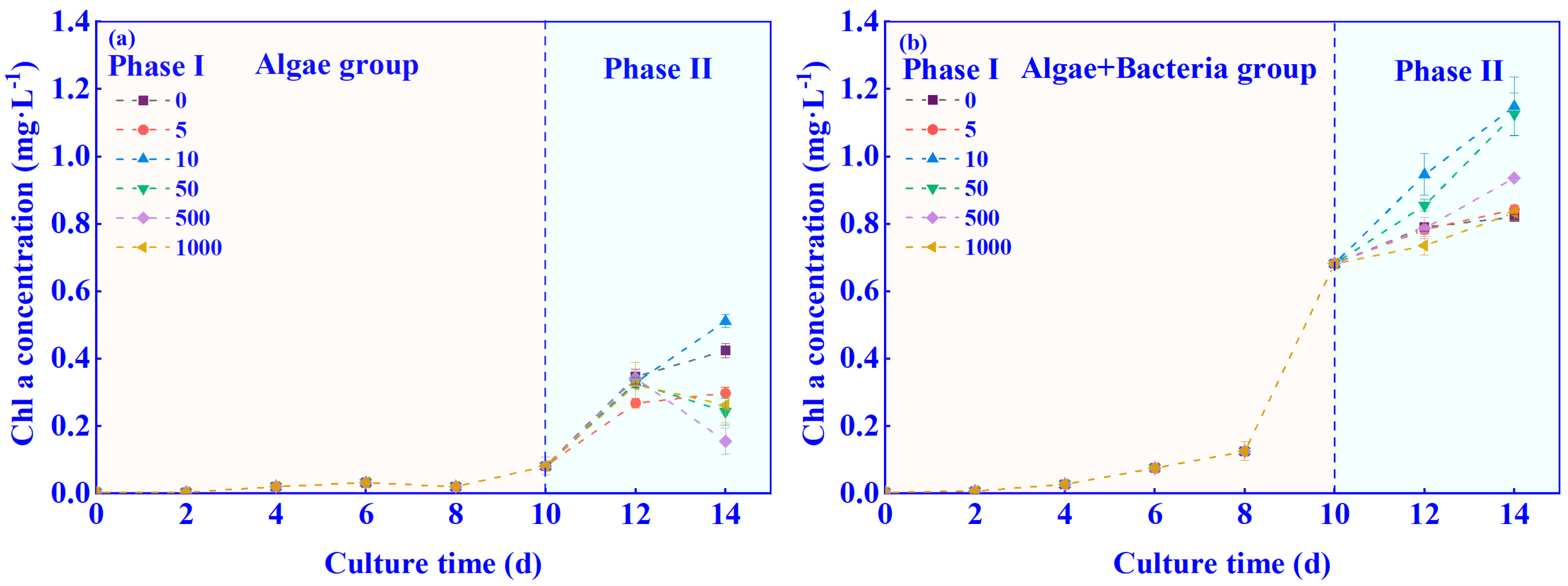
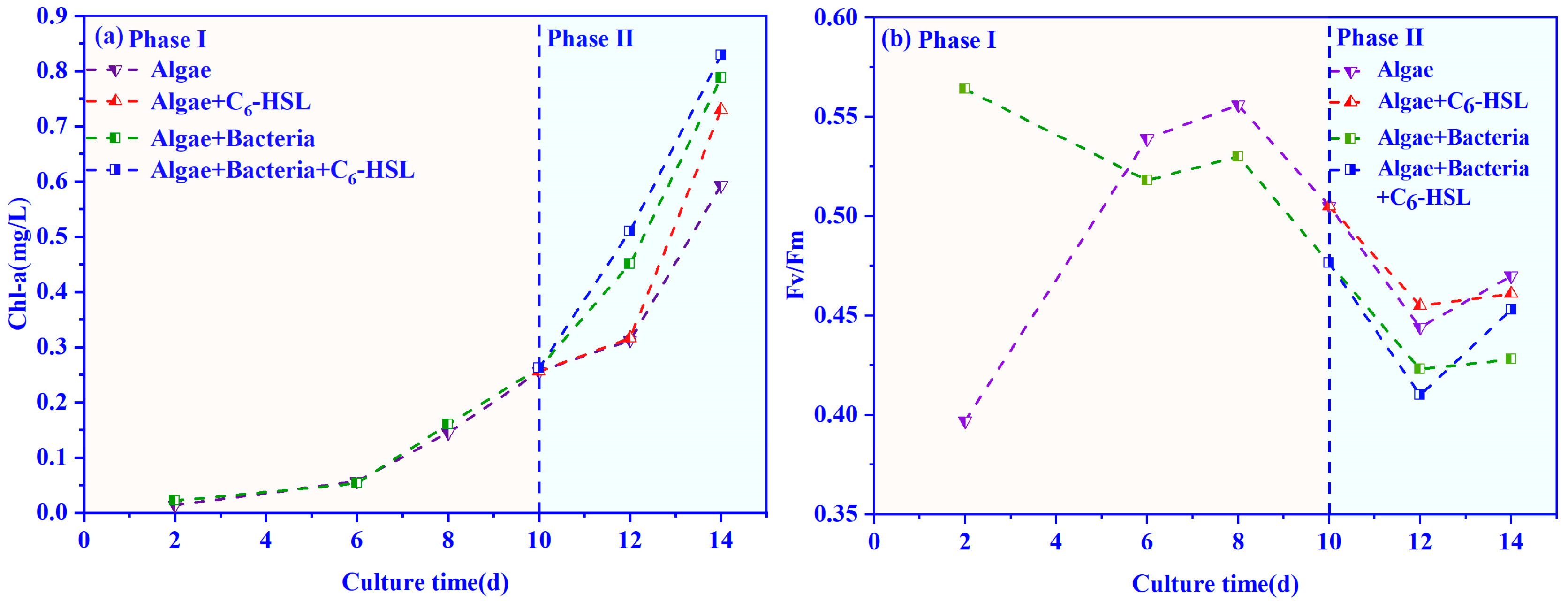
3.1. Effects of C6-HSL on the Growth of Algae–Bacteria System in Exp. I
3.2. Effects of C6-HSL on the Growth of Algae–Bacteria System in Exp. II
3.3. Changes in TIC and TOC Concentrations in the Algae–Bacteria System
3.4. The carbon Fixation Ability of Algae–Bacteria System Based on the CCM Model
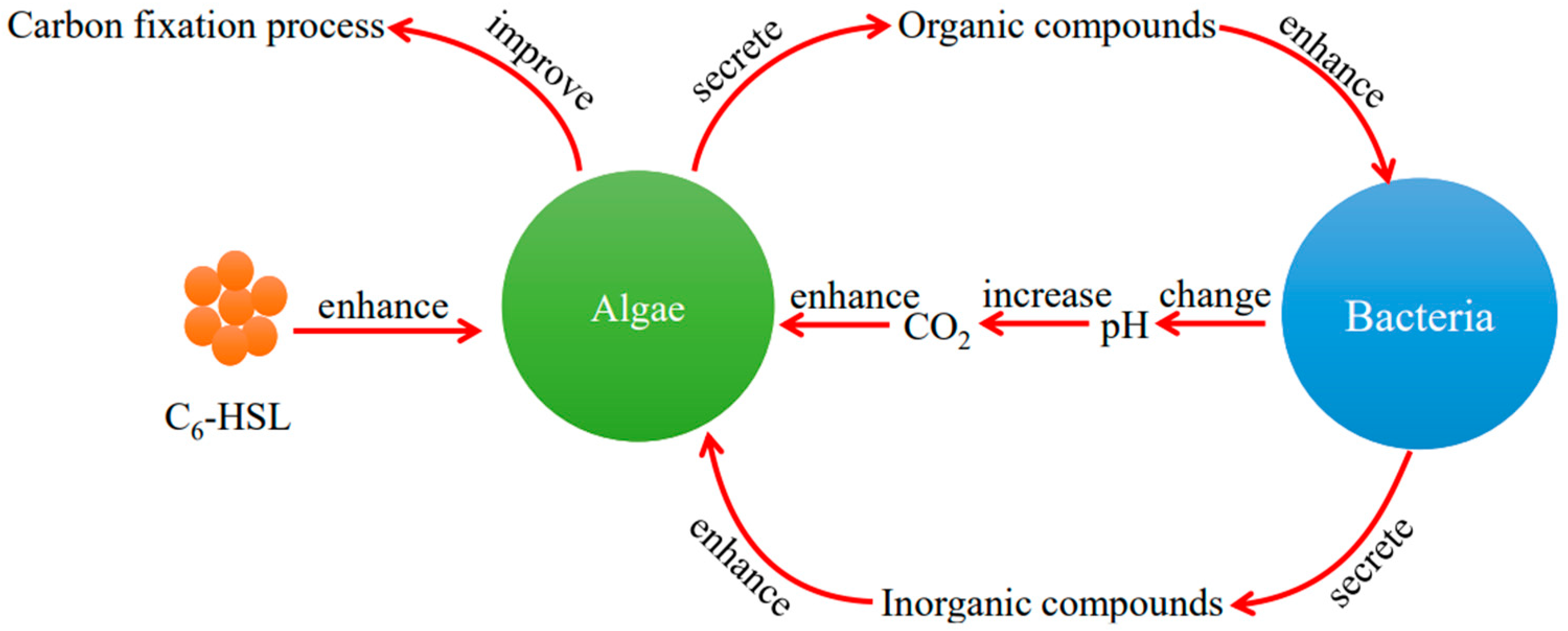
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | Define | Value |
|---|---|---|
| Number of carboxysomes per cell | 6 | |
| Radius of CO2 source zone (CA space) within carboxysomes | 0.01 μm | |
| Radius of carboxysomes | 0.2 μm | |
| Radius of the inner cytoplasmic region (up to thylakoid zone) | 1.73 μm | |
| Radius of inner+outer cytoplasmic regions (up to plasmalemma) | 1.77 μm | |
| Radius of cytoplasm + periplasmic space | 1.87 μm | |
| Radius of cell + unstirred layer | 3.54 μm | |
| Permeability coefficient of lipid bilayer membrane to CO2 | ||
| Coefficient for diffusion of CO2 in water | ||
| Coefficient for diffusion of HCO3− in water | ||
| Maximum rate of CO2 hydration per cell | ||
| Maximum rate of HCO3− dehydration | ||
| Km of CA for HCO3− | 1800 μM | |
| Km of CA for CO2 | 30,133 μM | |
| Overall rate constant for dehydration of HCO3− | 0.00263 | |
| Overall rate constant for hydration of CO2 | 0.0372 | |
| Maximum rate of CO2 conversion per cell | ||
| Apparent Km for CO2 conversion | 0.4 μM | |
| Maximum rate of CO2 fixation per cell | ||
| Apparent Km (CO2) for Rubisco | 250 μM | |
| Maximum rate of HCO3− transport per cell | ||
| Michaelis constants for transport in the inward directions | 80 μM | |
| Michaelis constants for transport in the outward directions | 200,000 μM | |
| General permeability coefficient through the six lipid membranes and the water channels in the thylakoid system | 5.83 |
References
- Montzka, S.A.; Dlugokencky, E.J.; Butler, J.H. Non-CO2 greenhouse gases and climate change. Nature 2011, 476, 43–50. [Google Scholar] [CrossRef]
- Hu, M.J.; Sardans, J.; Yang, X.Y.; Penuelas, J.; Tong, C. Patterns and environmental drivers of greenhouse gas fluxes in the coastal wetlands of China: A systematic review and synthesis. Environ. Res. 2020, 186, 109576. [Google Scholar] [CrossRef]
- Kumar, A.; Ergas, S.; Yuan, X.; Sahu, A.; Zhang, Q.O.; Dewulf, J.; Malcata, F.X.; van Langenhove, H. Enhanced CO2 fixation and biofuel production via microalgae: Recent developments and future directions. Trends Biotechnol. 2010, 28, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Kabir, F.; Gulfraz, M.; Raja, G.K.; Inam-Ul-Haq, M.; Awais, M.; Mustafa, M.S.; Khan, S.U.; Tlili, I.; Shadloo, M.S. Screening of native hyper-lipid producing microalgae strains for biomass and lipid production. Renew. Energy 2020, 160, 1295–1307. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Mathimani, T.; Rene, E.R.; Shanmugam, S.; Chi, N.T.L.; Pugazhendhi, A. Impact of cultivation conditions on the biomass and lipid in microalgae with an emphasis on biodiesel. Fuel 2021, 284, 119058. [Google Scholar] [CrossRef]
- Gao, X.Y.; Sun, T.; Pei, G.S.; Chen, L.; Zhang, W.W. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 2016, 100, 3401–3413. [Google Scholar] [CrossRef]
- Kouzuma, A.; Watanabe, K. Exploring the potential of algae/bacteria interactions. Curr. Opin. Biotech. 2015, 33, 125–129. [Google Scholar] [CrossRef]
- Takemura, A.E.; Chien, D.M.; Polz, M.E. Associations and dynamics of Vibrionaceae in the environment, from the genus to the population level. Front. Microbiol. 2014, 5, 38. [Google Scholar] [CrossRef]
- Ramanan, R.; Kim, B.H.; Cho, D.H.; Oh, H.M.; Kim, H.S. Algae-bacteria interactions: Evolution, ecology and emerging applications. Biotechnol. Adv. 2016, 34, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Amini, E.; Babaei, A.; Mehrnia, M.R.; Shayegan, J.; Safdari, M.S. Municipal wastewater treatment by semi -continuous and membrane algal-bacterial photo-bioreactors. J. Water Process. Eng. 2020, 36, 101274. [Google Scholar] [CrossRef]
- Wang, M.; Chen, S.B.; Zhou, W.G.; Yuan, W.Q.; Wang, D. Algal cell lysis by bacteria: A review and comparison to conventional methods. Algal Res. 2020, 46, 101794. [Google Scholar] [CrossRef]
- Xie, B.; Bishop, S.; Stessman, D.; Wright, D.; Spalding, M.H.; Halverson, L.J. Chlamydomonas reinhardtii thermal tolerance enhancement mediated by a mutualistic interaction with vitamin B12-producing bacteria. ISME J. 2013, 7, 1544–1555. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A. The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul. 2014, 73, 57–66. [Google Scholar] [CrossRef]
- Liu, J.Y.; Qiu, W.; Song, Y.M. Stimulatory effect of auxins on the growth and lipid productivity of Chlorella pyrenoidosa and Scenedesmus quadricauda. Algal Res. 2016, 18, 273–280. [Google Scholar] [CrossRef]
- Peng, H.X.; de-Bashan, L.E.; Higgins, B.T. Comparison of algae growth and symbiotic mechanisms in the presence of plant growth promoting bacteria and non-plant growth promoting bacteria. Algal Res. 2021, 53, 102156. [Google Scholar] [CrossRef]
- Huerlimann, R.; de Nys, R.; Heimann, K. Growth, Lipid Content, Productivity, and Fatty Acid Composition of Tropical Microalgae for Scale-Up Production. Biotechnol. Bioeng. 2010, 107, 245–257. [Google Scholar] [CrossRef]
- Fallahi, A.; Rezvani, F.; Asgharnejad, H.; Nazloo, E.K.; Hajinajaf, N.; Higgins, B. Interactions of microalgae-bacteria consortia for nutrient removal from wastewater: A review. Chemosphere 2021, 272, 129878. [Google Scholar] [CrossRef]
- Johnston, A.M.; Maberly, S.C.; Raven, J.A. The acquisition of inorganic carbon by four red macroalgae. Oecologia 1992, 92, 317–326. [Google Scholar] [CrossRef]
- Dobretsov, S.; Teplitski, M.; Paul, V. Mini-review: Quorum sensing in the marine environment and its relationship to biofouling. Biofouling 2009, 25, 413–427. [Google Scholar] [CrossRef]
- Willenbacher, J.; Mohr, T.; Henkel, M.; Gebhard, S.; Mascher, T.; Syldatk, C.; Hausmann, R. Substitution of the native srfA promoter by constitutive Pveg in two B. subtilis strains and evaluation of the effect on Surfactin production. J. Biotechnol. 2016, 224, 14–17. [Google Scholar] [CrossRef]
- Chen, X.; Schauder, S.; Potier, N.; Van Dorsselaer, A.; Pelczer, I.; Bassler, B.L.; Hughson, F.M. Structural identification of a bacterial quorum-sensing signal containing boron. Nature 2002, 415, 545–549. [Google Scholar] [CrossRef]
- Ren, T.T.; Yu, H.Q.; Li, X.Y. The quorum-sensing effect of aerobic granules on bacterial adhesion, biofilm formation, and sludge granulation. Appl. Microbiol. Biotechnol. 2010, 88, 789–797. [Google Scholar] [CrossRef]
- Withers, H.; Swift, S.; Williams, P. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Curr. Opin. Microbiol. 2001, 4, 186–193. [Google Scholar] [CrossRef]
- Takayama, Y.; Kato, N. In Vitro Analysis of Essential Binding Sites on the Promoter of the Serratia marcescens spn Operon With the Quorum-Sensing Receptor SpnR. Biotechnol. Bioeng. 2016, 113, 2513–2517. [Google Scholar] [CrossRef]
- Qixin, L.; Xuan, F.; Zhiya, S.; Wenxin, S.; Shuo, W.; Ji, L. Enhanced wastewater treatment performance by understanding the interaction between algae and bacteria based on quorum sensing. Bioresour. Technol. 2022, 354, 127161. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Lens, P.N.L.; Zhang, Z.Q.; Shi, W.X.; Cui, F.Y.; Tay, J.H. Effect of light intensity on the characteristics of algal-bacterial granular sludge and the role of N-acyl-homoserine lactone in the granulation. Sci. Total. Environ. 2019, 659, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, C.; Fu, L.; Xu, L.; Cui, X.; Li, Q.; Crittenden, J.C. Responses of the Microalga Chlorophyta sp. to Bacterial Quorum Sensing Molecules (N-Acylhomoserine Lactones): Aromatic Protein-Induced Self-Aggregation. Environ. Sci. Technol. 2017, 51, 3490–3498. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.F.; Li, Q.C.; Fu, L.; Zhou, D.D.; Crittenden, J.C. Quorum sensing molecules in activated sludge could trigger microalgae lipid synthesis. Bioresour. Technol. 2018, 263, 576–582. [Google Scholar] [CrossRef]
- Zhang, C.F.; Ho, S.H.; Li, A.R.; Fu, L.; Zhou, D.D. Co-culture of Chlorella and Scenedesmus could enhance total lipid production under bacteria quorum sensing molecule stress. J. Water Process. Eng. 2021, 39, 101739. [Google Scholar] [CrossRef]
- Canizares, R.O.; Dominguez, A.R.; Rivas, L.; Montes, M.C.; Travieso, L.; Benitez, F. Free and Immobilized Cultures of Spirulina-Maxima for Swine Waste Treatment. Biotechnol. Lett. 1993, 15, 321–326. [Google Scholar] [CrossRef]
- Beltran-Lopez, J.I.; Romero-Maldonado, A.; Monreal-Escalante, E.; Banuelos-Hernandez, B.; Paz-Maldonado, L.M.T.; Rosales-Mendoza, S. Chlamydomonas reinhardtii chloroplasts express an orally immunogenic protein targeting the p210 epitope implicated in atherosclerosis immunotherapies. Plant Cell Rep. 2016, 35, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.H.; Xu, H.; Luo, Y.C.; Wan, M.X.; Huang, J.K.; Wang, W.L.; Li, Y.G. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella. Appl. Microbiol. Biot. 2015, 99, 2451–2462. [Google Scholar] [CrossRef]
- Lines, T.; Beardall, J. Carbon Acquisition Characteristics of Six Microalgal Species Isolated from a Subtropical Reservoir: Potential Implications for Species Succession. J. Phycol. 2018, 54, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.L.; Pires, J.C.M.; Simoes, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Fridlyand, L.; Kaplan, A.; Reinhold, L. Quantitative evaluation of the role of a putative CO2-scavenging entity in the cyanobacterial CO2-concentrating mechanism. Biosystems 1996, 37, 229–238. [Google Scholar] [CrossRef]
- Purkayastha, J.; Bora, A.; Gogoi, H.K.; Singh, L. Growth of high oil yielding green alga Chlorella ellipsoidea in diverse autotrophic media, effect on its constituents. Algal Res. 2017, 21, 81–88. [Google Scholar] [CrossRef]
- Mohite, B. Isolation and characterization of indole acetic acid (IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Soil Sci. Plant Nutr. 2013, 13, 638–649. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The Solubilities of Calcite, Aragonite and Vaterite in CO2-H2O Solutions between 0-Degrees-C and 90-Degrees-C, and an Evaluation of the Aqueous Model for the System CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Anderson, N.G.; Wilbur, K.M. Electrometric and Colorimetric Determination of Carbonic Anhydrase. Anat. Rec. 1948, 101, 685. [Google Scholar]
- Tang, C.C.; Tian, Y.; He, Z.W.; Zuo, W.; Zhang, J. Performance and mechanism of a novel algal-bacterial symbiosis system based on sequencing batch suspended biofilm reactor treating domestic wastewater. Bioresour. Technol. 2018, 265, 422–431. [Google Scholar] [CrossRef]
- Fang, Y.K.; Hu, Z.; Zou, Y.N.; Zhang, J.; Zhu, Z.R.; Zhang, J.D.; Nie, L.C. Improving nitrogen utilization efficiency of aquaponics by introducing algalbacterial consortia. Bioresour. Technol. 2017, 245, 358–364. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef]
- Taga, M.E.; Bassler, B.L. Chemical communication among bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 14549–14554. [Google Scholar] [CrossRef] [PubMed]
- Young, E.B.; Beardall, J. Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle. J. Phycol. 2003, 39, 897–905. [Google Scholar] [CrossRef]
- Engel, A.; Schulz, K.G.; Riebesell, U.; Bellerby, R.; Delille, B.; Schartau, M. Effects of CO2 on particle size distribution and phytoplankton abundance during a mesocosm bloom experiment (PeECE II). Biogeosciences 2008, 5, 509–521. [Google Scholar] [CrossRef]
- Hu, H.H.; Gao, K.S. Impacts of CO2 enrichment on growth and photosynthesis in freshwater and marine diatoms. Chin. J. Oceanol. Limnol. 2008, 26, 407–414. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Figueroa, F.L.; Niell, F.X.J.P. Photon- and carbon-use efficiency in Ulva rigida at different CO2 and N levels. Planta 2003, 218, 315–322. [Google Scholar] [CrossRef]
- Rotatore, C.; Colman, B.; Kuzma, M. The Active Uptake of Carbon-Dioxide by the Marine Diatoms Phaeodactylum-Tricornutum and Cyclotella Sp. Plant Cell Environ. 1995, 18, 913–918. [Google Scholar] [CrossRef]
- Lade, H.; Paul, D.; Kweon, J.H. Quorum Quenching Mediated Approaches for Control of Membrane Biofouling. Int. J. Biol. Sci. 2014, 10, 547–562. [Google Scholar] [CrossRef]
- Shrout, J.D.; Nerenberg, R. Monitoring Bacterial Twitter: Does Quorum Sensing Determine the Behavior of Water and Wastewater Treatment Biofilms? Environ. Sci. Technol. 2012, 46, 1995–2005. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, L.; Chen, B.; Deng, K.; He, Q.; Lin, G.; Guo, J.; Yan, P. Effect of the N-hexanoyl-L-homoserine Lactone on the Carbon Fixation Capacity of the Algae–Bacteria System. Int. J. Environ. Res. Public Health 2023, 20, 5047. https://doi.org/10.3390/ijerph20065047
Liao L, Chen B, Deng K, He Q, Lin G, Guo J, Yan P. Effect of the N-hexanoyl-L-homoserine Lactone on the Carbon Fixation Capacity of the Algae–Bacteria System. International Journal of Environmental Research and Public Health. 2023; 20(6):5047. https://doi.org/10.3390/ijerph20065047
Chicago/Turabian StyleLiao, Lei, Bin Chen, Kaikai Deng, Qiang He, Guijiao Lin, Jinsong Guo, and Peng Yan. 2023. "Effect of the N-hexanoyl-L-homoserine Lactone on the Carbon Fixation Capacity of the Algae–Bacteria System" International Journal of Environmental Research and Public Health 20, no. 6: 5047. https://doi.org/10.3390/ijerph20065047
APA StyleLiao, L., Chen, B., Deng, K., He, Q., Lin, G., Guo, J., & Yan, P. (2023). Effect of the N-hexanoyl-L-homoserine Lactone on the Carbon Fixation Capacity of the Algae–Bacteria System. International Journal of Environmental Research and Public Health, 20(6), 5047. https://doi.org/10.3390/ijerph20065047








