An Ultrasound–Fenton Process for the Degradation of 2,4,6-Trinitrotoluene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Process
2.3. Analytical Methods
3. Results and Discussion
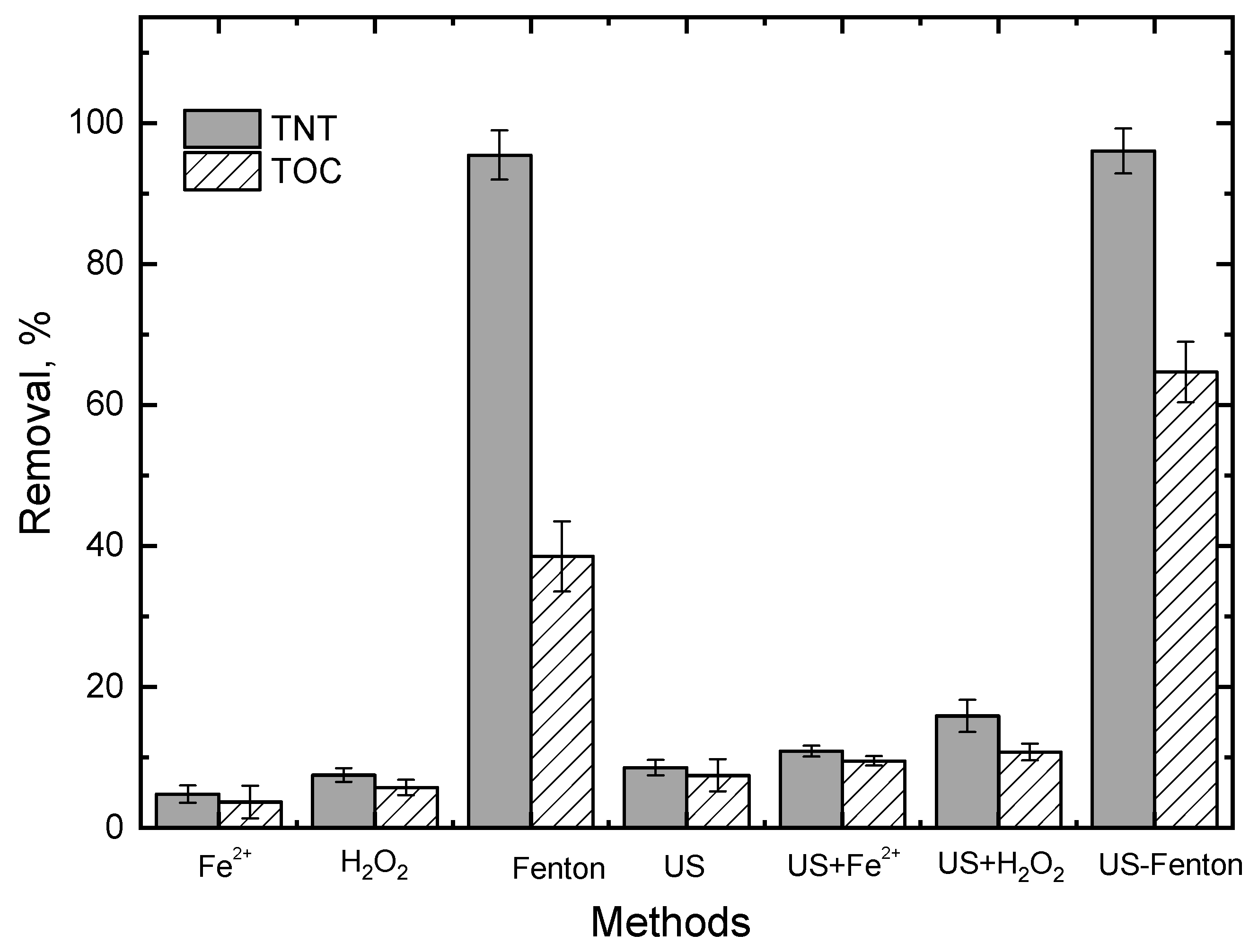
3.1. Screening of Various Treatment Processes

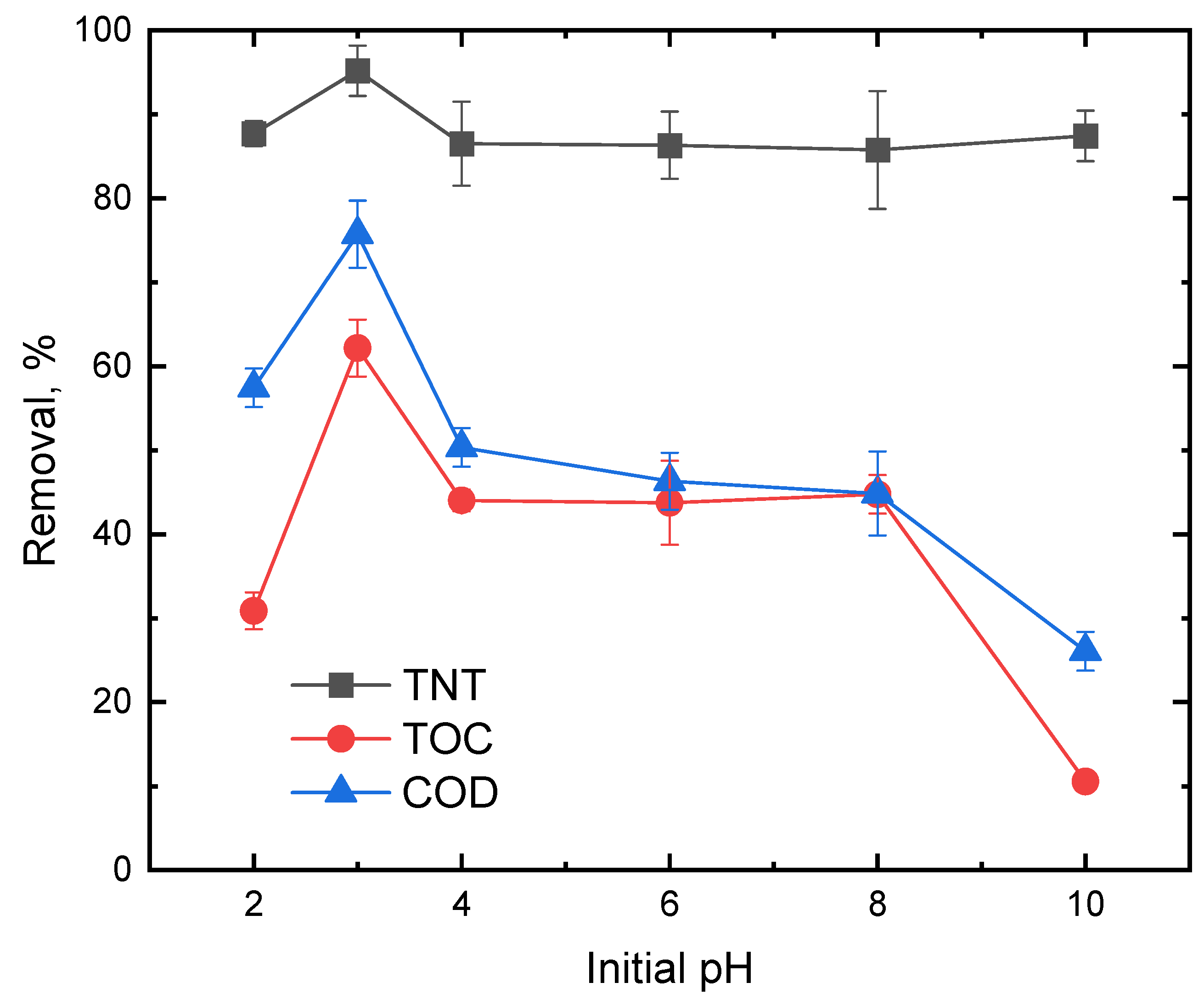
3.2. Effect of Initial pH on TNT Degradation
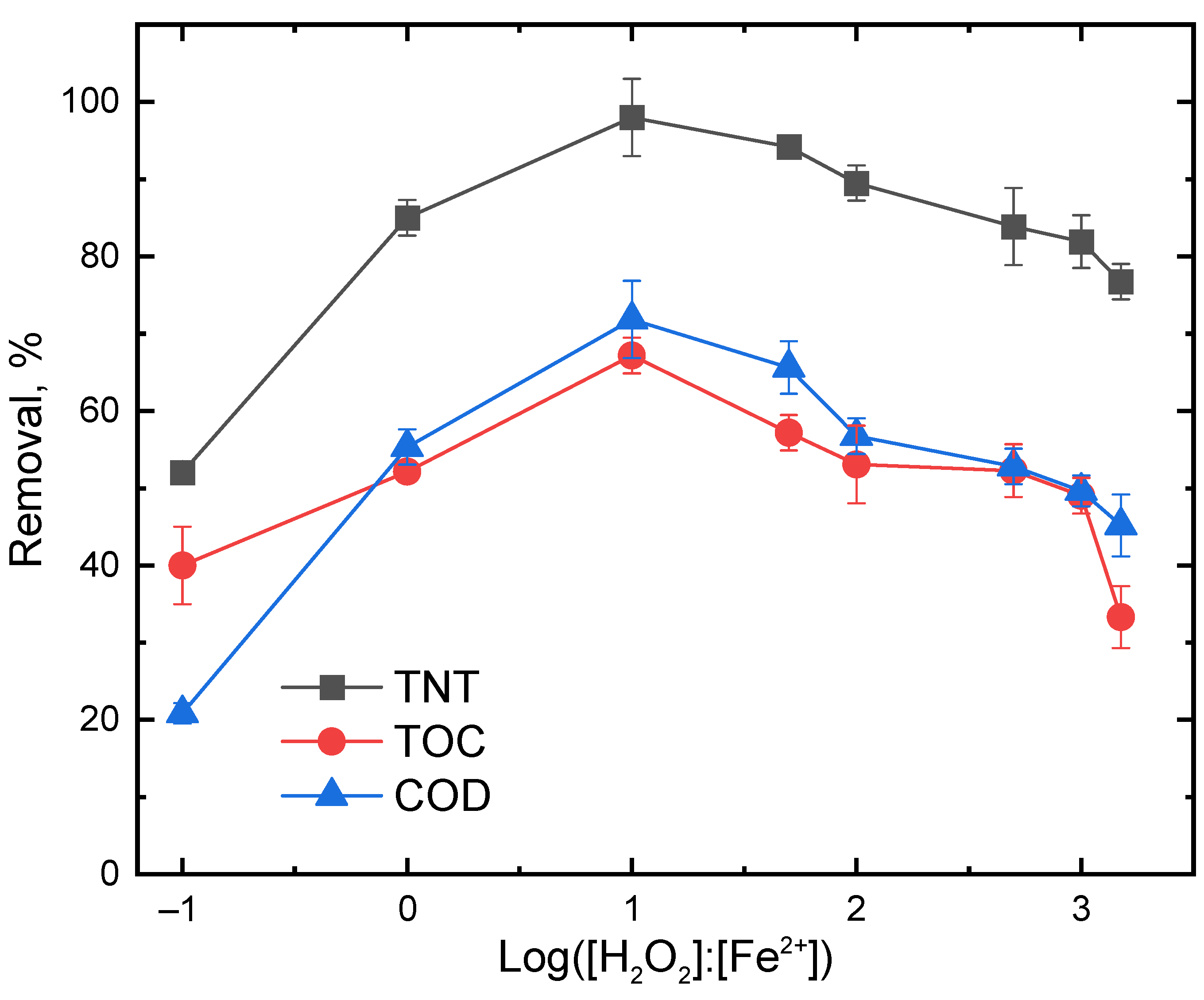
3.3. Effect of H2O2 to Fe2+ Molar Ratio
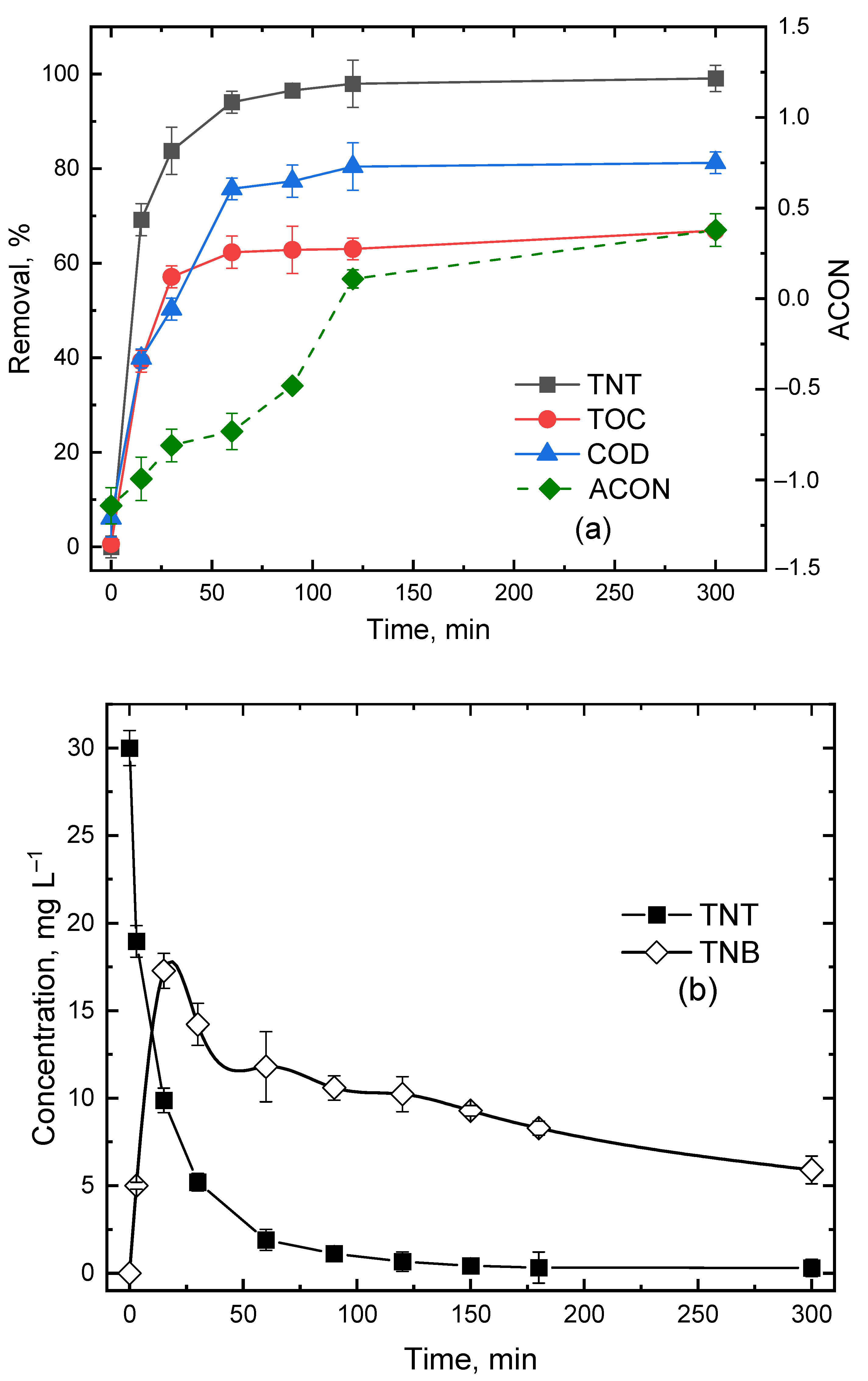
3.4. Effect of Reaction Time
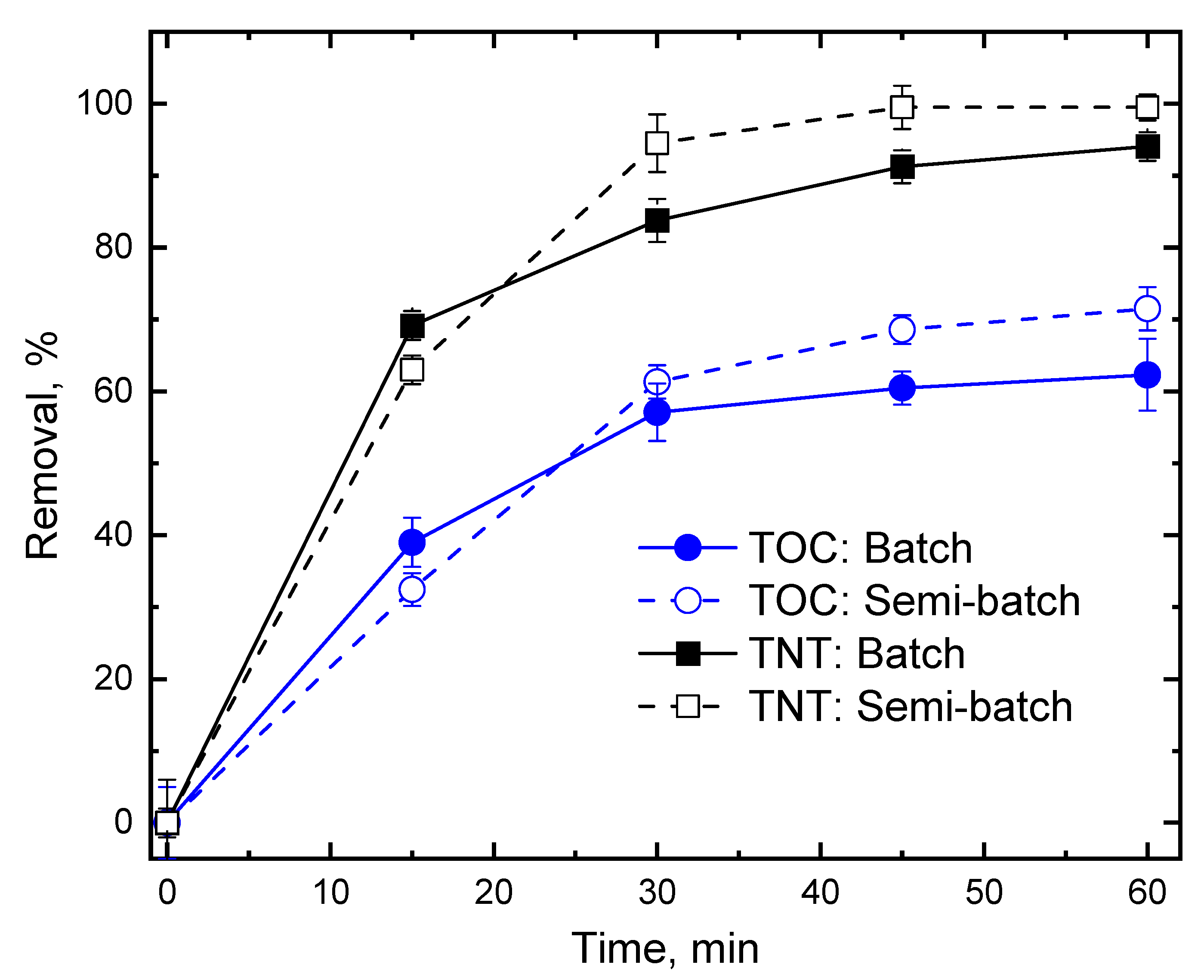
3.5. TNT Degradation in Semi-Batch Mode
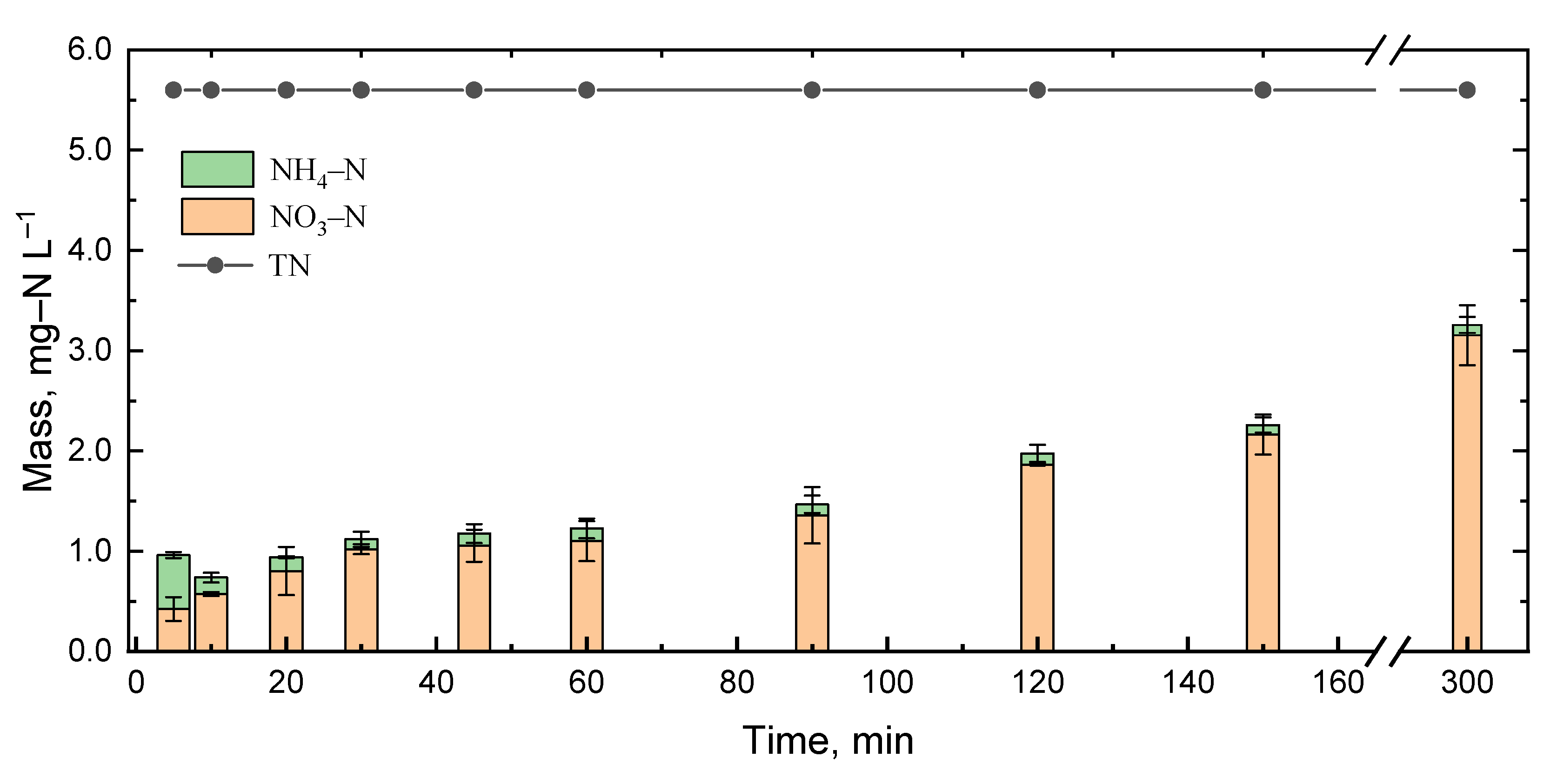
3.6. Nitrogen Mass Balance
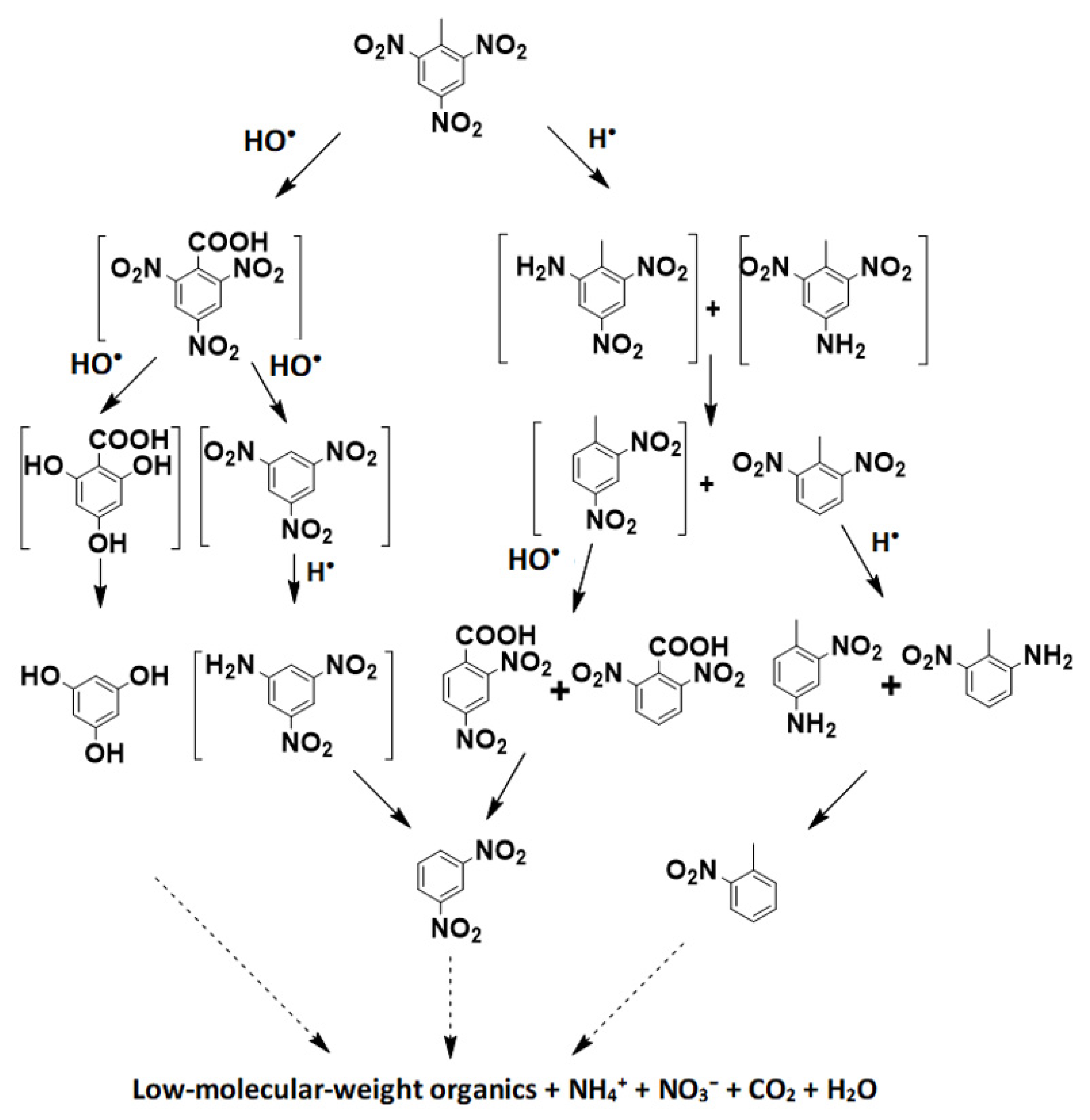
3.7. Degradation Pathways
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lachance, B.; Robidoux, P.Y.; Hawari, J.; Ampleman, G.; Thiboutot, S.; Sunahara, G.I. Cytotoxic and genotoxic effects of energetic compounds on bacterial and mammalian cells in vitro. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 1999, 444, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, J.D.; Bunce, N.J. Treatment methods for the remediation of nitroaromatic explosives. Water Res. 2001, 35, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for 2,4,6-Trinitrotoluene; ATSDR Toxicological Profile; EUA, Department of Health and Human Services: Atlanta, GA, USA, 1995.
- Alnaizy, R.; Akgerman, A. Oxidative treatment of high explosives contaminated wastewater. Water Res. 1999, 33, 2021–2030. [Google Scholar] [CrossRef]
- Kang, K.H.; Lim, D.M.; Shin, H. Oxidative-coupling reaction of TNT reduction products by manganese oxide. Water Res. 2006, 40, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Mercimek, H.A.; Dincer, S.; Guzeldag, G.; Ozsavli, A.; Matyar, F. Aerobic Biodegradation of 2,4,6-Trinitrotoluene (TNT) by Bacillus cereus A degrees solated from Contaminated Soil. Microb. Ecol. 2013, 66, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Vasilyeva, G.K.; Kreslavski, V.D.; Shea, P.J. Catalytic oxidation of TNT by activated carbon. Chemosphere 2002, 47, 311–317. [Google Scholar] [CrossRef]
- Ayoub, K.; Nelieu, S.; Hullebusch, E.D.; Maia-Grondard, A.; Cassir, M.; Bermond, A. TNT oxidation by Fenton reaction: Reagent ratio effect on kinetics and early stage degradation pathways. Chem. Eng. J. 2011, 173, 309–317. [Google Scholar] [CrossRef]
- Bruhn, C.; Lenke, H.; Knackmuss, H.J. Nitrosubstituted aromatic compounds as nitrogen source for bacteria. Appl. Environ. Microbiol. 1987, 53, 208–210. [Google Scholar] [CrossRef]
- Dulova, N.; Trapido, M.; Dulov, A. Catalytic degradation of picric acid by heterogeneous Fenton-based processes. Environ. Technol. 2011, 32, 439–446. [Google Scholar] [CrossRef]
- Gogate, P.R.; Mujumdar, S.; Pandit, A.B. Sonochemical reactors for waste water treatment: Comparison using formic acid degradation as a model reaction. Adv. Environ. Res. 2003, 7, 283–299. [Google Scholar] [CrossRef]
- Hamdaoui, O.; Naffrechoux, E. Sonochemical and photosonochemical degradation of 4-chlorophenol in aqueous media. Ultrason. Sonochem. 2008, 15, 981–987. [Google Scholar] [CrossRef]
- Kim, I.K.; Huang, C.P.; Chiu, P.C. Sonochemical decomposition of dibenzothiophene in aqueous solution. Water Res. 2001, 35, 4370–4378. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Lee, D.S.; Kang, N.; Yoon, J. Characteristics of p-chlorophenol oxidation by Fenton’s reagent. Water Res. 1999, 33, 2110–2118. [Google Scholar] [CrossRef]
- Li, Z.M.; Comfort, S.D.; Shea, P.J. Destruction of 2,4,6-trinitrotoluene by fenton oxidation. J. Environ. Qual. 1997, 26, 480–487. [Google Scholar] [CrossRef]
- Sun, H.H.; Sun, S.P.; Sun, J.Y.; Sun, R.X.; Qiao, L.P.; Guo, H.Q.; Fan, M.H. Degradation of azo dye Acid black 1 using low concentration iron of Fenton process facilitated by ultrasonic irradiation. Ultrason. Sonochem. 2007, 14, 761–766. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Effects of important reaction conditions on landfill leachate treatment by Fenton process. Fresenius Environ. Bull. 2006, 15, 43–47. [Google Scholar]
- Moholkar, V.S. Mechanistic optimization of a dual frequency sonochemical reactor. Chem. Eng. Sci. 2009, 64, 5255–5267. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T.; Krishna, C.M. Sonochemistry of volatile and nonvolatile solutes in aqueous-solutions-EPR and spin trapping studies. Ultrasonics 1990, 28, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Basturk, E.; Karatas, M. Advanced oxidation of Reactive Blue 181 solution: A comparison between Fenton and Sono-Fenton Process. Ultrason. Sonochem. 2014, 21, 1881–1885. [Google Scholar] [CrossRef]
- Grcic, I.; Obradovic, M.; Vujevic, D.; Koprivanac, N. Sono-Fenton oxidation of formic acid/formate ions in an aqueous solution: From an experimental design to the mechanistic modeling. Chem. Eng. J. 2010, 164, 196–207. [Google Scholar] [CrossRef]
- Segura, Y.; Martinez, F.; Melero, J.A.; Molina, R.; Chand, R.; Bremner, D.H. Enhancement of the advanced Fenton process (Fe-0/H2O2) by ultrasound for the mineralization of phenol. Appl. Catal. B 2012, 113, 100–106. [Google Scholar] [CrossRef]
- Li, Y.G.; Hsiehb, W.P.; Mahmudov, R.; Wei, X.M.; Huang, C.P. Combined ultrasound and Fenton (US-Fenton) process for the treatment of ammunition wastewater. J. Hazard. Mater. 2013, 244–245, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.S.; Juan, C.N.; Wei, K.M. Mineralization of dinitrotoluenes and trinitrotoluene of spent acid in toluene nitration process by Fenton oxidation. Chemosphere 2005, 60, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Yi, H.; Zhou, Z.J.; Zhuo, Q.F.; Bing, Y.X.; Guo, Q.W.; Xu, Z.C. Fenton Oxidation Kinetics and Intermediates of Nonylphenol Ethoxylates. Environ. Eng. Sci. 2014, 31, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dowd, K.; Pillai, S. Photo-Fenton disinfection at near neutral pH: Process, parameter optimization and recent advances. J. Environ. Chem. Eng. 2020, 8, 104063. [Google Scholar] [CrossRef]
- Nam, K.; Rodriguez, W.; Kukor, J.J. Enhanced degradation of polycyclic aromatic hydrocarbons by biodegradation combined with a modified Fenton reaction. Chemosphere 2001, 45, 11–20. [Google Scholar] [CrossRef]
- Zhang, H.; Choi, H.J.; Huang, C.P. Treatment of landfill leachate by Fenton’s reagent in a continuous stirred tank reactor. J. Hazard. Mater. 2006, 136, 618–623. [Google Scholar] [CrossRef]
- Lyman, W.J.; Reehl, W.F.; Rosenblatt, D.H. Handbook of Chemical Property Estimation Methods: Environmental Behavior of Organic Compounds; McGraw-Hill: New York, NY, USA, 1982. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry, 3rd ed.; John Willey & Sons: New York, NY, USA, 1996. [Google Scholar]
- Zhang, H.; Choi, H.J.; Huang, C.P. Optimization of Fenton process for the treatment of landfill leachate. J. Hazard. Mater. 2005, 125, 166–174. [Google Scholar] [CrossRef]
- Potter, F.J.; Roth, J.A. Oxidation of chlorinated phenols using Fenton reagent, Hazard. Waste Hazard. Mater. 1993, 10, 151–170. [Google Scholar] [CrossRef]
- Oh, S.Y.; Chiu, P.C.; Kim, B.J.; Cha, D.K. Enhancing Fenton oxidation of TNT and RDX through pretreatment with zero-valent iron. Water Res. 2003, 37, 4275–4283. [Google Scholar] [CrossRef]
- Ayoub, K.; Hullebusch, E.D.; Cassir, M.; Bermond, A. Application of advanced oxidation processes for TNT removal: A review. J. Hazard. Mater. 2010, 178, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Liou, M.J.; Lu, M.C.; Chen, J.N. Oxidation of explosives by Fenton and photo-Fenton processes. Water Res. 2003, 37, 3172–3179. [Google Scholar] [CrossRef] [PubMed]
- Schmelling, D.C.; Gray, K.A. Photocatalytic treansformation and mineralization of 2,4,6-trinitrotoluene (TNT) in TiO2 slurries. Water Res. 1995, 29, 2651–2662. [Google Scholar] [CrossRef]
- Doppalapudi, R.B.; Sorial, G.A.; Maloney, S.W. Electrochemical reduction of simulated munitions wastewater in a bench-scale batch reactor. Environ. Eng. Sci. 2002, 19, 115–130. [Google Scholar] [CrossRef]
- Chen, W.S.; Liang, J.S. Electrochemical destruction of dinitrotoluene isomers and 2,4,6-trinitrotoluene in spent acid from toluene nitration process. J. Hazard. Mater. 2009, 161, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Palaniswamy, D.K.; Sorial, G.A.; Maloney, S.W. Electrochemical reduction of 2,4,6-trinitrotoluene. Environ. Eng. Sci. 2004, 21, 203–218. [Google Scholar] [CrossRef]
- Schmelling, D.C.; Gray, K.A.; Kamat, P.V. Role of reduction in the photocatalytic degradation of TNT. Environ. Sci. Technol. 1996, 30, 2547–2555. [Google Scholar] [CrossRef]
- Huang, C.P.; Dong, C.; Tang, Z. Advanced chemical oxidation: Its present role and potential future in hazardous waste treatment. Waste Manag. 1993, 13, 361–377. [Google Scholar] [CrossRef]

| Group | [Fe2+]0 (mol/L) a | [H2O2]0 (mol/L) c | [H2O2]/[Fe2+] f | US Intensity (W/m3) | Initial pH | Reaction Time (min) |
|---|---|---|---|---|---|---|
| Screening of various treatment processes | ||||||
| I | 5 × 10−4 | – | – | – | 3.0 ± 0.1 | 60 |
| II | – | 5 × 10−3 | – | – | 3.0 ± 0.1 | 60 |
| III | 5 × 10−4 | 5 × 10−3 | 10 | – | 3.0 ± 0.1 | 60 |
| IV | – | – | – | 8571 | 3.0 ± 0.1 | 60 |
| V | 5 × 10−4 | – | – | 8571 | 3.0 ± 0.1 | 60 |
| VI | – | 5 × 10−3 | – | 8571 | 3.0 ± 0.1 | 60 |
| VII | 5 × 10−4 | 5 × 10−3 | 10 | 8571 | 3.0 ± 0.1 | 60 |
| Influencing factors of TNT degradation by US–Fenton | ||||||
| VIII | 5 × 10−4 | 5 × 10−3 | 10 | 8571 | 2.0–10.0 h | 60 |
| IX | 5 × 10−4 | 5 × 10−5–0.75 d | 0.1–1500 g | 8571 | 3.0 ± 0.1 | 60 |
| X | 5 × 10−4 | 5 × 10−3 | 10 | 8571 | 3.0 ± 0.1 | 15–300 i |
| XI | 5 × 10−4 | 5 × 10−3 | 10 | 8571 | 3.0 ± 0.1 | 60 |
| XII | 2.5 × 10−7–7.5 × 10−7 b | 2.5 × 10−6–7.5 × 10−6 e | 10 | 8571 | 3.0 ± 0.1 | 60 |
| Nitrogen balance and TNT degradation pathways | ||||||
| XIII | 5 × 10−4 | 5 × 10−3 | 10 | 8571 | 3.0 ± 0.1 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, W.; Mu, K.; Li, S.; Wang, J.; Zhang, S.; Wang, L. An Ultrasound–Fenton Process for the Degradation of 2,4,6-Trinitrotoluene. Int. J. Environ. Res. Public Health 2023, 20, 3102. https://doi.org/10.3390/ijerph20043102
Li Y, Zhang W, Mu K, Li S, Wang J, Zhang S, Wang L. An Ultrasound–Fenton Process for the Degradation of 2,4,6-Trinitrotoluene. International Journal of Environmental Research and Public Health. 2023; 20(4):3102. https://doi.org/10.3390/ijerph20043102
Chicago/Turabian StyleLi, Yangang, Wenzhen Zhang, Kelei Mu, Shangkun Li, Jiawei Wang, Shujun Zhang, and Lu Wang. 2023. "An Ultrasound–Fenton Process for the Degradation of 2,4,6-Trinitrotoluene" International Journal of Environmental Research and Public Health 20, no. 4: 3102. https://doi.org/10.3390/ijerph20043102
APA StyleLi, Y., Zhang, W., Mu, K., Li, S., Wang, J., Zhang, S., & Wang, L. (2023). An Ultrasound–Fenton Process for the Degradation of 2,4,6-Trinitrotoluene. International Journal of Environmental Research and Public Health, 20(4), 3102. https://doi.org/10.3390/ijerph20043102





