ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures
Abstract
1. Introduction
- (1)
- They require enrollment: Patient-specific deployment of discriminative model methods presupposes the existence of prior recordings of a patient’s seizures, as fitting classifier models requires samples from both inter-ictal and pre-ictal classes. This means that when designing a patient-specific system, a patient would have to enroll by undergoing a strenuous ECG recording session, in order to experience one or several seizures to personalize the device with specific pre-ictal heart rate parameters.
- (2)
- They are data hungry: Patient-agnostic deployment, on the other hand, would be dependent on the existence of large, difficult-to-obtain datasets. To train robust classifiers with the ability to generalize across different patient and seizure cases, a large amount of seizure recordings would need to be acquired and reliably labeled. This, in turn, gives rise to the necessity of conducting lengthy data acquisition sessions that require ethical clearance.
- (3)
- They are trained with unreliable labels: ANS disturbances occur at different time intervals for each epileptic seizure, making the procedure of reliably labeling ECG segments subjective to the human ability to distinguish the patterns of these disturbances.
- (4)
- They are potentially error-prone when presented with novel samples: There is no guarantee that novel ANS disturbances not included in a dataset will be detected using these methods. It is possible that in a two-class classifier that discriminates ECG segments as inter-ictal or pre-ictal, certain ECG abnormalities remain within the boundary of the wrong class when mapped onto the feature space. However, an efficient prediction system that is sensitive to all deviations from a pre-defined normal HRV pattern is not compromised in this scenario.
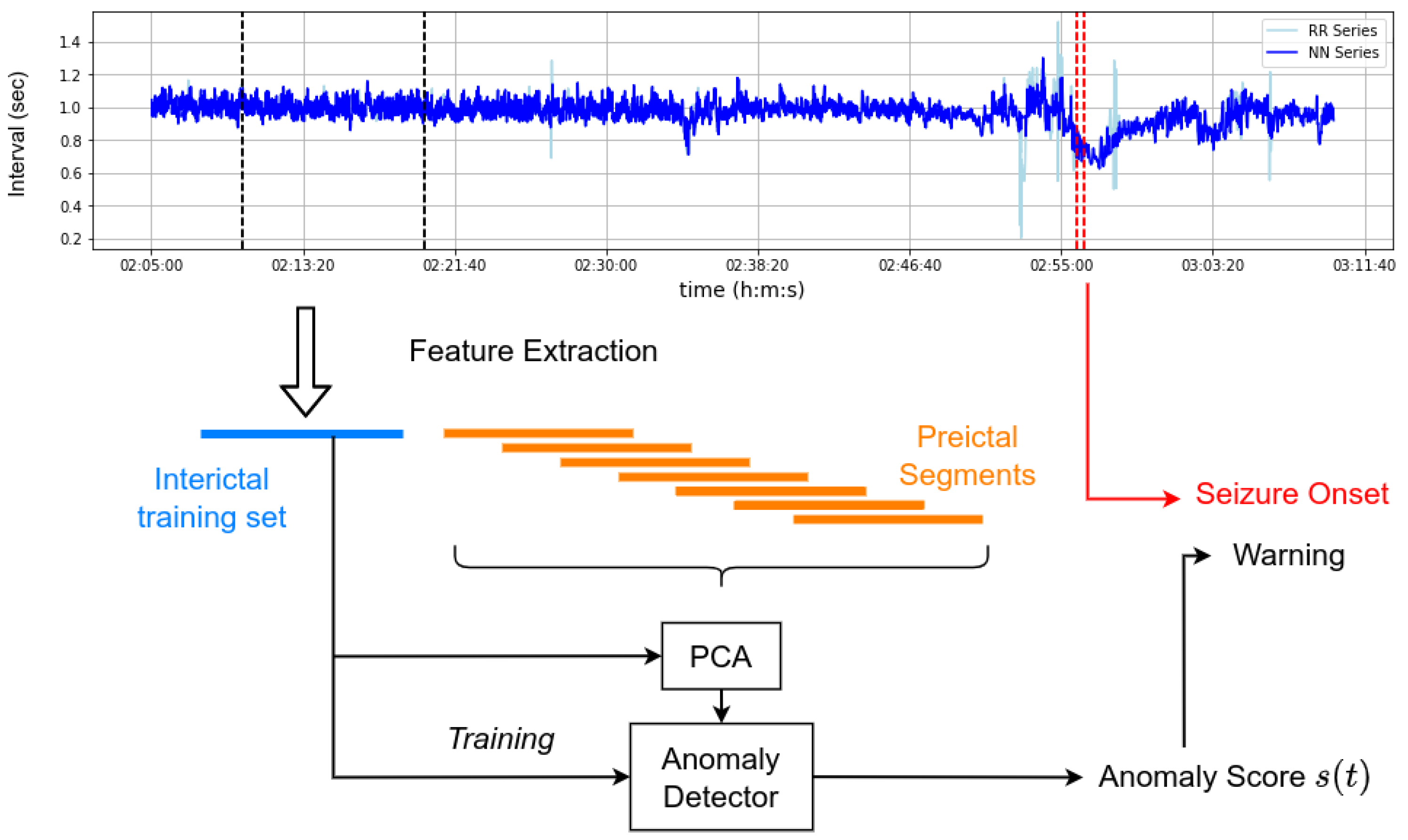
2. Methods and Recordings
2.1. Pre-Processing
2.2. Feature Extraction
2.3. Anomaly Detection
2.3.1. Minimum Covariance Determinant
2.3.2. One-Class Support Vector Machine
2.3.3. Local Outlier Factor
2.4. Novelty Scores
2.5. Available Recordings
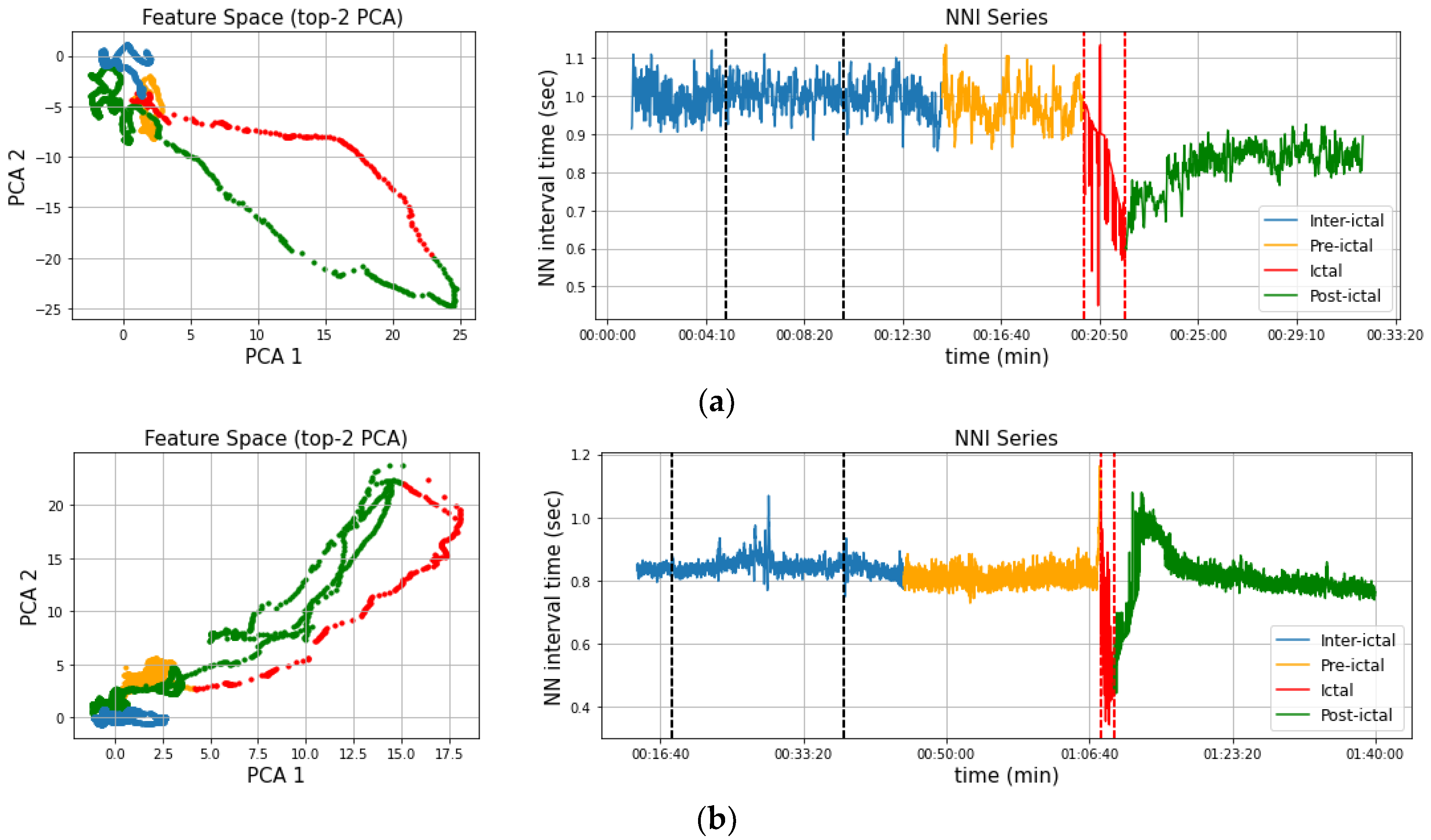
2.6. Segment Labeling
2.7. Experiment Procedure
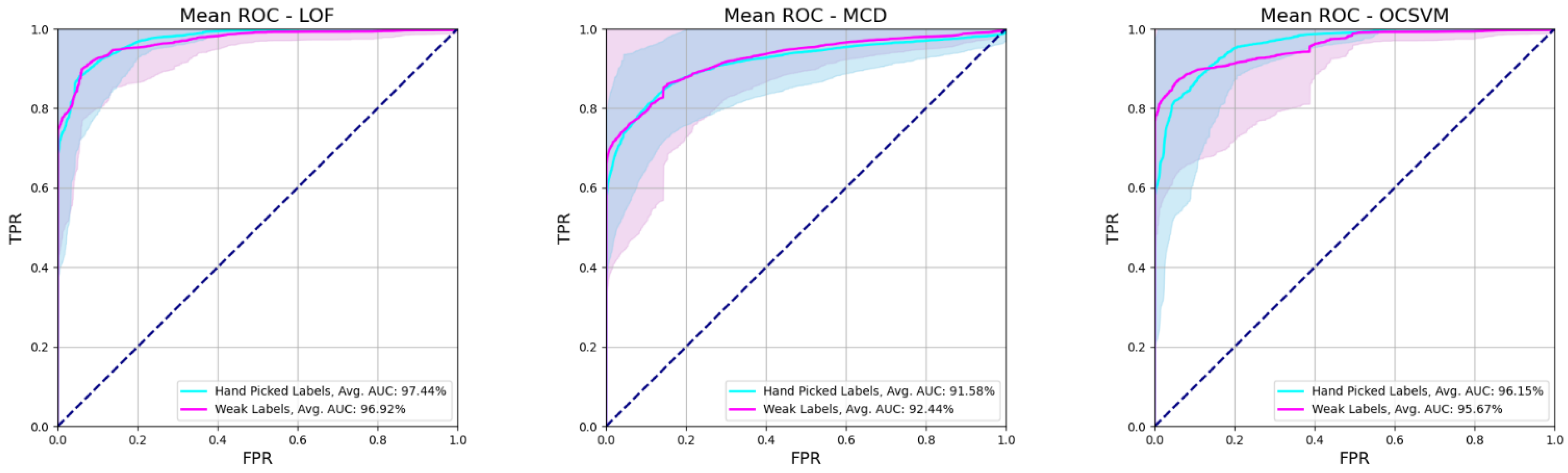
2.8. Performance Scores
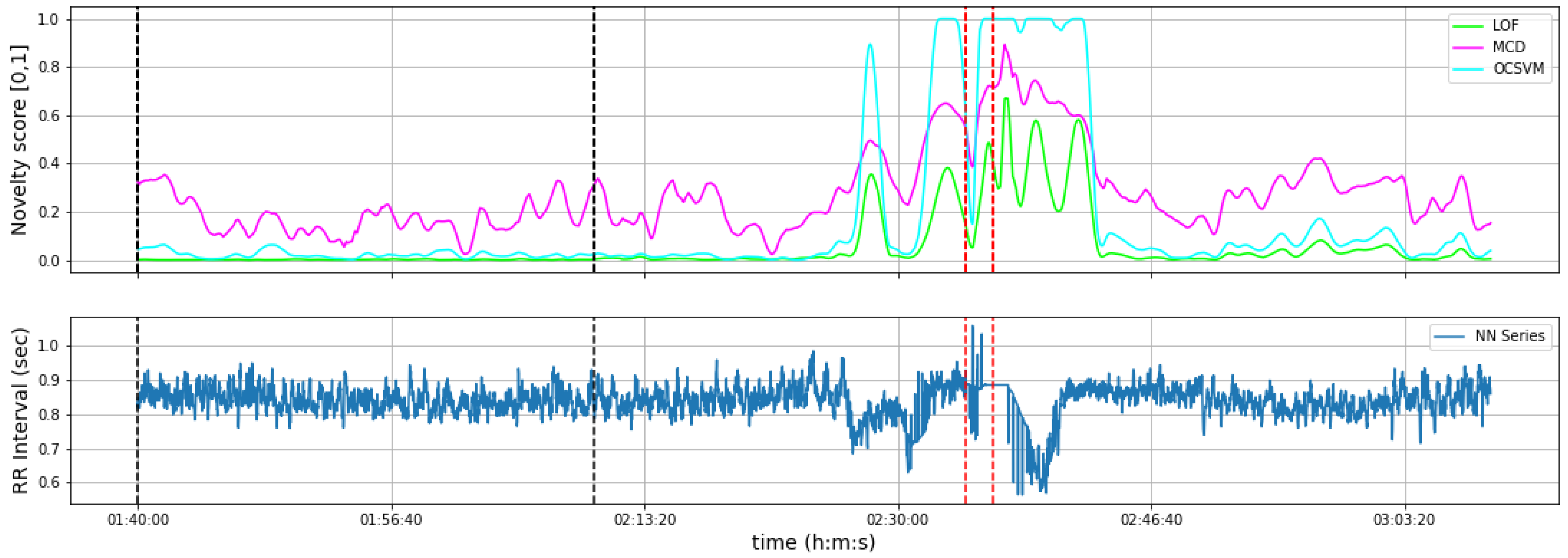
3. Results
3.1. Experimental Results
3.2. Post-Ictal Monitoring
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization Epilepsy. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 2 December 2022).
- Epilepsy Across the Spectrum: Promoting Health and Understanding. In The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press (US): Washington, DC, USA, 2012.
- Fischer, B.A.; Tedrus, G.M.A.S. Seizure-Related Injuries in Adults: A Prospective Case-Controlled Study on Risk Factors, Seizure Severity, and Quality of Life. Epilepsy Behav. 2022, 134, 108849. [Google Scholar] [CrossRef]
- Gaitatzis, A.; Johnson, A.L.; Chadwick, D.W.; Shorvon, S.D.; Sander, J.W. Life Expectancy in People with Newly Diagnosed Epilepsy. Brain 2004, 127, 2427–2432. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.; Lauersen, T.; Tomson, T.; Plana-Ripoll, O.; Christensen, J. Cause-Specific Mortality and Life Years Lost in People with Epilepsy: A Danish Cohort Study. Brain 2022, 146, 124–134. [Google Scholar] [CrossRef] [PubMed]
- Nashef, L. Sudden Unexpected Death in Epilepsy: Terminology and Definitions. Epilepsia 1997, 38, S6–S8. [Google Scholar] [CrossRef]
- Massey, C.A.; Sowers, L.P.; Dlouhy, B.J.; Richerson, G.B. Sudden Unexpected Death in Epilepsy: Current Knowledge and Future Directions. Lancet Neurol. 2008, 7, 1021–1031. [Google Scholar]
- Velagapudi, P.; Turagam, M.; Laurence, T.; Kocheril, A. Cardiac Arrhythmias and Sudden Unexpected Death in Epilepsy (SUDEP). Pacing Clin. Electrophysiol. (PACE) 2012, 35, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Finsterer, J.; Stöllberger, C. Cardiopulmonary Surveillance to Prevent SUDEP. Lancet Neurol. 2009, 8, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Mazzola, L.; Rheims, S. Ictal and Interictal Cardiac Manifestations in Epilepsy. A Review of Their Relation with an Altered Central Control of Autonomic Functions and With the Risk of SUDEP. Front. Neurol. 2021, 12, 642645. [Google Scholar] [CrossRef]
- Massey, C.A.; Sowers, L.P.; Dlouhy, B.J.; Richerson, G.B. Mechanisms of Sudden Unexpected Death in Epilepsy: The Pathway to Prevention. Nat. Rev. Neurol. 2014, 10, 271–282. [Google Scholar] [CrossRef]
- Kanner, A.M. Peri-Ictal Cardiac and Respiratory Disturbances in Epilepsy: Incidental Finding or Culprit of SUDEP? Epilepsy Curr. 2011, 11, 16–18. [Google Scholar] [CrossRef]
- A Guide to Wearable Electrocardiogram (ECG) Devices: Types, Advantages and Disadvantages. Available online: https://apacmed.org/wearable-ecg-device/ (accessed on 2 December 2022).
- Pavei, J.; Heinzen, R.G.; Novakova, B.; Walz, R.; Serra, A.J.; Reuber, M.; Ponnusamy, A.; Marques, J.L.B. Early Seizure Detection Based on Cardiac Autonomic Regulation Dynamics. Front. Physiol. 2017, 8, 765. [Google Scholar] [CrossRef]
- Varon, C.; Jansen, K.; Lagae, L.; Huffel, S.V. Detection of Epileptic Seizures by Means of Morphological Changes in the ECG. Comput. Cardiol. 2013, 2013, 863–866. [Google Scholar]
- Moridani, M.K.; Farhadi, H. Heart Rate Variability as a Biomarker for Epilepsy Seizure Prediction. Bratisl. Lek Listy 2017, 118, 3–8. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Johansen, P.; Christensen, J.; Wüstenhagen, S.; Tankisi, H.; Qerama, E.; Beniczky, S. Seizure Detection Using Heart Rate Variability: A Prospective Validation Study. Epilepsia 2020, 61 (Suppl. S1), S41–S46. [Google Scholar] [CrossRef]
- Nunan, D.; Sandercock, G.R.H.; Brodie, D.A. A Quantitative Systematic Review of Normal Values for Short-Term Heart Rate Variability in Healthy Adults. Pacing Clin. Electrophysiol. 2010, 33, 1407–1417. [Google Scholar] [CrossRef]
- Sandercock, G.R.H.; Bromley, P.D.; Brodie, D.A. The Reliability of Short-Term Measurements of Heart Rate Variability. Int. J. Cardiol. 2005, 103, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Nunan, D.; Donovan, G.; Jakovljevic, D.G.; Hodges, L.D.; Sandercock, G.R.H.; Brodie, D.A. Validity and Reliability of Short-Term Heart-Rate Variability from the Polar S810. Med. Sci. Sport. Exerc. 2009, 41, 243–250. [Google Scholar] [CrossRef]
- Vandecasteele, K.; De Cooman, T.; Gu, Y.; Cleeren, E.; Claes, K.; Paesschen, V.; Wim; Huffel, V.; Sabine; Hunyadi, B. Automated Epileptic Seizure Detection Based on Wearable ECG and PPG in a Hospital Environment. Sensors 2017, 10, 2338. [Google Scholar] [CrossRef]
- Jeppesen, J.; Fuglsang-Frederiksen, A.; Johansen, P.; Christensen, J.; Wüstenhagen, S.; Tankisi, H.; Qerama, E.; Hess, A.; Beniczky, S. Seizure Detection Based on Heart Rate Variability Using a Wearable Electrocardiography Device. Epilepsia 2019, 60, 2105–2113. [Google Scholar] [CrossRef]
- Beniczky, S.; Karoly, P.; Nurse, E.; Ryvlin, P.; Cook, M. Machine Learning and Wearable Devices of the Future. Epilepsia 2021, 62 (Suppl. S2), S116–S124. [Google Scholar] [CrossRef] [PubMed]
- Perez-Sanchez, A.V.; Perez-Ramirez, C.A.; Valtierra-Rodriguez, M.; Dominguez-Gonzalez, A.; Amezquita-Sanchez, J.P. Wavelet Transform-Statistical Time Features-Based Methodology for Epileptic Seizure Prediction Using Electrocardiogram Signals. Mathematics 2020, 8, 2125. [Google Scholar] [CrossRef]
- Giannakakis, G.; Tsiknakis, M.; Vorgia, P. Focal Epileptic Seizures Anticipation Based on Patterns of Heart Rate Variability Parameters. Comput. Methods Programs Biomed. 2019, 178, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Billeci, L.; Marino, D.; Insana, L.; Vatti, G.; Varanini, M. Patient-Specific Seizure Prediction Based on Heart Rate Variability and Recurrence Quantification Analysis. PLoS ONE 2018, 13, e0204339. [Google Scholar] [CrossRef]
- Fujiwara, K.; Miyajima, M.; Yamakawa, T.; Abe, E.; Suzuki, Y.; Sawada, Y.; Kano, M.; Maehara, T.; Ohta, K.; Sasai-Sakuma, T.; et al. Epileptic Seizure Prediction Based on Multivariate Statistical Process Control of Heart Rate Variability Features. IEEE Trans. Biomed. Eng. 2016, 63, 1321–1332. [Google Scholar]
- Leal, A.; Mauro Pinto, F.; Lopes, F.; Anna Bianchi, M.; Henriques, J.; Maria Ruano, G.; de Carvalho, P.; Dourado, A.; Teixeira, A. César Heart Rate Variability Analysis for the Identification of the Preictal Interval in Patients with Drug-Resistant Epilepsy. Nat. Sci. Rep. 2021, 11, 5987. [Google Scholar]
- Wen, T.; Zhang, Z. Deep Convolution Neural Network and Autoencoders-Based Unsupervised Feature Learning of EEG Signals. IEEE Access 2018, 6, 25399–25410. [Google Scholar] [CrossRef]
- Asghar, M.Z.; Ahmad, I.; Zhu, M.; Wang, C.; Pi, Y.; Khan, J.A.; Khan, S.; Samuel, O.W.; Chen, S.; Li, G. EEG-Based Epileptic Seizure Detection via Machine/Deep Learning Approaches: A Systematic Review. Comput. Intell. Neurosci. 2022, 2022, 6486570. [Google Scholar]
- Smart, O.; Chen, M. Semi-Automated Patient-Specific Scalp EEG Seizure Detection with Unsupervised Machine Learning. In Proceedings of the 2015 IEEE Conference on Computational Intelligence in Bioinformatics and Computational Biology (CIBCB), Niagara Falls, ON, Canada, 12–15 August 2015; pp. 1–7. [Google Scholar]
- Tsiouris, K.M.; Konitsiotis, S.; Markoula, S.; Rigas, G.; Koutsouris, D.D.; Fotiadis, D.I. Unsupervised Detection of Epileptic Seizures from EEG Signals: A Channel-Specific Analysis of Long-Term Recordings. In Proceedings of the 2018 IEEE EMBS International Conference on Biomedical & Health Informatics (BHI), Las Vegas, NV, USA, 4–7 March 2018; pp. 92–95. [Google Scholar]
- Birjandtalab, J.; Pouyan, M.B.; Nourani, M. Unsupervised EEG Analysis for Automated Epileptic Seizure Detection. Int. Workshop Pattern Recognit. 2016, 10011, 124–128. [Google Scholar]
- Markus, B.M.; Kriegel H-P Ng, R.T.; Sander, J. LOF: Identifying Density-Based Local Outliers. SIGMOD Rec. 2000, 29, 93–104. [Google Scholar]
- Hubert, M.; Debruyne, M.; Rousseeuw, P. Minimum Covariance Determinant and Extensions. WIREs Comput. Stat. 2017, 10, e1421. [Google Scholar] [CrossRef]
- Manevitz, L.M. Malik Yousef One-Class SVMs for Document Classification. J. Mach. Learn. Res. 2001, 2, 139–154. [Google Scholar]
- Carreiras, C.; Alves, A.P.; Lourenço, A.; Canento, F.; Silva, H.; Fred, A. Others BioSPPy: Biosignal Processing in Python. Available online: https://github.com/PIA-Group/BioSPPy/ (accessed on 2 December 2022).
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef]
- Gomes, P.; Margaritoff, P.; Silva, H. PyHRV: Development and Evaluation of an Open-Source Python Toolbox for Heart Rate Variability (HRV). In Proceedings of the (Ic)ETRAN 2019: 6th International Conference on Electrical, Electronic and Computing Engineering, Veliko Gradište, Serbia, 3–6 June 2019. [Google Scholar]
- Lake, E.D.; Richman, S.J.; Griffin, P.M.; Moorman, R.J. Sample Entropy Analysis of Neonatal Heart Rate Variability. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2002, 283, R789–R797. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Tsai, M.-Y.; Huang, G.-S.; Lin, T.-C.; Chen, K.-P.; Ho, S.-T.; Shyu, L.-Y.; Li, C.-Y. Poincaré Plot Indexes of Heart Rate Variability Detect Dynamic Autonomic Modulation during General Anesthesia Induction. Acta Anaesthesiol. Taiwan 2012, 50, 12–18. [Google Scholar] [CrossRef]
- Katz, M.J. Fractals and the Analysis of Waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Pedro Gomes Highlights—PyHRV—OpenSource Python Toolbox for Heart Rate Variability 0.4 Documentation. Available online: https://pyhrv.readthedocs.io/en/latest/ (accessed on 2 December 2022).
- Estévez, M.; Machado, C.; Leisman, G.; Estévez-Hernández, T.; Arias-Morales, A.; Machado, A.; Montes-Brown, J. Spectral Analysis of Heart Rate Variability. Int. J. Disabil. Hum. Dev. 2016, 5–17, 5–17. [Google Scholar] [CrossRef]
- Muller, L.R.; Kauffmann, J.R.; Vandermeulen, R.A.; Montavon, G.; Samek, W.; Kloft, M.; Dietterich, T.G.; Robert, K. A Unifying Review of Deep and Shallow Anomaly Detection. Proc. IEEE 2021, 109, 756–795. [Google Scholar]
- Bendre, M.S.; Kale, K.B. Masking Effect on Tests for Outliers in Normal Samples. Biometrika 1987, 74, 891–896. [Google Scholar] [CrossRef]
- Roelant, E.; Van Aelst, S. The Minimum Weighted Covariance Determinant Estimator. Metrika 2009, 70, 177–204. [Google Scholar] [CrossRef]
- Seliya, N.; Abdollah, A.Z.; Khoshgoftaar, M.T. A Literature Review on One-Class Classification and Its Potential Applications in Big Data. Big Data 2021, 8, 2021. [Google Scholar] [CrossRef]
- Scholkopf, B. Learning with Kernels: Support Vector Machines, Regularization, Optimization, and Beyond; MIT Press: London, UK, 2001. [Google Scholar]
- Al-Aweel, I.C.; Krishnamurthy, K.B.; Hausdorff, J.M.; Mietus, J.E.; Ives, J.R.; Blum, A.S.; Schomer, D.L.; Goldberger, A.L. Post-Ictal Heart Rate Oscillations in Partial Epilepsy. Neurology 1999, 53, 1590–1592. [Google Scholar] [CrossRef] [PubMed]
- Goldberger, A.; Amaral, L.; Glass, L.; Hausdorff, J.; Ivanov, P.C.; Mark, R.; Mietus, J.E.; Moody, G.B.; Peng, C.K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Peikari, M.; Salama, S.; Nofech-Mozes, S.; Martel, L. Anne A Cluster-Then-Label Semi-Supervised Learning Approach for Pathology Image Classification. Nat. Sci. Rep. 2018, 8, 7193. [Google Scholar]
- Chapelle, O.; Schoelkopf, B.; Zien, A. (Eds.) Semi-Supervised Learning; Adaptive Computation and Machine Learning Series; MIT Press: London, UK, 2019. [Google Scholar]
- Rousseeuw, P.J. Silhouettes: A Graphical Aid to the Interpretation and Validation of Cluster Analysis. Comput. Appl. Math. 1987, 20, 53–65. [Google Scholar] [CrossRef]
- Steinley, D. Properties of the Hubert-Arabie Adjusted Rand Index. Psychol. Methods 2004, 9, 386–396. [Google Scholar] [CrossRef]
- Popov, A.; Panichev, O.; Karplyuk, Y.; Smirnov, Y.; Zaunseder, S.; Kharytonov, V. Heart Beat-to-Beat Intervals Classification for Epileptic Seizure Prediction. In Proceedings of the Signal Process: Symposium (SPSympo 2017), Jachranka, Poland, 12–14 September 2017. [Google Scholar]
- Arthurs, S.; Zaveri, H.P.; Frei, M.G.; Osorio, I. Patient and Caregiver Perspectives on Seizure Prediction. Epilepsy Behav. 2010, 19, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Manis, G.; Alexandridi, A.; Nikolopoulos, S.; Davos, C. The Effect of White Noise and False Peak Detection on HRV Analysis; SciTePress: Setúbal, Portugal, 2005. [Google Scholar]
- Nikolic-Popovic, J.; Goubran, R. Towards Increased Usability of Noisy ECG Signals in HRV-Based Classifiers. In Proceedings of the 2014 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Lisbon, Portugal, 11 June 2014; pp. 1–4. [Google Scholar]
- An, X.; Liu, Y.; Zhao, Y.; Lu, S.; Stylios, G.K.; Liu, Q. Adaptive Motion Artifact Reduction in Wearable ECG Measurements Using Impedance Pneumography Signal. Sensors 2022, 22, 5493. [Google Scholar] [CrossRef] [PubMed]
- Ghaleb, F.A.; Kamat, M.B.; Salleh, M.; Rohani, M.F.; Abd Razak, S. Two-Stage Motion Artefact Reduction Algorithm for Electrocardiogram Using Weighted Adaptive Noise Cancelling and Recursive Hampel Filter. PLoS ONE 2018, 13, e0207176. [Google Scholar] [CrossRef]
- Vijayarangan, S.; Vignesh, R.; Murugesan, B.; Preejith, S.P.; Joseph, J.; Sivaprakasam, M. RPnet: A Deep Learning Approach for Robust R Peak Detection in Noisy ECG. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; Volume 2020, p. 348. [Google Scholar]
- Zahid, M.U.; Kiranyaz, S.; Ince, T.; Devecioglu, O.C.; Chowdhury, M.E.H.; Khandakar, A.; Tahir, A.; Gabbouj, M. Robust R-Peak Detection in Low-Quality Holter ECGs Using 1D Convolutional Neural Network. IEEE Trans. Biomed. Eng. 2022, 69, 119–128. [Google Scholar] [CrossRef]
- Yun, D.; Lee, H.-C.; Jung, C.-W.; Kwon, S.; Lee, S.-R.; Kim, K.; Kim, Y.S.; Han, S.S. Robust R-Peak Detection in an Electrocardiogram with Stationary Wavelet Transformation and Separable Convolution. Sci. Rep. 2022, 12, 19638. [Google Scholar] [CrossRef]
- Voss, A.; Schroeder, R.; Heitmann, A.; Peters, A.; Perz, S. Short-Term Heart Rate Variability—Influence of Gender and Age in Healthy Subjects. PLoS ONE 2015, 10, e0118308. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.-C.; Lin, H.-C.; Chuang, Y.-C.; Hsu, C.-Y. Exploring Factors Associated with Interictal Heart Rate Variability in Patients with Medically Controlled Focal Epilepsy. Seizure 2021, 92, 24–28. [Google Scholar] [CrossRef]
- Behbahani, S.; Jafarnia Dabanloo, N.; Motie Nasrabadi, A.; Dourado, A. Gender-Related Differences in Heart Rate Variability of Epileptic Patients. Am. J. Mens. Health 2018, 12, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Greene, B.R.; Boylan, G.B.; Reilly, R.B.; de Chazal, P.; Connolly, S. Combination of EEG and ECG for Improved Automatic Neonatal Seizure Detection. Clin. Neurophysiol. 2007, 118, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, I.; Zafar, M.; Sinha, S.R.; Hu, X. Seizures Detection Using Multimodal Signals: A Scoping Review. Physiol. Meas. 2022, 43, 07TR01. [Google Scholar] [CrossRef] [PubMed]
| Feature | Description | Formula 1 | Unit 2 |
|---|---|---|---|
| MEAN | Mean of NN intervals | ||
| SD | Standard deviation | ||
| SKEW | Skewness of NN intervals | ||
| KURT | Kurtosis of NN intervals | ||
| NNX | Number of NN exceeding the threshold X | ||
| SDSD | Standard deviation of NN | ||
| RMSSD | Root mean of squared differences of NNI | ||
| SAMPEN | Sample entropy [40] | (See cited paper) | |
| SD1 | Standard deviation of major axis in Poincaré plot [41] | ||
| SD2 | Standard deviation of minor axis in Poincare plot | ||
| SD1SD2 | Ratio between SD1, SD2 | ||
| ELLIPSE | Ellipse area of Poincare plot | ||
| KFD | Katz fractal dimension [42] | ||
| LF | Low-frequency band energy (0.04–0.15 Hz) | [] | |
| HF | High-frequency band energy (0.15–0.4 Hz) | ||
| LFHF | Low-frequency to high-frequency band energy ratio | ||
| LFPEAK | Peak LF band frequency | ||
| HFPEAK | Peak HF band frequency |
| Case | Seizure 1 | Reference 1 | OPP 2 | ||
|---|---|---|---|---|---|
| Start | End | Start | End | ||
| sz01-1 | 00:14:36 | 00:16:12 | 00:04:00 | 00:08:00 | 6:30 |
| sz02-1 | 01:02:43 | 01:03:43 | 00:15:00 | 00:25:00 | 30:00 |
| sz02-2 | 02:55:51 | 02:56:16 | 02:10:00 | 02:20:00 | 21:00 |
| sz03-1 | 01:24:34 | 01:26:22 | 00:30:00 | 00:50:00 | 22:00 |
| sz03-2 | 02:34:27 | 02:36:17 | 01:40:00 | 02:10:00 | 7:20 |
| sz04-1 | 00:20:10 | 00:21:55 | 00:05:00 | 00:10:00 | 6:00 |
| sz05-1 | 00:24:07 | 00:25:30 | 00:03:30 | 00:10:00 | 13:30 |
| sz06-1 | 00:51:2 | 00:52:19 | 00:30:00 | 00:35:00 | 7:00 |
| sz06-2 | 02:04:45 | 02:06:10 | 01:43:00 | 01:50:00 | 6:30 |
| sz07-1 | 01:08:02 | 01:09:31 | 00:18:00 | 00:38:00 | 23:00 |
| Case | Silhouette | ARI | |
|---|---|---|---|
| Weak | Hand-Picked | ||
| sz01-1 | 0.533 | 0.503 | 0.741 |
| sz02-1 | 0.414 | 0.356 | 0.588 |
| sz02-2 | 0.400 | 0.357 | 0.674 |
| sz03-1 | 0.367 | 0.313 | 0.758 |
| sz03-2 | 0.758 | 0.604 | 0.644 |
| sz04-1 | 0.552 | 0.498 | 0.664 |
| sz05-1 | 0.631 | 0.005 | −0.054 |
| sz06-1 | 0.418 | 0.247 | 0.131 |
| sz06-2 | 0.782 | 0.475 | 0.344 |
| sz07-1 | 0.723 | 0.714 | 0.970 |
| Model | Case | Threshold | AUC (%) | Accuracy (%) | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|---|---|---|---|
| LOF | Hand-picked | −1.77 ± 0.64 | 14:07 ± 8:40 | 97.4 ± 3.1 | 94.8 ± 4.8 | 93.0 ± 7.7 | 95.8 ± 5.3 |
| Weak | −2.53 ± 1.17 | 14:01 ± 8:32 | 96.9 ± 3.8 | 93.5 ± 7.7 | 95.6 ± 5.2 | 93.1 ± 9.3 | |
| MCD | Hand-picked | −2.07 ± 0.54 | 14:06 ± 8:41 | 91.6 ± 7.9 | 86.9 ± 9.4 | 88.2 ± 8.3 | 85.7 ± 14.7 |
| Weak | −2.20 ± 0.53 | 13:37 ± 8:02 | 92.4 ± 8.9 | 88.9 ± 9.3 | 91.1 ± 9.2 | 87.8 ± 10.5 | |
| OCSVM | Hand-picked | 1.85 ± 0.50 | 14:05 ± 8:38 | 96.1 ± 5.5 | 93.2 ± 6.5 | 92.7 ± 7.9 | 93.4 ± 7.5 |
| Weak | 1.47 ± 0.63 | 14:00 ± 8:35 | 95.6 ± 7.9 | 95.6 ± 4.2 | 92.4 ± 15.7 | 96.6 ± 4.6 |
| (a) Hand-Picked Labels | ||||||||
| LOF (%) | Pre-Ictal | Inter-Ictal | MCD (%) | Pre-Ictal | Inter-Ictal | OCSVM (%) | Pre-Ictal | Inter-Ictal |
| + | 36.60 | 2 | + | 34.30 | 8 | + | 36.48 | 4 |
| - | 5.47 | 56 | - | 7.74 | 50 | - | 5.85 | 54 |
| (b) Weak Labels | ||||||||
| LOF (%) | Pre-Ictal | Inter-Ictal | MCD (%) | Pre-Ictal | Inter-Ictal | OCSVM (%) | Pre-Ictal | Inter-Ictal |
| + | 33.78 | 4 | + | 31.87 | 9 | + | 34.91 | 2 |
| - | 1.60 | 60 | - | 3.69 | 56 | - | 1.81 | 61 |
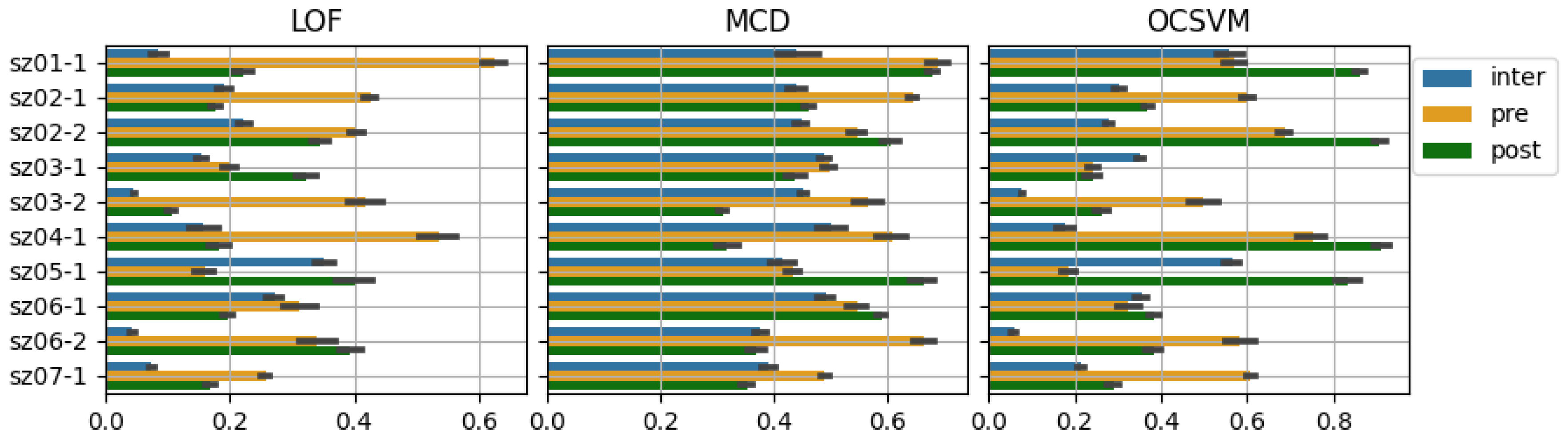
| Case | State | LOF | MCD | OCSVM |
|---|---|---|---|---|
| Inter-ictal | 0.09 ± 0.12 | 0.19 ± 0.28 | 0.57 ± 0.27 | |
| sz01-1 | Post-ictal | 0.22 ± 0.24 | 0.17 ± 0.21 | 0.92 ± 0.19 |
| Pre-ictal | 0.63 ± 0.22 | 0.41 ± 0.29 | 0.69 ± 0.25 | |
| Inter-ictal | 0.19 ± 0.18 | 0.20 ± 0.24 | 0.36 ± 0.20 | |
| sz02-1 | Post-ictal | 0.18 ± 0.19 | 0.15 ± 0.18 | 0.70 ± 0.26 |
| Pre-ictal | 0.43 ± 0.24 | 0.28 ± 0.19 | 0.86 ± 0.22 | |
| Inter-ictal | 0.22 ± 0.20 | 0.15 ± 0.18 | 0.36 ± 0.18 | |
| sz02-2 | Post-ictal | 0.35 ± 0.21 | 0.30 ± 0.23 | 0.93 ± 0.19 |
| Pre-ictal | 0.40 ± 0.22 | 0.28 ± 0.23 | 0.87 ± 0.15 | |
| Inter-ictal | 0.15 ± 0.18 | 0.19 ± 0.20 | 0.40 ± 0.22 | |
| sz03-1 | Post-ictal | 0.32 ± 0.25 | 0.19 ± 0.24 | 0.51 ± 0.27 |
| Pre-ictal | 0.20 ± 0.20 | 0.14 ± 0.19 | 0.60 ± 0.23 | |
| Inter-ictal | 0.05 ± 0.06 | 0.16 ± 0.16 | 0.16 ± 0.12 | |
| sz03-2 | Post-ictal | 0.11 ± 0.18 | 0.02 ± 0.09 | 0.47 ± 0.32 |
| Pre-ictal | 0.42 ± 0.35 | 0.30 ± 0.31 | 0.68 ± 0.40 | |
| Inter-ictal | 0.16 ± 0.28 | 0.16 ± 0.26 | 0.32 ± 0.29 | |
| sz04-1 | Post-ictal | 0.18 ± 0.25 | 0.17 ± 0.22 | 0.92 ± 0.22 |
| Pre-ictal | 0.53 ± 0.28 | 0.41 ± 0.27 | 0.89 ± 0.20 | |
| Inter-ictal | 0.35 ± 0.21 | 0.13 ± 0.21 | 0.60 ± 0.22 | |
| sz05-1 | Post-ictal | 0.40 ± 0.34 | 0.34 ± 0.33 | 0.91 ± 0.19 |
| Pre-ictal | 0.16 ± 0.28 | 0.09 ± 0.15 | 0.29 ± 0.29 | |
| Inter-ictal | 0.27 ± 0.21 | 0.13 ± 0.15 | 0.41 ± 0.21 | |
| sz06-1 | Post-ictal | 0.20 ± 0.23 | 0.16 ± 0.19 | 0.70 ± 0.26 |
| Pre-ictal | 0.31 ± 0.28 | 0.34 ± 0.18 | 0.51 ± 0.30 | |
| Inter-ictal | 0.04 ± 0.09 | 0.04 ± 0.08 | 0.21 ± 0.14 | |
| sz06-2 | Post-ictal | 0.39 ± 0.25 | 0.10 ± 0.14 | 0.68 ± 0.20 |
| Pre-ictal | 0.34 ± 0.32 | 0.34 ± 0.22 | 0.80 ± 0.24 | |
| Inter-ictal | 0.07 ± 0.10 | 0.10 ± 0.21 | 0.27 ± 0.20 | |
| sz07-1 | Post-ictal | 0.17 ± 0.19 | 0.08 ± 0.20 | 0.54 ± 0.32 |
| Pre-ictal | 0.26 ± 0.15 | 0.15 ± 0.11 | 0.94 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karasmanoglou, A.; Antonakakis, M.; Zervakis, M. ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures. Int. J. Environ. Res. Public Health 2023, 20, 5000. https://doi.org/10.3390/ijerph20065000
Karasmanoglou A, Antonakakis M, Zervakis M. ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures. International Journal of Environmental Research and Public Health. 2023; 20(6):5000. https://doi.org/10.3390/ijerph20065000
Chicago/Turabian StyleKarasmanoglou, Apostolos, Marios Antonakakis, and Michalis Zervakis. 2023. "ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures" International Journal of Environmental Research and Public Health 20, no. 6: 5000. https://doi.org/10.3390/ijerph20065000
APA StyleKarasmanoglou, A., Antonakakis, M., & Zervakis, M. (2023). ECG-Based Semi-Supervised Anomaly Detection for Early Detection and Monitoring of Epileptic Seizures. International Journal of Environmental Research and Public Health, 20(6), 5000. https://doi.org/10.3390/ijerph20065000








