Accumulative Effects of Multifrequency Microwave Exposure with 1.5 GHz and 2.8 GHz on the Structures and Functions of the Immune System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Microwave Exposure
2.2. Histopathological Analysis
2.3. Transmission Electron Microscopy (TEM)
2.4. Analysis of Immune Cells in Peripheral Blood
2.5. Cytokine Release
2.6. Concentration of Immunoglobulins and Complement Proteins in Sera
2.7. Statistical Analysis
3. Results
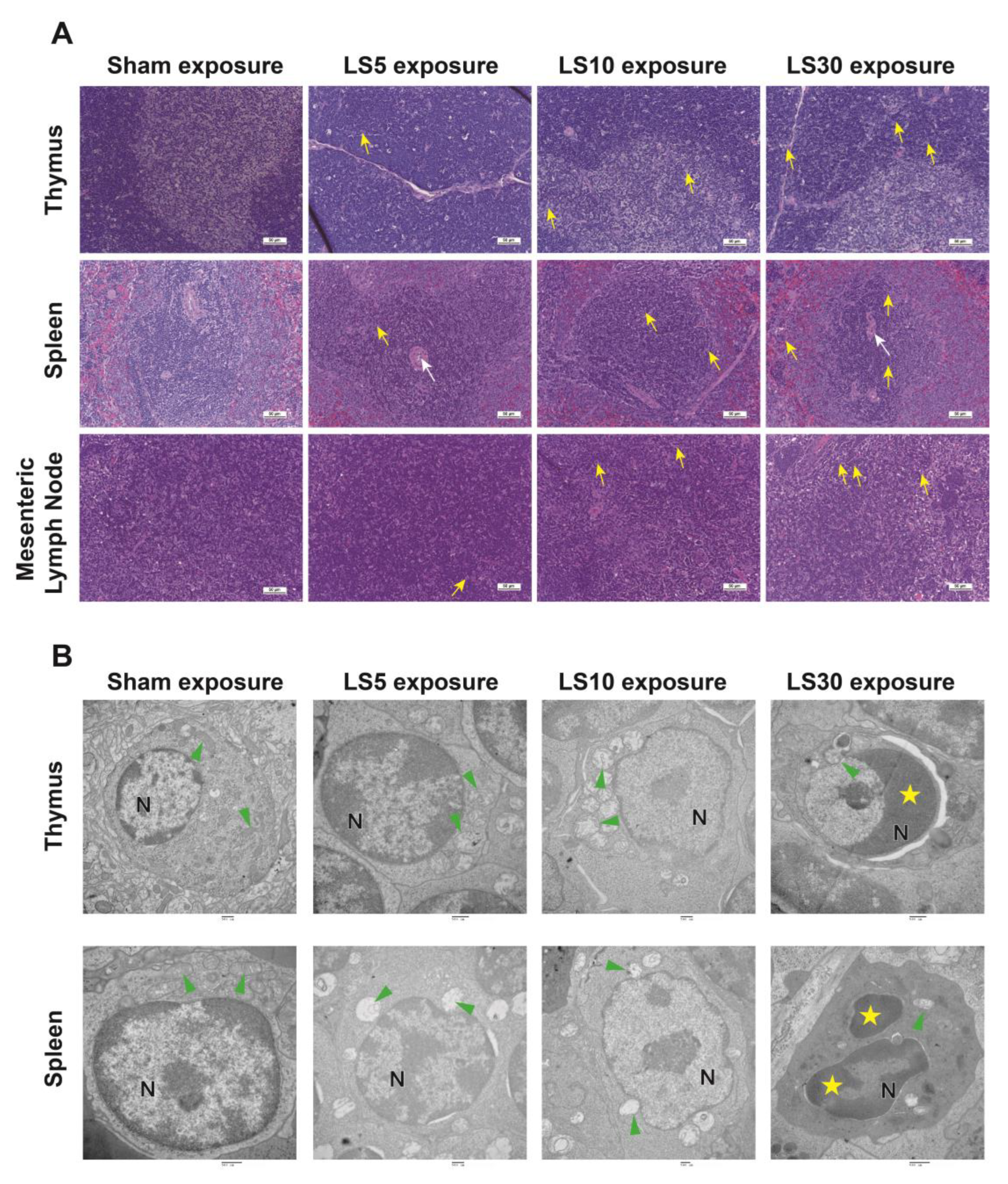
3.1. Multifrequency Microwave Dose-Dependently Caused Structural and Ultrastructural Injuries
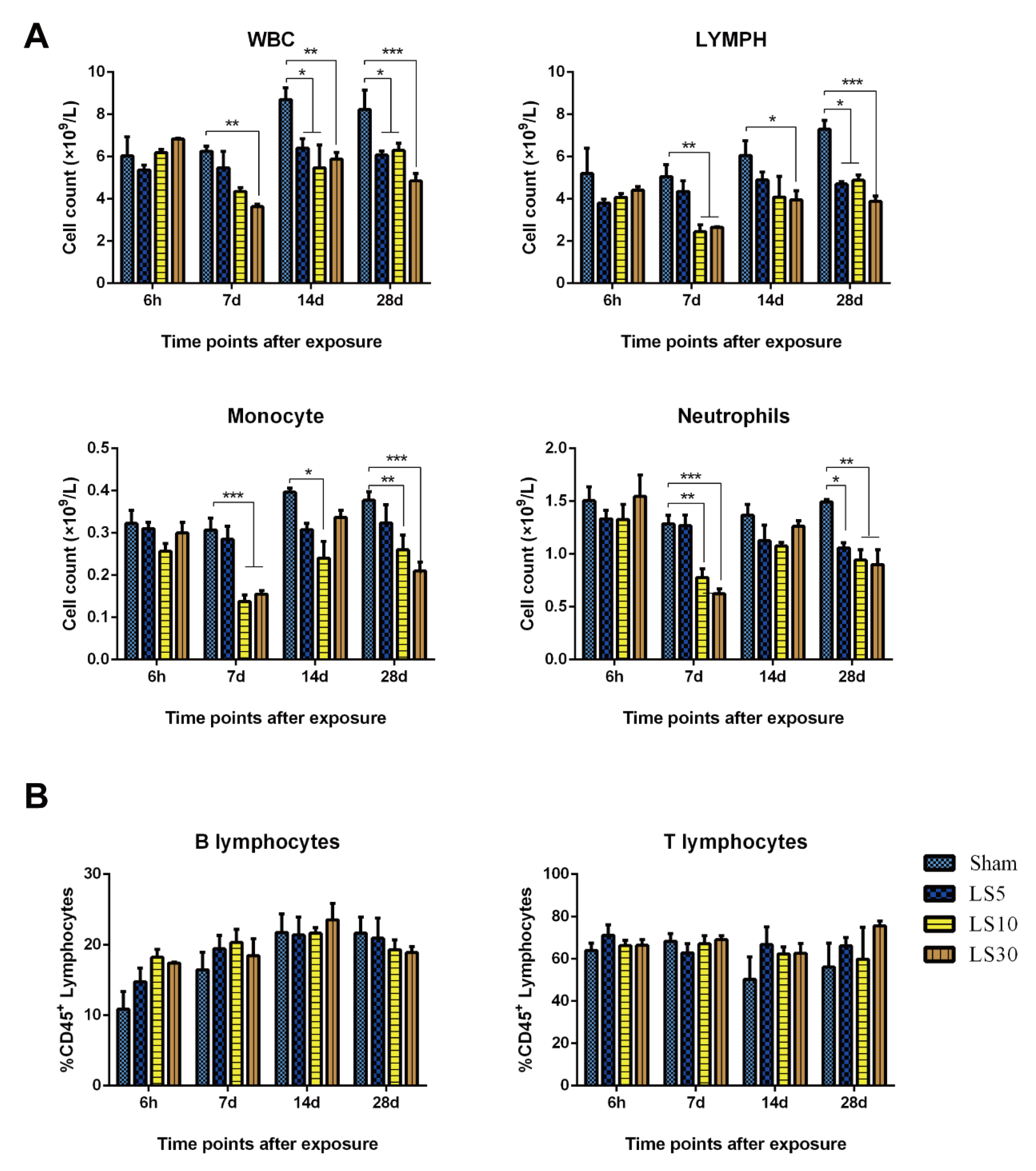
3.2. Multifrequency Microwave Exposure Decreased Immune Cells in Peripheral Blood
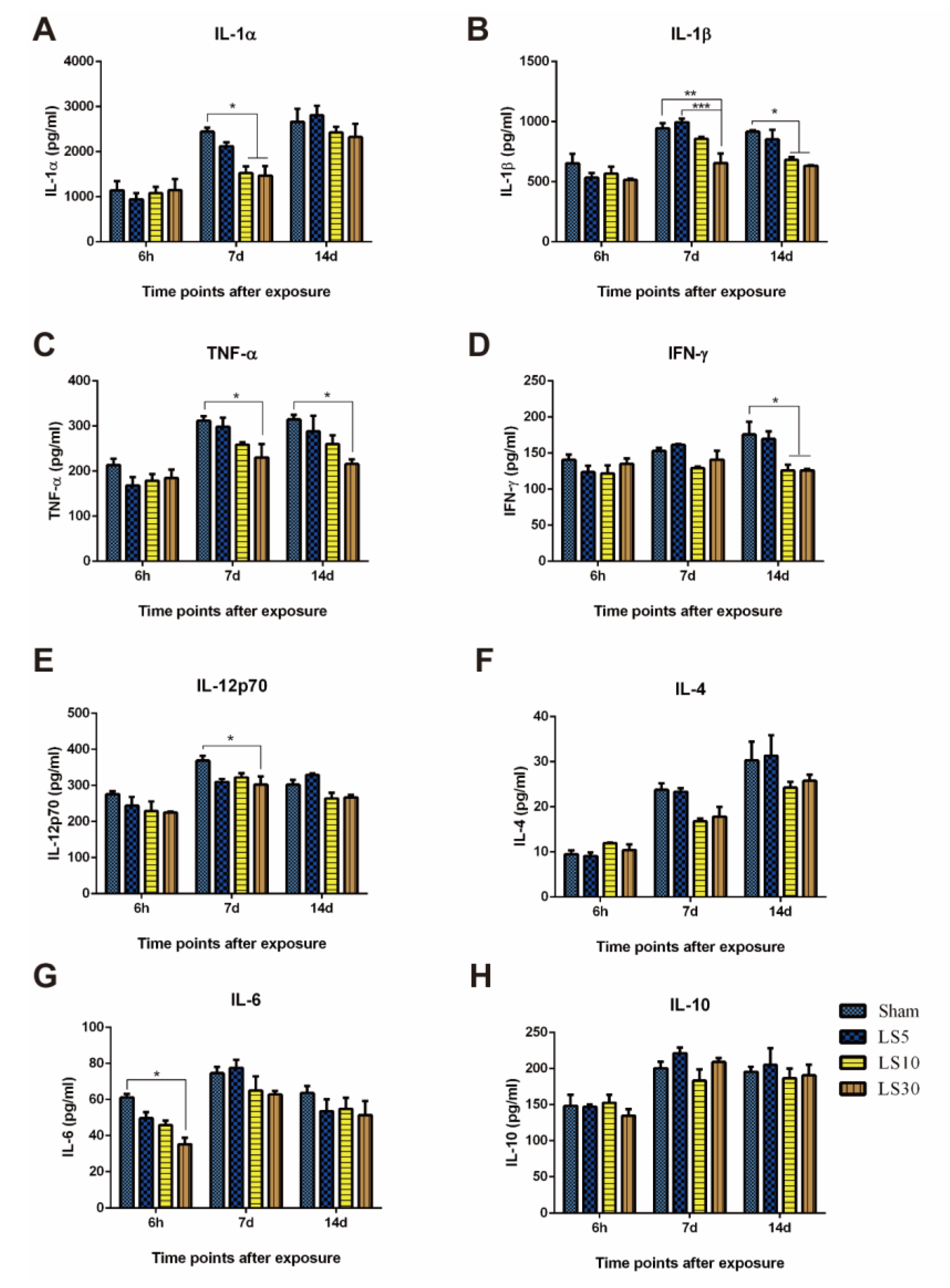
3.3. Multifrequency Microwave Exposure Reduced Th1 Cytokines in Peripheral Blood
3.4. Multifrequency Microwave Exposure Dose-Dependently Reduced the Levels of IgG and IgM in Peripheral Blood
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kesari, K.K.; Siddiqui, M.H.; Meena, R.; Verma, H.N.; Kumar, S. Cell phone radiation exposure on brain and associated biological systems. Indian J. Exp. Biol. 2013, 51, 187–200. [Google Scholar]
- Szmigielski, S. Reaction of the immune system to low-level RF/MW exposures. Sci. Total Environ. 2013, 454–455, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef] [PubMed]
- Shahin, S.; Banerjee, S.; Singh, S.P.; Chaturvedi, C.M. 2.45 GHz Microwave Radiation Impairs Learning and Spatial Memory via Oxidative/Nitrosative Stress Induced p53-Dependent/Independent Hippocampal Apoptosis: Molecular Basis and Underlying Mechanism. Toxicol. Sci. 2015, 148, 380–399. [Google Scholar] [CrossRef] [PubMed]
- Banik, S.; Bandyopadhyay, S.; Ganguly, S. Bioeffects of microwave–a brief review. Bioresour. Technol. 2003, 87, 155–159. [Google Scholar] [CrossRef]
- Friedman, J.; Kraus, S.; Hauptman, Y.; Schiff, Y.; Seger, R. Mechanism of short-term ERK activation by electromagnetic fields at mobile phone frequencies. Biochem. J. 2007, 405, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.R.; Swicord, M.L.; Balzano, Q. Quantitative evaluations of mechanisms of radiofrequency interactions with biological molecules and processes. Health Phys. 2008, 95, 365–396. [Google Scholar] [CrossRef]
- Szmigielski, S.; Szudzinski, A.; Pietraszek, A.; Bielec, M.; Janiak, M.; Wrembel, J.K. Accelerated development of spontaneous and benzopyrene-induced skin cancer in mice exposed to 2450-MHz microwave radiation. Bioelectromagnetics 1982, 3, 179–191. [Google Scholar] [CrossRef]
- Lin, Y.; Gao, P.; Guo, Y.; Chen, Q.; Lang, H.; Guo, Q.; Miao, X.; Li, J.; Zeng, L.; Guo, G. Effects of Long-Term Exposure to L-Band High-Power Microwave on the Brain Function of Male Mice. BioMed Res. Int. 2021, 2021, 2237370. [Google Scholar] [CrossRef]
- Yin, Y.; Xu, X.; Gao, Y.; Wang, J.; Yao, B.; Zhao, L.; Wang, H.; Wang, H.; Dong, J.; Zhang, J.; et al. Abnormal Expression of Connexin43 in Cardiac Injury Induced by S-Band and X-Band Microwave Exposure in Rats. J. Immunol. Res. 2021, 2021, 3985697. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Wang, S.M.; Peng, R.Y.; Gao, Y.B.; Wang, L.F.; Zhao, L.; Zuo, H.Y.; Dong, J.; Su, Z.T. Long-term microwave radiation affects male reproduction in rats. Natl. J. Androl. 2011, 17, 214–218. [Google Scholar] [PubMed]
- Tripathi, A.; Attri, L.; Tiwari, R.K. Spaceborne C-band SAR remote sensing-based flood mapping and runoff estimation for 2019 flood scenario in Rupnagar, Punjab, India. Environ. Monit. Assess. 2021, 193, 110. [Google Scholar] [CrossRef]
- Tanase, M.A.; Villard, L.; Pitar, D.; Apostol, B.; Petrila, M.; Chivulescu, S.; Leca, S.; Borlaf-Mena, I.; Pascu, I.S.; Dobre, A.C.; et al. Synthetic aperture radar sensitivity to forest changes: A simulations-based study for the Romanian forests. Sci. Total Environ. 2019, 689, 1104–1114. [Google Scholar] [CrossRef]
- Tan, S.; Wang, H.; Xu, X.; Zhao, L.; Zhang, J.; Dong, J.; Yao, B.; Wang, H.; Zhou, H.; Gao, Y.; et al. Study on dose-dependent, frequency-dependent, and accumulative effects of 1.5 GHz and 2.856 GHz microwave on cognitive functions in Wistar rats. Sci. Rep. 2017, 7, 10781. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, H.; Xu, X.; Zhao, L.; Zhang, J.; Dong, J.; Yao, B.; Wang, H.; Zhou, H.; Gao, Y.; et al. Effects of 1.5 and 4.3 GHz microwave radiation on cognitive function and hippocampal tissue structure in Wistar rats. Sci. Rep. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Rangamuwa, K.; Leong, T.; Weeden, C.; Asselin-Labat, M.L.; Bozinovski, S.; Christie, M.; John, T.; Antippa, P.; Irving, L.; Steinfort, D. Thermal ablation in non-small cell lung cancer: A review of treatment modalities and the evidence for combination with immune checkpoint inhibitors. Transl. Lung Cancer Res. 2021, 10, 2842–2857. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, Y.; Bie, Z.; Li, B.; Ma, J.; Li, X. Combination of thermal ablation and activated functional killer cells immunotherapy for cancer: A retrospective study. J. Cancer Res. Ther. 2021, 17, 797–802. [Google Scholar] [CrossRef]
- Duan, X.; Wang, M.; Han, X.; Ren, J.; Huang, G.; Ju, S.; Zhang, Q. Combined use of microwave ablation and cell immunotherapy induces nonspecific immunity of hepatocellular carcinoma model mice. Cell Cycle 2020, 19, 3595–3607. [Google Scholar] [CrossRef]
- Slovak, R.; Ludwig, J.M.; Gettinger, S.N.; Herbst, R.S.; Kim, H.S. Immuno-thermal ablations-boosting the anticancer immune response. J. Immunother. Cancer 2017, 5, 78. [Google Scholar] [CrossRef]
- Yu, M.; Pan, H.; Che, N.; Li, L.; Wang, C.; Wang, Y.; Ma, G.; Qian, M.; Liu, J.; Zheng, M.; et al. Microwave ablation of primary breast cancer inhibits metastatic progression in model mice via activation of natural killer cells. Cell. Mol. Immunol. 2021, 18, 2153–2164. [Google Scholar] [CrossRef]
- Wang, H.; Peng, R.; Zhou, H.; Wang, S.; Gao, Y.; Wang, L.; Yong, Z.; Zuo, H.; Zhao, L.; Dong, J.; et al. Impairment of long-term potentiation induction is essential for the disruption of spatial memory after microwave exposure. Int. J. Radiat. Biol. 2013, 89, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.B.; Morgan, L.L.; Udasin, I.; Davis, D.L. Cancer epidemiology update, following the 2011 IARC evaluation of radiofrequency electromagnetic fields (Monograph 102). Environ. Res. 2018, 167, 673–683. [Google Scholar] [CrossRef]
- Stein, Y.; Udasin, I.G. Electromagnetic hypersensitivity (EHS, microwave syndrome)-Review of mechanisms. Environ. Res. 2020, 186, 109445. [Google Scholar] [CrossRef]
- Baan, R.; Grosse, Y.; Lauby-Secretan, B.; El Ghissassi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Islami, F.; Galichet, L.; Straif, K. Carcinogenicity of radiofrequency electromagnetic fields. Lancet Oncol. 2011, 12, 624–626. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.F.; Wang, H.Y.; Peng, R.Y. Establishment of injury models in studies of biological effects induced by microwave radiation. Mil. Med. Res. 2021, 8, 12. [Google Scholar] [CrossRef]
- Jonwal, C.; Sisodia, R.; Saxena, V.K.; Kesari, K.K. Effect of 2.45 GHz microwave radiation on the fertility pattern in male mice. Gen. Physiol. Biophys. 2018, 37, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Bayat, M.; Karimi, N.; Karami, M.; Haghighi, A.B.; Bayat, K.; Akbari, S.; Haghani, M. Chronic exposure to 2.45 GHz microwave radiation improves cognition and synaptic plasticity impairment in vascular dementia model. Int. J. Neurosci. 2023, 133, 111–122. [Google Scholar] [CrossRef]
- Dong, G.; Zhou, H.; Gao, Y.; Zhao, X.; Liu, Q.; Li, Z.; Zhao, X.; Yin, J.; Wang, C. Effects of 1.5-GHz high-power microwave exposure on the reproductive systems of male mice. Electromagn. Biol. Med. 2021, 40, 311–320. [Google Scholar] [CrossRef]
- Rui, G.; Liu, L.Y.; Guo, L.; Xue, Y.Z.; Lai, P.P.; Gao, P.; Xing, J.L.; Li, J.; Ding, G.R. Effects of 5.8 GHz microwave on hippocampal synaptic plasticity of rats. Int. J. Environ. Health Res. 2022, 32, 2247–2259. [Google Scholar] [CrossRef]
- Gao, X.F.; Wang, S.M.; Peng, R.Y.; Gao, Y.B.; Li, X.; Dong, H.Y.; Ma, J.J. High power microwave radiation damages blood-testis barrier in rats. Zhonghua Nan Ke Xue 2008, 14, 579–582. [Google Scholar]
- Wang, H.; Tan, S.; Dong, J.; Zhang, J.; Yao, B.; Xu, X.; Hao, Y.; Yu, C.; Zhou, H.; Zhao, L.; et al. iTRAQ quantitatively proteomic analysis of the hippocampus in a rat model of accumulative microwave-induced cognitive impairment. Environ. Sci. Pollut. Res. Int. 2019, 26, 17248–17260. [Google Scholar] [CrossRef]
- Hao, Y.; Li, W.; Wang, H.; Zhang, J.; Yu, C.; Tan, S.; Wang, H.; Xu, X.; Dong, J.; Yao, B.; et al. Autophagy mediates the degradation of synaptic vesicles: A potential mechanism of synaptic plasticity injury induced by microwave exposure in rats. Physiol. Behav. 2018, 188, 119–127. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, J.; Yao, B.W.; Xu, X.P.; Wang, H.; Zhao, L.; Dong, J.; Wang, H.Y.; Tan, S.Z.; Peng, R.Y. Dose-Dependent, Frequency-Dependent, and Cumulative Effects on Cardiomyocyte Injury and Autophagy of 2.856 GHz and 1.5 GHz Microwave in Wistar Rats. Biomed. Environ. Sci. 2022, 35, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, A.A.; Grigor’ev Iu, G.; Mal’tsev, V.N.; Ulanova, A.M.; Stavrakova, N.M.; Skachkova, V.G.; Grigor’ev, O.A. Autoimmune processes after long-term low-level exposure to electromagnetic fields (the results of an experiment). Part 3. The effect of the long-term non-thermal RF EMF exposure on complement-fixation antibodies against homologenous tissue. Radiatsionnaia Biol. Radioecol. 2010, 50, 17–21. [Google Scholar]
- Zhao, L.; Li, J.; Hao, Y.H.; Gao, Y.B.; Wang, S.M.; Zhang, J.; Dong, J.; Zhou, H.M.; Liu, S.C.; Peng, R.Y. Microwave-induced Apoptosis and Cytotoxicity of NK Cells through ERK1/2 Signaling. Biomed. Environ. Sci. 2017, 30, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yao, C.; Wang, H.; Dong, J.; Zhang, J.; Xu, X.; Wang, H.; Yao, B.; Ren, K.; Sun, L.; et al. Immune Responses to Multi-Frequencies of 1.5 GHz and 4.3 GHz Microwave Exposure in Rats: Transcriptomic and Proteomic Analysis. Int. J. Mol. Sci. 2022, 23, 6949. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.F.; Dupuy, D.E. Thermal ablation of tumours: Biological mechanisms and advances in therapy. Nat. Rev. Cancer 2014, 14, 199–208. [Google Scholar] [CrossRef]
- García-Tejedor, A.; Guma, A.; Soler, T.; Valdivieso, A.; Petit, A.; Contreras, N.; Chappuis, C.G.; Falo, C.; Pernas, S.; Amselem, A.; et al. Radiofrequency Ablation Followed by Surgical Excision versus Lumpectomy for Early Stage Breast Cancer: A Randomized Phase II Clinical Trial. Radiology 2018, 289, 317–324. [Google Scholar] [CrossRef]
- Kutlu, O.C.; Chan, J.A.; Aloia, T.A.; Chun, Y.S.; Kaseb, A.O.; Passot, G.; Yamashita, S.; Vauthey, J.N.; Conrad, C. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer 2017, 123, 1817–1827. [Google Scholar] [CrossRef]
- Sandler, K.A.; Abtin, F.; Suh, R.; Cook, R.R.; Felix, C.; Lee, J.M.; Garon, E.B.; Wu, J.; Luterstein, E.M.; Agazaryan, N.; et al. A Prospective Phase 2 Study Evaluating Safety and Efficacy of Combining Stereotactic Body Radiation Therapy With Heat-based Ablation for Centrally Located Lung Tumors. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 564–573. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, H.; Li, Y.; Xiao, W.; Liu, Y.; Chen, R.; Zhu, Y.; Zheng, X.; Wu, C.; Chen, L. TIGIT Blockade Exerts Synergistic Effects on Microwave Ablation Against Cancer. Front. Immunol. 2022, 13, 832230. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, M.; Pan, H.; Qiu, W.; Wang, H.; Qian, M.; Che, N.; Zhang, K.; Mao, X.; Li, L.; et al. Microwave ablation induces Th1-type immune response with activation of ICOS pathway in early-stage breast cancer. J. Immunother. Cancer 2021, 9, e002343. [Google Scholar] [CrossRef] [PubMed]
- Jauchem, J.R. Effects of low-level radio-frequency (3 kHz to 300 GHz) energy on human cardiovascular, reproductive, immune, and other systems: A review of the recent literature. Int. J. Hyg. Environ. Health 2008, 211, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Bland, R.D.; Clarke, T.L.; Harden, L.B. Rapid infusion of sodium bicarbonate and albumin into high-risk premature infants soon after birth: A controlled, prospective trial. Am. J. Obstet. Gynecol. 1976, 124, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Choi, H.D.; Kim, B.C.; Pack, J.K.; Kim, N.; Lee, Y.S. Effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic fields on serum hormone levels in rats. J. Radiat. Res. 2013, 54, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.B.; Pyun, B.J.; Jin, H.; Choi, H.D.; Pack, J.K.; Kim, N.; Lee, Y.S. Effects of simultaneous combined exposure to CDMA and WCDMA electromagnetic field on immune functions in rats. Int. J. Radiat. Biol. 2012, 88, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Li, W.; Wang, H.; Zhang, J.; Wang, H.; Dong, J.; Yao, B.; Xu, X.; Zhao, L.; Peng, R. Microwave radiation induces neuronal autophagy through miR-30a-5p/AMPKα2 signal pathway. Biosci. Rep. 2022, 42, BSR20212584. [Google Scholar] [CrossRef]
- Wu, H.; Wang, D.; Meng, Y.; Ning, H.; Liu, X.; Xie, Y.; Cui, L.; Wang, S.; Xu, X.; Peng, R. Activation of TLR signalling regulates microwave radiation-mediated impairment of spermatogenesis in rat testis. Andrologia 2018, 50, e12828. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Vyas, A.; Agnihotri, S.K.; Chattopadhyay, N.; Sachdev, M. Small secretory proteins of immune cells can modulate gynecological cancers. Semin. Cancer Biol. 2022, 86, 513–531. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Xu, H.; Yu, Y.; Xu, Z. Regulatory roles of cytokines in T and B lymphocytes-mediated immunity in teleost fish. Dev. Comp. Immunol. 2023, 104621. [Google Scholar] [CrossRef]
- Yao, C.; Wang, H.; Sun, L.; Ren, K.; Dong, J.; Wang, H.; Zhang, J.; Xu, X.; Yao, B.; Zhou, H.; et al. The Biological Effects of Compound Microwave Exposure with 2.8 GHz and 9.3 GHz on Immune System: Transcriptomic and Proteomic Analysis. Cells 2022, 11, 3849. [Google Scholar] [CrossRef]
- Panagopoulos, D.J.; Karabarbounis, A.; Yakymenko, I.; Chrousos, G.P. Human-made electromagnetic fields: Ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage (Review). Int. J. Oncol. 2021, 59, 92. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, A.; Mazurek, P.A.; Wdowiak, A.; Bojar, I. Effect of electromagnetic waves on human reproduction. Ann. Agric. Environ. Med. 2017, 24, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Yang, Y.F.; Gao, Y.B.; Wang, S.M.; Wang, L.F.; Zuo, H.Y.; Dong, J.; Xu, X.P.; Su, Z.T.; Zhou, H.M.; et al. Upregulation of HIF-1alpha via activation of ERK and PI3K pathway mediated protective response to microwave-induced mitochondrial injury in neuron-like cells. Mol. Neurobiol. 2014, 50, 1024–1034. [Google Scholar] [CrossRef]
- Hao, Y.H.; Zhang, J.; Wang, H.; Wang, H.Y.; Dong, J.; Xu, X.P.; Yao, B.W.; Wang, L.F.; Zhou, H.M.; Zhao, L.; et al. HIF-1alpha regulates COXIV subunits, a potential mechanism of self-protective response to microwave induced mitochondrial damages in neurons. Sci. Rep. 2018, 8, 10403. [Google Scholar] [CrossRef]
- Hao, Y.H.; Zhao, L.; Peng, R.Y. Effects of microwave radiation on brain energy metabolism and related mechanisms. Mil. Med. Res. 2015, 2, 4. [Google Scholar] [CrossRef]
- Durdik, M.; Kosik, P.; Markova, E.; Somsedikova, A.; Gajdosechova, B.; Nikitina, E.; Horvathova, E.; Kozics, K.; Davis, D.; Belyaev, I. Microwaves from mobile phone induce reactive oxygen species but not DNA damage, preleukemic fusion genes and apoptosis in hematopoietic stem/progenitor cells. Sci. Rep. 2019, 9, 16182. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S.; Choi, E.H.; Han, I. ROS production in response to high-power microwave pulses induces p53 activation and DNA damage in brain cells: Radiosensitivity and biological dosimetry evaluation. Front. Cell Dev. Biol. 2023, 11, e1067861. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Dong, J.; Ren, K.; Sun, L.; Wang, H.; Zhang, J.; Wang, H.; Xu, X.; Yao, B.; Zhou, H.; et al. Accumulative Effects of Multifrequency Microwave Exposure with 1.5 GHz and 2.8 GHz on the Structures and Functions of the Immune System. Int. J. Environ. Res. Public Health 2023, 20, 4988. https://doi.org/10.3390/ijerph20064988
Yao C, Dong J, Ren K, Sun L, Wang H, Zhang J, Wang H, Xu X, Yao B, Zhou H, et al. Accumulative Effects of Multifrequency Microwave Exposure with 1.5 GHz and 2.8 GHz on the Structures and Functions of the Immune System. International Journal of Environmental Research and Public Health. 2023; 20(6):4988. https://doi.org/10.3390/ijerph20064988
Chicago/Turabian StyleYao, Chuanfu, Ji Dong, Ke Ren, Liu Sun, Hui Wang, Jing Zhang, Haoyu Wang, Xinping Xu, Binwei Yao, Hongmei Zhou, and et al. 2023. "Accumulative Effects of Multifrequency Microwave Exposure with 1.5 GHz and 2.8 GHz on the Structures and Functions of the Immune System" International Journal of Environmental Research and Public Health 20, no. 6: 4988. https://doi.org/10.3390/ijerph20064988
APA StyleYao, C., Dong, J., Ren, K., Sun, L., Wang, H., Zhang, J., Wang, H., Xu, X., Yao, B., Zhou, H., Zhao, L., & Peng, R. (2023). Accumulative Effects of Multifrequency Microwave Exposure with 1.5 GHz and 2.8 GHz on the Structures and Functions of the Immune System. International Journal of Environmental Research and Public Health, 20(6), 4988. https://doi.org/10.3390/ijerph20064988




