Bioremediation of Heavy Metals by the Genus Bacillus
Abstract
1. Introduction
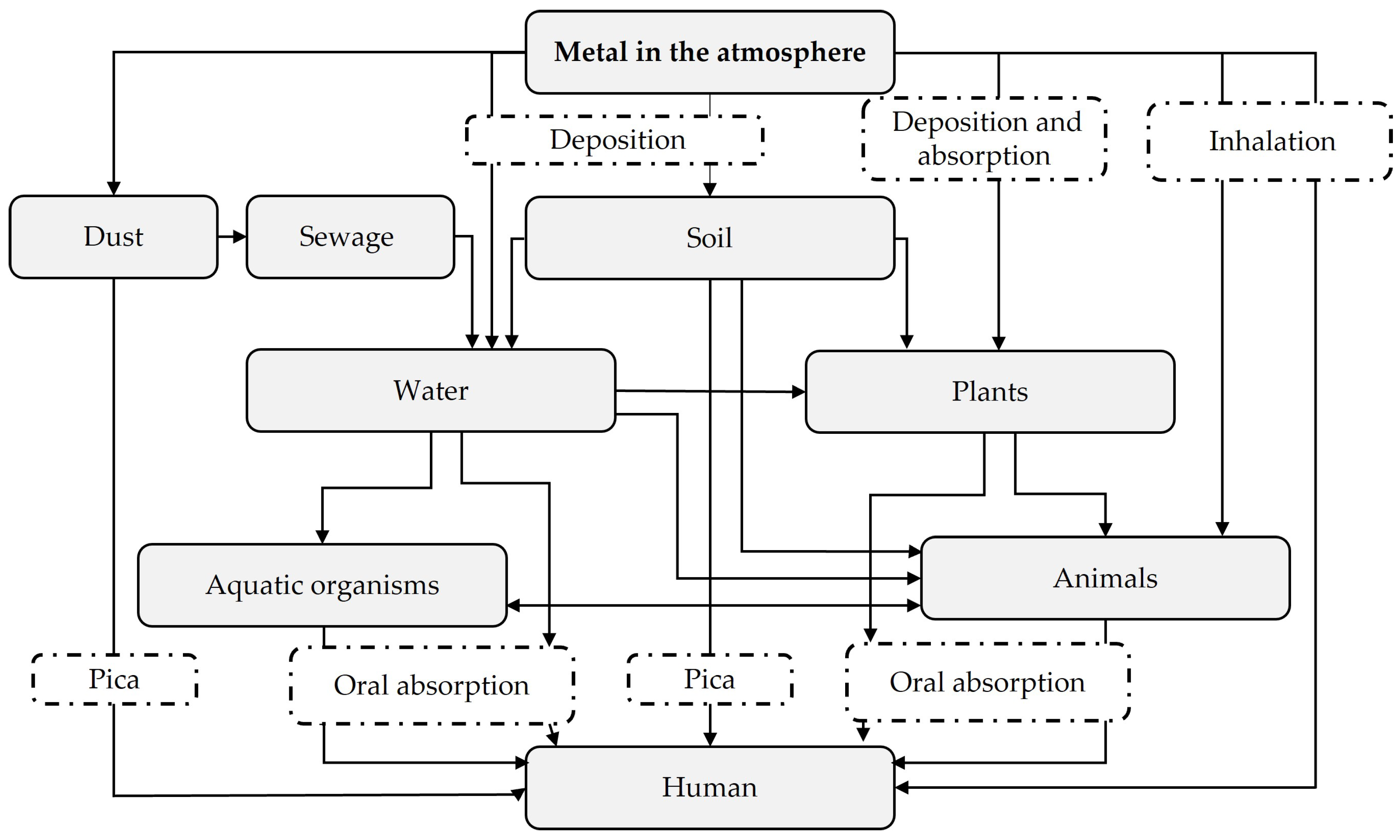
2. Scale of Heavy Metal Contamination
3. Impacts of Heavy Metal Pollution on Environment and Human Health
4. Heavy Metal Bioremediation Strategies Detected in Bacillus
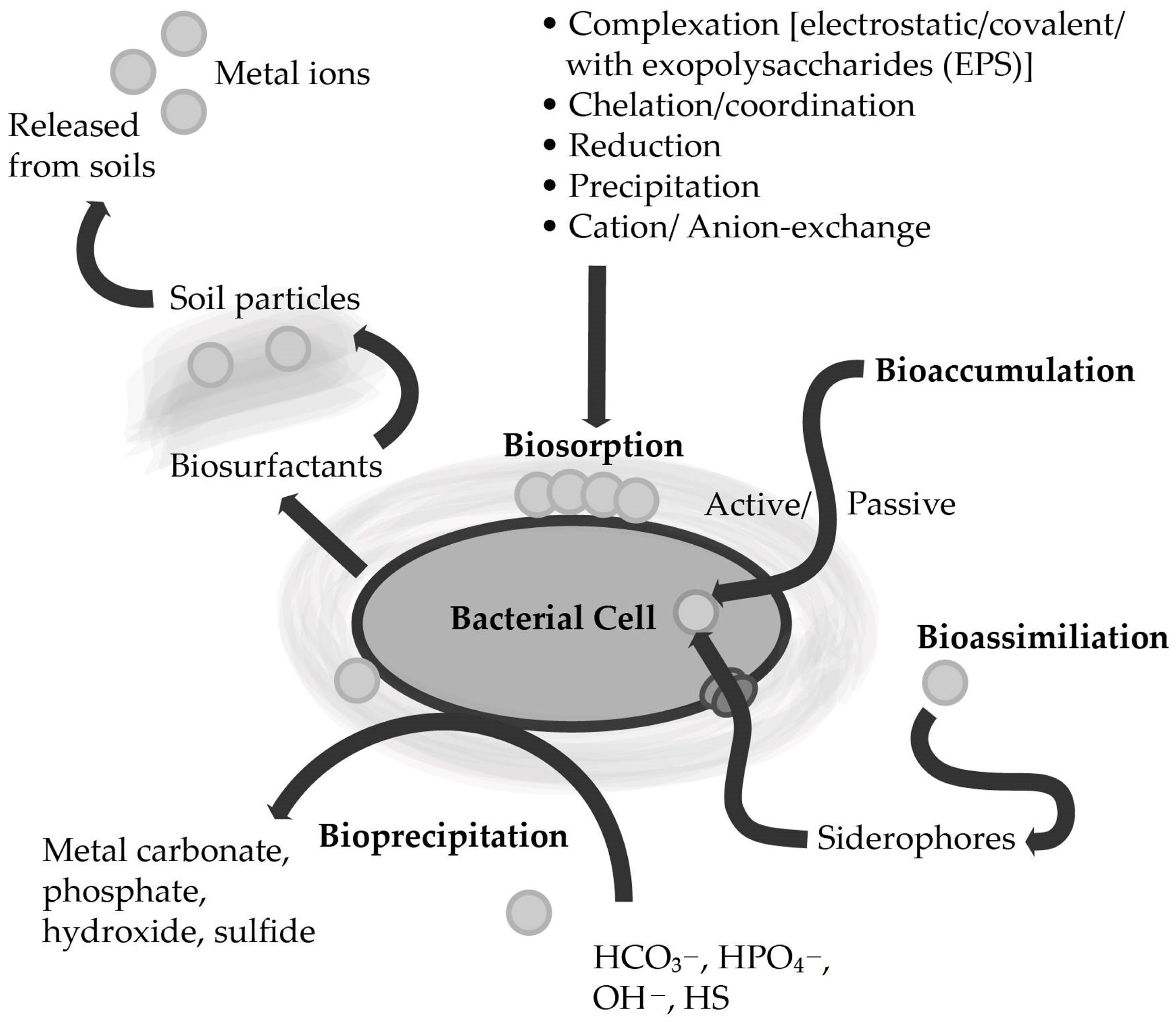
4.1. Biosorption
4.2. Bioremediation by Extracellular Polymeric Substances (EPS)
4.3. Bioaccumulation
4.4. Bioprecipitation
4.5. Biological Removal of Heavy Metals Using Plant Growth-Promoting Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cegiełkowska, W.; Michalska-Kacymirow, M.; Wierzbicka, M. Heavy metals in the environment. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale], 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; pp. 22–23, 31–36. ISBN 978-83-235-1854-9. [Google Scholar]
- Gholizadeh, M.; Hu, X. Removal of heavy metals from soil with biochar composite: A critical review of the mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Kacprzak, M.; Fijałkowski, K. Trace elements and heavy metals. Occurrence, toxicity. In Potential of Plants to Clean Up the Environment [Fitoremediacja. Potencjał Roślin do Oczyszczania Środowiska], 1st ed.; Charitonow, E., Ed.; Scientific Publishers PWN: Warsaw, Poland, 2020; pp. 6–11. ISBN 978-830-121-170-7. [Google Scholar]
- Ociepa-Kubicka, A.; Ociepa, E. Toxic effects of heavy metals on plants, animals and humans. Inż. Ochr. Śr. 2012, 15, 169–180. Available online: https://www.infona.pl/resource/bwmeta1.element.baztech-article-LODD-0002-0015/tab/summary (accessed on 11 March 2015).
- Rostański, A.; Cabala, J.; Slota, M. Environmental exposure to heavy metals. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale], 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; pp. 522–541. ISBN 978-83-235-1854-9. [Google Scholar]
- Kumar, A.; Kumar, A.; Cabral-Pinto, M.; Chaturvedi, A.K.; Shabnam, A.A.; Subrahmanyam, G.; Mondal, R.; Gupta, D.K.; Malyan, S.K.; Kumar, S.S.; et al. Lead toxicity: Health hazards, influence on food chain, and sustainable remediation approaches. Int. J. Environ. Res. Public Health 2020, 17, 2179. [Google Scholar] [CrossRef]
- Liu, X.; Shunbin, G.; Shiyan, Y.; Jinsong, D. Heavy metals in soil-vegetable system around E-waste site and the health risk assessment. Sci. Total Environ. 2021, 779, 146438. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Chung, W. Bacterial biosorbents, an efficient heavy metals green clean-up strategy: Prospects, challenges, and opportunities. Microorganisms 2022, 10, 610. [Google Scholar] [CrossRef]
- Dell’Anno, F.; Brunet, C.; van Zyl, L.J.; Trindade, M.; Golyshin, P.N.; Dell’Anno, A.; Ianora, A.; Sansone, C. Degradation of hydrocarbons and heavy metal reduction by marine bacteria in highly contaminated sediments. Microorganisms 2020, 8, 1402. [Google Scholar] [CrossRef]
- United Nations Home Page. Available online: https://www.un.org/sustainabledevelopment/sustainable-development-goals/ (accessed on 25 September 2015).
- Wang, L.; Rinklebe, J.; Tack, F.M.; Hou, D. A review of green remediation strategies for heavy metal contaminated soil. Soil Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Shamim, S. Biosorption of Heavy Metals; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Das, A.; Osborne, J.W. Bioremediation of heavy metals. In Nanotechnology, Food Security and Water Treatment. Environmental Chemistry for a Sustainable World, 1st ed.; Gothandam, K.M., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2018; Volume 9, pp. 277–311. ISBN 978-331-970-165-3. [Google Scholar]
- Guo, H.; Hanjun, S.; Chen, L.; Xiao, X.; Xi, Q.; Wei, W.; Zeng, G.; Liu, C.; Wan, Y.; Chen, J.; et al. Bioremediation of heavy metals by growing hyperaccumulator endophytic bacterium Bacillus sp. L14. Bioresour. Technol. 2010, 101, 8599–8605. [Google Scholar] [CrossRef]
- Sadowska, A.; Obidoska, G.; Rumowska, M. Heavy metals. In Ecotoxicology. Toxic Environmental Agents and Methods of Their Detection [Ekotoksykologia. Toksyczne Czynniki Środowiskowe i Metody ich Wykrywania]; SGGW Publishing: Warsaw, Poland, 2000; pp. 54–57. ISBN 837-244-171-5. [Google Scholar]
- Niklińska, M.; Stefanowicz, A.M. Soil microorganisms in metal-bearing areas. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale], 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; p. 210. ISBN 978-83-235-1854-9. [Google Scholar]
- Dadrasnia, A.; Chuan Wei, K.S.; Shahsavari, N.; Azirun, M.S.; Ismail, S. Biosorption potential of Bacillus salmalaya strain 139SI for removal of Cr (VI) from aqueous solution. Int. J. Environ. Res. 2015, 12, 15321–15338. [Google Scholar] [CrossRef]
- Ontañon, O.M.; Fernandez, M.; Agostini, E.; González, P.S. Identification of the main mechanisms involved in the tolerance and bioremediation of Cr (VI) by Bacillus sp. SFC 500-1E. Environ. Sci. Pollut. Res. 2018, 25, 16111–16120. [Google Scholar] [CrossRef]
- Heidari, P.; Panico, A. Sorption mechanism and optimization study for the bioremediation of Pb (II) and Cd (II) contamination by two novel isolated strains Q3 and Q5 of Bacillus sp. Int. J. Environ. Res. Public Health 2020, 17, 4059. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Khan, M.; Shamim, S. Unraveling the underlying heavy metal detoxification mechanisms of Bacillus species. Microorganisms 2021, 9, 1628. [Google Scholar] [CrossRef]
- Babar, Z.; Khan, M.; Chotana, G.A.; Murtaza, G.; Shamim, S. Evaluation of the potential role of Bacillus altitudinis MT422188 in nickel bioremediation from contaminated industrial effluents. Sustainability 2021, 13, 7353. [Google Scholar] [CrossRef]
- Alou, M.T.; Rathored, J.; Khelaifia, S.; Michelle, C.; Brah, S.; Diallo, B.A.; Raoult, D.; Lagier, J.-C. Bacillus rubiinfantis sp. nov. strain mt2T, a new bacterial species isolated from human gut. New Microbes New Infect. 2015, 8, 51–60. [Google Scholar] [CrossRef]
- Kotb, E. Purification and partial characterization of serine fibrinolytic enzyme from Bacillus megaterium KSK-07 isolated from kishk, a traditional Egyptian fermented food. Appl. Biochem. Microbiol. 2015, 51, 34–43. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus probiotics: An alternative to antibiotics for livestock production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Qin, Y.; Angelini, L.L.; Chai, Y. Bacillus subtilis cell differentiation, biofilm formation and environmental prevalence. Microorganisms 2022, 10, 1108. [Google Scholar] [CrossRef]
- Zocca, V.F.B.; Corrêa, G.G.; Lins, M.R.D.C.R.; de Jesus, V.N.; Tavares, L.F.; Amorim, L.A.D.S.; Kundlatsch, G.E.; Pedrolli, D.B. The CRISPR toolbox for the gram-positive model bacterium Bacillus subtilis. Crit. Rev. Biotechnol. 2022, 42, 813–826. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Islam, N.U.; Ullah, M.; Mahnashi, M.H.; Ali, A.A.; Jalal, N.A.; et al. Bacillus subtilis: As an efficient bacterial strain for the reclamation of water loaded with textile azo dye, orange II. Int. J. Mol. Sci. 2022, 23, 10637. [Google Scholar] [CrossRef]
- Elshaghabee, F.M.F.; Rokana, N.; Gulhane, R.D.; Sharma, C.; Panwar, H. Bacillus as potential probiotics: Status, concerns, and future perspectives. Front. Microbiol. 2017, 8, 1490. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Biotechnol. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef]
- Fira, D.; Dimkić, I.; Berić, T.; Lozo, J.; Stanković, S. Biological control of plant pathogens by Bacillus species. J. Biotechnol. 2018, 285, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Kuebutornye, F.K.; Abarike, E.D.; Lu, Y. A review on the application of Bacillus as probiotics in aquaculture. Fish. Shellfish. Immunol. 2019, 87, 820–828. [Google Scholar] [CrossRef]
- Miljaković, D.; Marinković, J.; Balešević-Tubić, S. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms 2020, 8, 1037. [Google Scholar] [CrossRef] [PubMed]
- Ngalimat, M.S.; Yahaya, R.S.R.; Baharudin, M.M.A.-A.; Yaminudin, S.M.; Karim, M.; Ahmad, S.A.; Sabri, S. A review on the biotechnological applications of the operational group Bacillus amyloliquefaciens. Microorganisms 2021, 9, 614. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Tran, C.; Cock, I.E.; Chen, X.; Feng, Y. Antimicrobial Bacillus: Metabolites and their mode of action. Antibiotics 2022, 11, 88. [Google Scholar] [CrossRef]
- Tirloni, E.; Stella, S.; Celandroni, F.; Mazzantini, D.; Bernardi, C.; Ghelardi, E. Bacillus cereus in dairy products and production plants. Foods 2022, 11, 2572. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Qi, X.; Gao, H.; Wang, M.; Guan, H.; Yu, B. Metabolic engineering of Bacillus subtilis for riboflavin production: A review. Microorganisms 2023, 11, 164. [Google Scholar] [CrossRef]
- Hodgson, E. Introduction to toxicology. In A Textbook of Modern Toxicology, 4th ed.; Hodgson, E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 3–14. ISBN 978-0-470-46206-5. [Google Scholar]
- European Environment Agency Home Page. Heavy Metal Emissions in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/eea32-heavy-metal-hm-emissions-1/assessment-10 (accessed on 4 September 2019).
- European Environment Agency Home Page. Industrial Pollutant Releases to Air in Europe. Available online: https://www.eea.europa.eu/data-and-maps/indicators/industrial-pollution-in-europe-3/assessment (accessed on 13 September 2019).
- Mohammadi, A.A.; Zarei, A.; Esmaeilzadeh, M.; Taghavi, M.; Yousefi, M.; Yousefi, Z.; Sedighi, F.; Javan, S. Assessment of heavy metal pollution and human health risks assessment in soils around an industrial zone in Neyshabur, Iran. Biol. Trace Elem. Res. 2020, 195, 343–352. [Google Scholar] [CrossRef]
- Sun, Y.; Li, H.; Guo, G.; Semple, K.T.; Jones, K.C. Soil contamination in China: Current priorities, defining background levels and standards for heavy metals. J. Environ. Manag. 2019, 251, 109512. [Google Scholar] [CrossRef]
- Barsova, N.; Yakimenko, O.; Tolpeshta, I.; Motuzova, G. Current state and dynamics of heavy metal soil pollution in Russian Federation–A review. Environ. Pollut. 2019, 249, 200–207. [Google Scholar] [CrossRef]
- Ramon, F.; Lull, C. Legal measures to prevent and manage soil contamination and to increase food safety for consumer health: The case of Spain. Environ. Pollut. 2019, 250, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Diarra, I.; Prasad, S. The current state of heavy metal pollution in Pacific Island Countries: A review. Appl. Spectrosc. Rev. 2020, 56, 27–51. [Google Scholar] [CrossRef]
- Solademi, F.; Thompson, S. Spatial analysis of heavy metal emissions in residential, commercial and industrial areas adjacent to a scrap metal shredder in Winnipeg, Canada. J. Geosci. Environ. Prot. 2020, 8, 359–386. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Biogeochemistry of Trace Elements [Biogeochemia Pierwiastków Śladowych], 2nd ed.; Rajpert, M., Ed.; Scientific Publishers PWN: Warsaw, Poland, 1999; ISBN 83-01128-23-2. [Google Scholar]
- Sher, S.; Abdul, R. Use of heavy metals resistant bacteria-a strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 2019, 103, 6007–6021. [Google Scholar] [CrossRef]
- Wierzbicka, M. Plant defense against heavy metals. In Ecotoxicology. Plants, Soils, Metals [Ekotoksykologia. Rośliny, Gleby, Metale], 1st ed.; Wierzbicka, M., Ed.; Warsaw University Press: Warsaw, Poland, 2015; p. 83. ISBN 978-83-235-1854-9. [Google Scholar]
- Seńczuk, W. Modern Toxicology [Toksykologia Współczesna], 2nd ed.; Seńczuk, W., Ed.; PZWL: Warsaw, Poland, 2012; ISBN 978-83-200-6099-7. [Google Scholar]
- Kozłowska, A.; Mikołajczyk, A.; Boroń, M.; Kasperczyk, S.; Pawlas, N. Effects of lead exposure on the concentration of cadmium, selenium and values of morphology in the blood. Environ. Med. 2015, 18, 17–25. [Google Scholar]
- International Agency for Research on Cancer Home Page. Available online: https://monographs.iarc.who.int/list-of-classifications (accessed on 7 September 2022).
- Sharma, B.; Shukla, P. Lead bioaccumulation mediated by Bacillus cereus BPS-9 from an industrial waste contaminated site encoding heavy metal resistant genes and their transporters. J. Hazard. Mater. 2021, 401, 123285. [Google Scholar] [CrossRef] [PubMed]
- Pande, V.; Pandey, S.C.; Sati, D.; Bhatt, P.; Samant, M. Microbial interventions in bioremediation of heavy metal contaminants in agroecosystem. Front. Microbiol. 2022, 13, 824084. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Li, M.; Teng, Z.; Qiu, B.; Huo, Y.; Zhang, K. Effects of Pb (II) and Cr (VI) stress on phosphate-solubilizing bacteria (Bacillus sp. strain MRP-3): Oxidative stress and bioaccumulation potential. Int. J. Environ. Res. Public Health. 2019, 16, 2172. [Google Scholar] [CrossRef]
- Ahemad, M. Remediation of metalliferous soils through the heavy metal resistant plant growth promoting bacteria: Paradigms and prospects. Arab. J. Chem. 2019, 12, 1365–1377. [Google Scholar] [CrossRef]
- Zabochnicka-Świątek, M.; Krzywonos, M. Potentials of biosorption and bioaccumulation processes for heavy metal removal. Pol. J. Environ. Stud. 2014, 23, 551–561. [Google Scholar]
- Tiquia-Arashiro, S.M. Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol. 2018, 102, 5437–5444. [Google Scholar] [CrossRef]
- Babák, L.; Šupinova, P.; Zichova, M.; Burdychova, R.; Vitova, E. Biosorption of Cu, Zn and Pb by thermophilic bacteria–effect of biomass concentration on biosorption capacity. Acta Univ. Agric. Silvic. Mendel. Brun. 2012, 60, 9–18. [Google Scholar] [CrossRef]
- Das, P.; Sinha, S.; Mukherjee, S.K. Nickel bioremediation potential of Bacillus thuringiensis KUNi1 and some environmental factors in nickel removal. Bioremediat. J. 2014, 18, 169–177. [Google Scholar] [CrossRef]
- Khan, M.; Ijaz, M.; Chotana, G.A.; Murtaza, G.; Malik, A.; Shamim, S. Bacillus altitudinis MT422188: A potential agent for zinc bioremediation. Bioremediat. J. 2022, 26, 228–248. [Google Scholar] [CrossRef]
- Chen, X.; Lin, H.; Dong, Y.; Li, B.; Liu, C.; Yin, T. Mechanisms underlying enhanced bioremediation of sulfamethoxazole and zinc (II) by Bacillus sp. SDB4 immobilized on biochar. J. Clean. Prod. 2020, 370, 133483. [Google Scholar] [CrossRef]
- Oves, M.; Khanm, M.S.; Zaidim, A.; Ahmadm, E. Soil contamination, nutritive value and human health risk assessment of heavy metals: An overview. In Toxicity of Heavy Metals to Legumes and Bioremediation, 1st ed.; Zaidi, A., Wani, P., Khan, M., Eds.; Springer: Vienna, Austria, 2012; pp. 1–27. ISBN 978-3-7091-0729-4. [Google Scholar]
- Sinha, A.; Pant, K.K.; Khare, S.K. Studies on mercury bioremediation by alginate immobilized mercury tolerant Bacillus cereus cells. Int. Biodeterior. Biodegrad. 2012, 2071, 1–8. [Google Scholar] [CrossRef]
- Chen, Z.; Pan, X.; Chen, H.; Lin, Z.; Guan, X. Investigation of lead (II) uptake by Bacillus thuringiensis 016. World J. Microbiol. Biotechnol. 2015, 31, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Hassan, S.H.; Oh, S.E. Comparative study of biosorption of Zn2+ by Pseudomonas aeruginosa and Bacillus cereus. Int. Biodeter. Biodegrad. 2010, 64, 734–741. [Google Scholar] [CrossRef]
- Giri, A.; Patel, R.K.; Mahapatra, S.S.; Mishra, P.C. Biosorption of arsenic (III) from aqueous solution by living cells of Bacillus cereus. Environ. Sci. Pollut. Res. 2013, 20, 1281–1291. [Google Scholar] [CrossRef] [PubMed]
- Altowayti, W.A.H.; Algaifi, H.A.; Bakar, S.A.; Shahir, S. The adsorptive removal of As (III) using biomass of arsenic resistant Bacillus thuringiensis strain WS3: Characteristics and modelling studies. Ecotoxicol. Environ. Saf. 2019, 172, 176–185. [Google Scholar] [CrossRef]
- Kumawat, T.K.; Kumawat, V.; Sharma, S.; Kandwani, N.; Biyani, M. Applications of EPS in environmental bioremediations. In Microbial Exopolysaccharides as Novel and Significant Biomaterials, 1st ed.; Nadda, A.K., Sajna, K.V., Sharma, S., Eds.; Springer: Cham, Switzerland, 2021; pp. 285–302. ISBN 978-3-030-75288-0. [Google Scholar]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Salehizadeh, H.; Shojaosadati, S.A. Removal of metal ions from aqueous solution by polysaccharide produced from Bacillus firmus. Water Res. 2003, 37, 4231–4235. [Google Scholar] [CrossRef]
- Krishnamurthy, M.; Uthaya, C.J.; Thangavel, M.; Annadurai, V.; Rajendran, R.; Gurusamy, A. Optimization, compositional analysis, and characterization of exopolysaccharides produced by multi-metal resistant Bacillus cereus KMS3-1. Carbohydr. Polym. 2020, 227, 115369. [Google Scholar] [CrossRef]
- Kalpana, R.; Angelaalincy, M.J.; Kamatchirajan, B.V.; Vasantha, V.S.; Ashokkumar, B.; Ganesh, V.; Varalakshmi, P. Exopolysaccharide from Bacillus cereus VK1: Enhancement, characterization and its potential application in heavy metal removal. Colloids Surf. B Biointerfaces 2018, 171, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Morillo Pérez, J.A.; García-Ribera, R.; Quesada, T.; Aguilera, M.; Ramos-Cormenzana, A.; Monteoliva-Sánchez, M. Biosorption of heavy metals by the exopolysaccharide produced by Paenibacillus jamilae. World J. Microbiol. Biotechnol. 2008, 24, 2699–2704. [Google Scholar] [CrossRef]
- Issazadeh, K.; Jahanpour, N.; Pourghorbanali, F.; Raeisi, G.; Faekhondeh, J. Heavy metals resistance by bacterial strains. Ann. Biol. Res. 2013, 4, 60–63. [Google Scholar]
- Vijayaraghavan, K.; Yun, Y.S. Bacterial biosorbents and biosorption. Biotechnol. Adv. 2008, 26, 266–291. [Google Scholar] [CrossRef]
- Srinath, T.; Verma, T.; Ramteke, P.W.; Garg, S.K. Chromium (VI) biosorption and bioaccumulation by chromate resistant bacteria. Chemosphere 2002, 48, 427–435. [Google Scholar] [CrossRef]
- Hamer, D.H. Metallothioneins. Annu. Rev. Biochem. 1986, 55, 913–951. [Google Scholar] [CrossRef]
- Blindauer, C.A.; Harrison, M.D.; Robinson, A.K.; Parkinson, J.A.; Bowness, P.W.; Sadler, P.J.; Robinson, N.J. Multiple bacteria encode metallothione in sand SmtA-like fingers. Mol. Microbiol. 2002, 45, 1421–1432. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Nakashima, S.; Hirose, K.; Uemura, Y.; Shibasaka, M.; Katsuhara, M.; Kasamo, K. A metallothionein and CPx-ATPase handle heavy-metal tolerance in the filamentous cyanobacteria Oscillatoria brevis. FEBS Lett. 2003, 542, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Dash, H.R.; Chakraborty, J. Genetic basis and importance of metal resistant genes in bacteria for bioremediation of contaminated environments with toxic metal pollutants. Appl. Microbiol. Biotechnol. 2016, 100, 2967–2984. [Google Scholar] [CrossRef]
- Yang, H.C.; Fu, H.L.; Lin, Y.F.; Rosen, B.P. Pathways of arsenic uptake and efflux. Curr. Top. Membr. 2012, 69, 325–358. [Google Scholar] [CrossRef]
- Borremans, B.; Hobman, J.L.; Provoost, A.; Brown, N.L.; van Der Lelie, D. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 2001, 183, 5651–5658. [Google Scholar] [CrossRef]
- Yu, P.; Yuan, J.; Deng, X.; Ma, M.; Zhang, H. Subcellular targeting of bacterial CusF enhances Cu accumulation and alters root to shoot Cu translocation in Arabidopsis. Plant Cell Physiol. 2014, 55, 1568–1581. [Google Scholar] [CrossRef]
- Kiyono, M.; Oka, Y.; Sone, Y.; Nakamura, R.; Sato, M.H.; Sakabe, K.; Pan-Hou, H. Bacterial heavy metal transporter MerC increases mercury accumulation in Arabidopsis thaliana. Biochem. Eng. J. 2013, 71, 19–24. [Google Scholar] [CrossRef]
- Murthy, S.; Geetha, B.; Sarangi, S.K. Effect of lead on metallothionein concentration in lead-resistant bacteria Bacillus cereus isolated from industrial effluent. Afr. J. Biotechnol. 2011, 10, 15966–15972. [Google Scholar] [CrossRef]
- Huang, F.; Wang, Z.H.; Cai, Y.X.; Chen, S.H.; Tian, J.H.; Cai, K.Z. Heavy metal bioaccumulation and cation release by growing Bacillus cereus RC-1 under culture conditions. Ecotoxicol. Environ. Saf. 2018, 157, 216–226. [Google Scholar] [CrossRef]
- Belapurkar, P.; Goyal, P.; Kar, A. In vitro evaluation of bioremediation capacity of a commercial probiotic, Bacillus coagulans, for chromium (VI) and lead (II) toxicity. J. Pharm. Bioallied Sci. 2016, 8, 272–276. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, S.; Marwa, N.; Pandey, V.; Verma, P.C.; Rathaur, S.; Singh, N. Arsenic mediated modifications in Bacillus aryabhattai and their biotechnological application for arsenic bioremediation. Chemosphere 2016, 164, 524–534. [Google Scholar] [CrossRef]
- Naskar, A.; Majumder, R.; Goswami, M. Bioaccumulation of Ni (II) on growing cells of Bacillus sp.: Response surface modeling and mechanistic insight. nviron. Technol. Innov. 2020, 20, 101057. [Google Scholar] [CrossRef]
- Kaksonen, A.H.; Puhakka, J.A. Sulfate reduction-based bioprocesses for the treatment of acid mine drainage and the recovery of metals. Eng. Life Sci. 2007, 7, 541–564. [Google Scholar] [CrossRef]
- De, J.; Ramaiah, N.; Vardanyan, L. Detoxification of toxic heavy metals by marine bacteria highly resistant to mercury. Mar. Biotechnol. 2008, 10, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, W.; Li, C.; Zhu, R.; Ge, F.; Zheng, Y.; Tang, Y. Self-mediated pH changes in culture medium affecting biosorption and biomineralization of Cd(2+) by Bacillus cereus Cd01. J. Hazard. Mater. 2018, 358, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Molokwane, P.E.; Meli, C.K.; Chirwa, E.M. Chromium (VI) reduction in activated sludge bacteria exposed to high chromium loading: Brits culture (South Africa). Water Sci. Technol. 2008, 58, 399–405. [Google Scholar] [CrossRef]
- Xiao, R.; Liu, X.; Ali, A.; Chen, A.; Zhang, M.; Li, R.; Chang, H.; Zhang, Z. Bioremediation of Cd-spiked soil using earthworms (Eisenia fetida): Enhancement with biochar and bacillus megatherium application. Chemosphere 2021, 264, 128517. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xing, Y.; Huang, B.; Chen, X.; Ji, L.; Fu, X.; Li, T.; Wang, J.; Chen, G.; Zhang, Q. Rhizospheric mechanisms of Bacillus subtilis bioaugmentation-assisted phytostabilization of cadmium-contaminated soil. Sci. Total Environ. 2022, 825, 154136. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zheng, Y.; Ding, C.; Ren, X.; Yuan, J.; Sun, F.; Li, Y. Integrated metagenomics and molecular ecological network analysis of bacterial community composition during the phytoremediation of cadmium-contaminated soils by bioenergy crops. Ecotoxicol. Environ. Saf. 2017, 145, 111–118. [Google Scholar] [CrossRef]
- Jing, R.; Kjellerup, B.V. Biogeochemical cycling of metals impacting by microbial mobilization and immobilization. J. Environ. Sci. 2018, 66, 146–154. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Hussain, I.; Rasheed, R.; Iqbal, M.; Riaz, M.; Arif, M.S. Advances in microbe-assisted reclamation of heavy metal contaminated soils over the last decade: A review. J. Environ. Manag. 2017, 198, 132–143. [Google Scholar] [CrossRef]
- Ren, X.-M.; Guo, S.-J.; Tian, W.; Chen, Y.; Han, H.; Chen, E.; Li, B.-L.; Li, Y.-Y.; Chen, Z.-J. Effects of Plant Growth-Promoting Bacteria (PGPB) inoculation on the growth, antioxidant activity, Cu uptake, and bacterial community structure of rape (Brassica napus L.) grown in Cu-contaminated agricultural soil. Front. Microbiol. 2019, 10, 1455. [Google Scholar] [CrossRef]
- Pandey, S.; Ghosh, P.K.; Ghosh, S.; De, T.K.; Maiti, T.K. Role of heavy metal resistant Ochrobactrum sp. and Bacillus spp. strains in bioremediation of a rice cultivar and their PGPR like activities. J. Microbiol. 2013, 51, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pishchik, V.N.; Filippova, P.S.; Mirskaya, G.V.; Khomyakov, Y.V.; Vertebny, V.E.; Dubovitskaya, V.I.; Ostankova, Y.V.; Semenov, A.V.; Chakrabarty, D.; Zuev, E.V.; et al. Epiphytic PGPB Bacillus megaterium AFI1 and Paenibacillus nicotianae AFI2 improve wheat growth and antioxidant status under Ni Stress. Plants 2021, 10, 2334. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.L.; Chen, L.; Chen, J.L.; Xiao, X.; Xu, T.Y.; Wan, Y.; Rao, C.; Liu, C.B.; Liu, Y.T.; Lai, C.; et al. Analysis and characterization of cultivable heavy metal-resistant bacterial endophytes isolated from Cd-hyperaccumulator Solanum nigrum L. and their potential use for phytoremediation. Chemosphere 2011, 85, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.R.; Ortiz, I.; Maymon, M.; Herbold, C.W.; Fujishige, N.A.; Vijanderan, J.A.; Villella, W.; Hanamoto, K.; Diener, A.; Sanders, E.R.; et al. Bacillus simplex—A little known PGPB with anti-fungal activity—Alters pea legume root architecture and nodule morphology when coinoculated with Rhizobium leguminosarum bv. viciae. Agronomy 2013, 3, 595–620. [Google Scholar] [CrossRef]
- Hiller, J.; Napora, A.; Grobelak, A. Growth promotion by PGBP bacteria. In Innovations in Technological Processes [Innowacje w Procesach Technologicznych], 1st ed.; Bajdur, W.M., Ed.; Wydawnictwo Wydziału Zarządzania Politechniki Częstochowskiej: Częstochowa, Poland, 2016; pp. 74–83. ISBN 978-83-65179-72-2. [Google Scholar]
- Hong, S.; Kim, T.Y.; Won, S.-J.; Moon, J.-H.; Ajuna, H.B.; Kim, K.Y.; Ahn, Y.S. Control of fungal diseases and fruit yield improvement of strawberry using Bacillus velezensis CE 100. Microorganisms 2022, 10, 365. [Google Scholar] [CrossRef]
- Mirskaya, G.V.; Khomyakov, Y.V.; Rushina, N.A.; Vertebny, V.E.; Chizhevskaya, E.P.; Chebotar, V.K.; Chesnokov, Y.V.; Pishchik, V.N. Plant development of early-maturing spring wheat (Triticum aestivum L.) under inoculation with Bacillus sp. V2026. Plants 2022, 11, 1817. [Google Scholar] [CrossRef]
- Mukhtar, T.; Rehman, S.U.; Smith, D.; Sultan, T.; Seleiman, M.F.; Alsadon, A.A.; Amna; Ali, S.; Chaudhary, H.J.; Solieman, T.H.I.; et al. Mitigation of heat stress in Solanum lycopersicum L. by ACC-deaminase and exopolysaccharide producing Bacillus cereus: Effects on biochemical profiling. Sustainability 2020, 12, 2159. [Google Scholar] [CrossRef]
- Mahdi, I.; Fahsi, N.; Hafidi, M.; Allaoui, A.; Biskri, L. Plant growth enhancement using rhizospheric halotolerant phosphate solubilizing bacterium Bacillus licheniformis QA1 and Enterobacter asburiae QF11 isolated from Chenopodium quinoa Willd. Microorganisms 2020, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Nithyapriya, S.; Lalitha, S.; Sayyed, R.Z.; Reddy, M.S.; Dailin, D.J.; El Enshasy, H.A.; Luh Suriani, N.; Herlambang, S. Production, purification, and characterization of bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in sesame. Sustainability 2021, 13, 5394. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Wierzchowski, P.S.; Stępień, W.; Górska, E.B. The reaction of cellulolytic and potentially cellulolytic spore-forming bacteria to various types of crop management and farmyard manure fertilization in bulk soil. Agronomy 2021, 11, 772. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Jakubowska, Z.; Dybek, B. Potential of Bacillus pumilus to directly promote plant growth. Front. Microbiol. 2022, 13, 1069053. [Google Scholar] [CrossRef]
- Khan, N.; Maymon, M.; Hirsch, A.M. Combating Fusarium infection using Bacillus-based antimicrobials. Microorganisms 2017, 5, 75. [Google Scholar] [CrossRef]
- Sibponkrung, S.; Kondo, T.; Tanaka, K.; Tittabutr, P.; Boonkerd, N.; Yoshida, K.-I.; Teaumroong, N. Co-inoculation of Bacillus velezensis strain S141 and Bradyrhizobium strains promotes nodule growth and nitrogen fixation. Microorganisms 2020, 8, 678. [Google Scholar] [CrossRef]
- Kwon, J.-H.; Won, S.-J.; Moon, J.-H.; Lee, U.; Park, Y.-S.; Maung, C.E.H.; Ajuna, H.B.; Ahn, Y.S. Bacillus licheniformis PR2 controls fungal diseases and increases production of jujube fruit under field conditions. Horticulturae 2021, 7, 49. [Google Scholar] [CrossRef]
- Dobrzyński, J.; Wróbel, B.; Górska, E.B. Cellulolytic properties of a potentially lignocellulose-degrading Bacillus sp. 8E1A strain isolated from bulk soil. Agronomy 2022, 12, 665. [Google Scholar] [CrossRef]
- Chiboub, M.; Jebara, S.H.; Saadani, O.; Fatnassi, I.C.; Abdelkerim, S.; Jebara, M. Physiological responses and antioxidant enzyme changes in Sulla coronaria inoculated by cadmium resistant bacteria. J. Plant Res. 2018, 131, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, A.; Khan, M.S. Heavy metal-induced oxidative damage and root morphology alterations of maize (Zea mays L.) plants and stress mitigation by metal tolerant nitrogen-fixing Azotobacter chroococcum. Ecotoxicol. Environ. Saf. 2018, 157, 9–20. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Chai, T.T.; Mohd Bahari, Z.; Abd Manan, F. The role of plant growth promoting bacteria on arsenic removal: A review of existing perspectives. Environ. Technol. Innov. 2020, 17, 100602. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace elements in the soil-plant interface: Phytoavailability, translocation, and phytoremediation–A review. Earth Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Kong, Z.; Glick, B.R. The role of plant growth-promoting bacteria in metal phytoremediation. Adv. Microb. Physiol. 2017, 71, 97–132. [Google Scholar] [CrossRef]
- Wu, J.; Kamal, N.; Hao, H.; Qian, C.; Liu, Z.; Shao, Y.; Zhong, X.; Xu, B. Endophytic Bacillus megaterium BM18-2 mutated for cadmium accumulation and improving plant growth in Hybrid Pennisetum. Biotechnol. Rep. 2019, 24, e00374. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. A plant growth promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998, 64, 3663–3668. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Penrose, D.M.; Li, J. A model for lowering of plant ethylene concentrations by plant-growth-promoting bacteria. J. Theor. Biol. 1998, 190, 63–68. [Google Scholar] [CrossRef]
- Hussein, K.A.; Joo, J.H. Potential of siderophore production by bacteria isolated from heavy metal: Polluted and rhizosphere soils. Curr. Microbiol. 2014, 68, 717–723. [Google Scholar] [CrossRef]
- Huo, Y.; Kang, J.P.; Ahn, J.C.; Kim, Y.J.; Piao, C.H.; Yang, D.U.; Yang, D.C. Siderophore-producing rhizobacteria reduce heavy metal-induced oxidative stress in Panax ginseng Meyer. J. Ginseng Res. 2021, 45, 218–227. [Google Scholar] [CrossRef]
- Caracciolo, A.B.; Terenzi, V. Rhizosphere microbial communities and heavy metals. Microorganisms 2021, 9, 1462. [Google Scholar] [CrossRef]
- Rajkumar, M.; Ae, N.; Prasad, M.N.V.; Freitas, H. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010, 28, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Roskova, Z.; Skarohlid, R.; McGachy, L. Siderophores: An alternative bioremediation strategy? Sci. Total Environ. 2022, 819, 153144. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of Bacillus subtilis strain SJ-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in Brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R. Using soil bacteria to facilitate phytoremediation. Biotechnol. Adv. 2010, 28, 367–374. [Google Scholar] [CrossRef]
- Sessitsch, A.; Kuffner, M.; Kidd, P.; Vangronsveld, J.; Wenzel, W.W.; Fallmann, K.; Puschenreiter, M. The role of plant-associated bacteria in the mobilization and phytoextraction of trace elements in contaminated soils. Soil Biol. Biochem. 2013, 60, 182–194. [Google Scholar] [CrossRef]
- He, L.Y.; Chen, Z.J.; Ren, G.D.; Zhang, Y.F.; Qian, M.; Sheng, X.F. Increased cadmium and lead uptake of a cadmium hyperaccumulator tomato by cadmium-resistant bacteria. Ecotoxicol. Environ. Saf. 2009, 72, 1343–1348. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.; Ghanem, K.; Ghanem, N.; Al-Kolaibe, A. The role of bacteria on heavy-metal extraction and uptake by plants growing on multi-metal-contaminated soils. World J. Microbiol. Biotechnol. 2008, 24, 253–262. [Google Scholar] [CrossRef]
- Saran, A.; Imperato, V.; Fernandez, L.; Gkorezis, P.; D′Haen, J.; Merini, L.J.; Vangronsveld, J.; Thijs, S. Phytostabilization of polluted military soil supported by bioaugmentation with PGP-trace element tolerant bacteria isolated from Helianthus petiolaris. Agronomy 2020, 10, 204. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Mohammed Ghilan, A.-K.; Valan Arasu, M. Optimizing the management of cadmium bioremediation capacity of metal-resistant Pseudomonas sp. strain Al-Dhabi-126 isolated from the industrial city of Saudi Arabian Environment. Int. J. Environ. Res. Public Health 2019, 16, 4788. [Google Scholar] [CrossRef]
- Malekzadeh, E.; Alikhani, H.A.; Savaghebi-Firoozabadi, G.R.; Zarei, M. Bioremediation of cadmium-contaminated soil through cultivation of maize inoculated with plant growth–promoting rhizobacteria. Bioremed. J. 2012, 16, 204–211. [Google Scholar] [CrossRef]
- Pinter, M.I.F.; Salomon, M.V.; Berli, F.; Gil, R.; Bottini, R.; Piccoli, P. Plant growth promoting rhizobacteria alleviate stress by AsIII in grapevine. Agric. Ecosyst. Environ. 2018, 267, 100–108. [Google Scholar] [CrossRef]
- He, C.Q.; Tan, G.; Liang, X.; Du, W.; Chen, Y.; Zhi, G.; Zhu, Y. Effect of Zn-tolerant bacterial strains on growth and Zn accumulation in Orychophragmus violaceus. Appl. Soil Ecol. 2010, 44, 1–5. [Google Scholar] [CrossRef]
- Ma, Y.; Rajkumar, M.; Freitas, H. Improvement of plant growth and nickel uptake by nickel resistant-plant-growth promoting bacteria. J. Hazard. Mater. 2009, 66, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; He, L.; Wang, Q.; Ye, H.; Jiang, C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008, 155, 17–22. [Google Scholar] [CrossRef] [PubMed]
| Strains | Plant | Bioremediated Metal | PGP Traits | PGP Effects | References |
|---|---|---|---|---|---|
| Bacillus sp. RJ16 | Solanum lycopersicum | Cd and Pb | IAA, siderophores and ACC deaminase | Stimulatation of tomato root growth | He et al. [135] |
| Bacillus cereus SRA10 | Brassica juncea | Ni | IAA, siderophores | Overall plant growth promotion | Ma et al. [142] |
| Bacillus sp. Ba32 | Brassica juncea | Cr | Siderophores | Increase in root and shoot length | Rajkumar et al. [143] |
| Bacillus proteolyticus ST89 | Helianthus annuus | Cd and Pb | IAA | Increase in biomass production | Saran et al. [137] |
| Bacillusparamycoides ST9 | Helianthus annuus | Cd and Pb | ⎼ | Increase in shoot biomass production | Saran, et al. [137] |
| Bacillus sp. J119 | Brassica napus Huiyou-50, Zea mays Denhai-11, Sorghum bicolor × Sorghum sudanense, Lycopersicon esculentum Shanghai-906 | Cd, Pb, Zn and Cu | IAA | Increase in stem length | Sheng et al. [144] |
| Bacillus subtilis SJ-101 | Brassica juncea | Ni | IAA | Increase in growth of above-ground tissue and root | Zaidi et al. [132] |
| Bacillus megaterium BM18-2 | Pennisetum americanum × Pennisetum purpureum Schumach | Cd | IAA | Increase in shoot and root length | Wu et al. [122] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróbel, M.; Śliwakowski, W.; Kowalczyk, P.; Kramkowski, K.; Dobrzyński, J. Bioremediation of Heavy Metals by the Genus Bacillus. Int. J. Environ. Res. Public Health 2023, 20, 4964. https://doi.org/10.3390/ijerph20064964
Wróbel M, Śliwakowski W, Kowalczyk P, Kramkowski K, Dobrzyński J. Bioremediation of Heavy Metals by the Genus Bacillus. International Journal of Environmental Research and Public Health. 2023; 20(6):4964. https://doi.org/10.3390/ijerph20064964
Chicago/Turabian StyleWróbel, Monika, Wojciech Śliwakowski, Paweł Kowalczyk, Karol Kramkowski, and Jakub Dobrzyński. 2023. "Bioremediation of Heavy Metals by the Genus Bacillus" International Journal of Environmental Research and Public Health 20, no. 6: 4964. https://doi.org/10.3390/ijerph20064964
APA StyleWróbel, M., Śliwakowski, W., Kowalczyk, P., Kramkowski, K., & Dobrzyński, J. (2023). Bioremediation of Heavy Metals by the Genus Bacillus. International Journal of Environmental Research and Public Health, 20(6), 4964. https://doi.org/10.3390/ijerph20064964






