Abstract
This study aimed to identify the development of hepatitis B or C infection in diabetes patients compared to those without and to elucidate factors associated with the prevalence of hepatitis B or C infection in diabetes. We conducted a cross-sectional study using data from the National Health and Nutrition Examination Survey (NHANES) 2013–2018. As evaluation factors, we included variables such as age, race, illicit drug use, and poverty. The diabetic group had a significantly higher prevalence of hepatitis B or C infection than the non-diabetic group (odds ratio (OR) = 1.73; 95% confidence interval (CI), 1.36–2.21, p < 0.01). In multivariate Cox regression, non-poverty and non-illicit drug use were lower risk factors contributing to hepatitis development in diabetes (hazard ratio (HR) = 0.50; 95% CI, 0.32–0.79, p < 0.01, and HR = 0.05; 95% CI, 0.03–0.08, p < 0.01, respectively). Logistic regression also showed that these factors were significant contributors to hepatitis development in the diabetic group (p < 0.01). In patients with diabetes, the development of hepatitis was higher than that in those without, and hepatitis development was influenced by poverty and illicit drug use. This may provide supporting evidence of response strategies for diabetes to care for hepatitis development in advance.
1. Introduction
Diabetes and hepatitis are among the most prevalent diseases worldwide [1]. The concomitant existence of diabetes and hepatitis potentially leads to a life-threatening status, increasing mortality by approximately 17% among diabetic patients without comparing to other patients, such as non-virus-infected diabetic patients [2]. With a considerably high prevalence, 865 outbreaks of patients infected with hepatitis B virus (HBV) through population-based surveillance for infectious diseases at eight Emerging Infections Program (EIP) sites, which are part of a network of 10 state health departments in the United States [3], have been reported among adults diagnosed with diabetes [4]. Regarding the increased prevalence of HBV in patients with diabetes and severe clinical outcomes, the Advisory Committee on Immunization Practices (ACIP) of the Centers for Disease Control and Prevention (CDC) recommends appropriate methods for preventing HBV infection in patients with diabetes based on the risk of an inadequate immune response [5]. In addition, for hepatitis C virus (HCV) infection, one of the major sources of morbidity and mortality in people, the CDC indicated that injection drug use (IDU) is a primary risk factor for infection [6]. Considering IDU use in diabetic patients, according to the guidelines for diabetes, diabetic patients are given insulin as the primary treatment and use self-monitoring of blood glucose (SMBG) to manage their blood glucose, so frequent exposure to needles increases the risk of HCV transmission [7,8]. Regarding the frequent exposure to IDU along with needles through multiple blood sampling and unsafe injection practices, patients with diabetes are more vulnerable to HCV infection than those without diabetes [9]. A previous cross-sectional study which analyzed the prevalence of HCV among diabetic patients reported that insulin users were 3.2 times more likely to have HCV infection than non-insulin users [10].
Furthermore, hepatitis and diabetes seem to be negatively associated with the development of each other. Schillie et al. [11] reported a higher prevalence rate of HBV infection among persons with diabetes than those without diabetes (odds ratio (OR) = 1.60; 95% confidence interval (CI), 1.30–1.90, p < 0.05). However, there is still controversy regarding the association between the development of diabetes and hepatic viral infection, as shown in a study in a tertiary hospital reporting low development of hepatitis C viral seropositivity among patients with type 2 diabetes mellitus [12]. To provide more substantial evidence explaining the association between diabetes and hepatitis, including HBV or HCV, large-scale epidemiological studies evaluating national data, such as the National Health and Nutrition Examination Survey (NHANES), are needed.
Currently, a limited number of studies have analyzed large-scale data, such as the NHANES, which might provide a clear explanation of the association between diabetes and hepatitis. Thus, to provide sufficient evidence to evaluate the impact of diabetes on HBV or HCV development compared to the population without diabetes, the current study examined the association between hepatitis infection and diabetes and aimed to characterize the factors related to the prevalence of hepatitis in the diabetic population using the NHANES database.
2. Materials and Methods
2.1. Study Design
We analyzed the 2013–2018 NHANES database. The NHANES is a cross-sectional monitoring program designed to assess the health and nutritional status of adults and children in the United States [13]. Multiple datasets were collected in this survey, including demographics, dietary, questionnaire, physical examinations, and laboratory testing of biologic samples in the U.S. populations [13]. The NHANES data have been collected in 2-year cycles without a break between cycles since 1999 [14]. The methodology for all databases is described on the NHANES website [14]. Since the participants of the NHANES are de-identified and assigned unique sequence numbers, details of the respondents and duplicate participation of people are unknown [15]. The NHANES is administered using a stratified multistage clustered probability sampling strategy to provide a nationally representative sample [15].
2.2. Definition of Diabetes and Hepatitis
Information on diabetes, HBV, and HCV were retrieved from the NHANES. Diabetes was defined according to the following criteria: HbA1c ≥ 6.5%, FPG ≥ 126 mg/dL, or participants being informed that they had diabetes by their doctor or other health professionals [16]. Both patients with type 1 and type 2 diabetes were included in our study. HBV was defined as positive for hepatitis B surface antigen (HBsAg) or if participants answered yes to the question, “Has a doctor or other health professional ever told you that you have hepatitis B?” [17]. HCV was defined as a positive for Hepatitis C virus ribonucleic acid (HCV-RNA) or if participants replied yes to the question “Has a doctor or other health professional ever told you that you have Hepatitis C?” [18]. HBV-infected and HCV-infected participants were assigned to the corresponding hepatitis B and C groups.
2.3. Covariates
We selected significant variables through logistic regression for variable selection to be included in the analysis dataset [19]. In our analysis, the confounding variables included age, sex, race, body mass index (BMI), the ratio of family income to poverty, and the use of illicit drugs. Demographic covariates included age (years) [20], sex (male or female), race (Mexican-American, other Hispanic, non-Hispanic White, non-Hispanic Black, non-Hispanic Asian, or other race) [21], and family income to poverty ratio (continuous from 0 to 4.99) [18]. We categorized age into four groups as follows: 0–19, 20–44, 45–64, and ≥65 years [20]. The family income to poverty ratio was divided into < or ≥1 [19]. A ratio < 1 indicated poverty, while a ratio ≥ 1 indicated without poverty [18]. BMI was collected from the laboratory dataset from the NHANES database and grouped as underweight (<18.5 kg/m²), normal weight (18.5~24.9 kg/m²), and overweight (≥25 kg/m²) [22]. Questionnaire covariates included the use of illicit drugs (yes or no) [23].
2.4. Statistical Analysis
Data are presented as frequency and percentage for categorical variables. Box plots consisted of quartiles for the prevalence over 2-year cycles of patients infected with hepatitis regarding diabetes status [24]. The OR with 95% CI was calculated using univariate and multivariate logistic regression models to elucidate the factors affecting the development of hepatitis in patients with diabetes. Multivariate logistic regression analysis was performed after adjusting for covariates of race, poverty, and illicit drug use. The Cox proportional hazards model determined the hazard rate to be roughly constant at all time points, so our study used this statistical method [25,26]. The Cox proportional hazards model was used to calculate the hazard ratio (HR) and 95% CI of the risk associated with hepatitis development in patients with diabetes. The univariate Cox proportional hazards model was used for all variables, and a multivariate model was utilized to adjust the race, poverty, and use of illicit drugs. All statistical analyses were performed using R software (version 4.2.1), with a p-value < 0.05 as statistically significant.
3. Results
3.1. General Characteristics of Participants
This study included 29,400 participants from the NHANES (2013–2018) database. Overall, 1160 participants with missing diabetes data were excluded. Then, 15,305 participants with missing data on illicit drug use, 28 participants with missing data on HBV, 3 participants with missing data on HCV, 1215 participants with missing data on the ratio of family income to poverty, and 88 participants with missing data on BMI were excluded in the present analysis. The additional analysis to clarify the variance between excluded data, including data related to illegal drug use, showed that the number of participants differed among age groups (Table S1). However, the distributions of excluded data for non-responding questions related to illegal drugs were not significantly different while the number of participants distributed among age groups differed. Ultimately, 11,601 participants were included in the final analysis (Figure 1 and Table 1). Of the 11,601 participants, 1709 were diagnosed with diabetes, whereas 9892 were classified in the non-diabetic group. The prevalence of HBV, HCV, and hepatitis B or C with diabetes was 2.3%, 3.2%, and 5.1%, respectively, whereas the prevalence in the non-diabetic group was 1.3%, 1.9%, and 3.0%, respectively (Table 2).
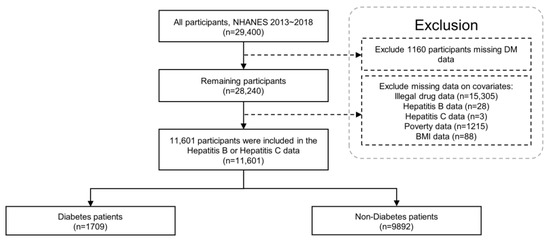
Figure 1.
Flowchart of the study participants using the NHANES 2013–2018. BMI: body mass index; NHANES: National Health and Nutrition Examination Survey.

Table 1.
Baseline characteristics of the study population from the National Health and Nutrition Examination Survey (NHANES) 2013–2018.

Table 2.
Characteristics of hepatitis infection status in with and without diabetes mellitus.
3.2. The Prevalence of Hepatitis According to Diabetes Status
The prevalence of HBV in participants without diabetes was 1.3%, whereas that in participants with diabetes was 2.3%, which indicated HBV development was 1.7721 odds more than in patients with diabetes, with a significant difference (OR = 1.77; 95% CI, 1.24–2.53, p < 0.05). The prevalence of HCV was 3.2% among patients with diabetes and 1.9% among those without diabetes. The development of HCV increased in patients with diabetes by 1.6751 odds compared to patients without diabetes, with a significant difference (OR = 1.68; 95% CI, 1.23–2.28, p < 0.05). Moreover, the prevalence of 5.1% for hepatitis B or C in patients with diabetes was significantly 1.7298 odds higher than that reported among non-diabetic subjects (OR = 1.73; 95% CI, 1.36–2.21, p < 0.0001) (Figure 2, Table 2 and Table S2). Furthermore, the prevalence of hepatitis infection was not significantly different based on the FPG or HbA1c levels (Figure S1 and Table S3).
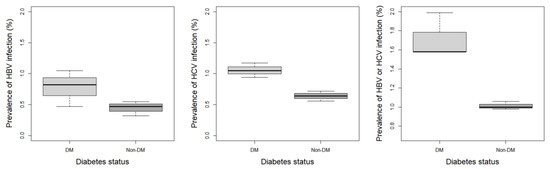
Figure 2.
Prevalence of each hepatitis regarding the diabetes mellitus status. DM, diabetes mellitus; HBV, hepatitis B virus; HCV, hepatitis C virus.
3.3. Characteristics of Hepatitis Development from 2013 to 2018
Analysis of the characteristics of hepatitis prevalence according to diabetes development at 2-year intervals (2013–2018) revealed that the prevalence of HBV in patients with diabetes increased from 1.5% to 3.0% in two cycles (2013–2016), and the non-diabetic group also showed an increased tendency from 1.3% to 1.7%. However, compared with the 2013–2016 period, the HBV prevalence in 2017–2018 decreased to 2.4% in patients with diabetes and 1.1% in the non-diabetic group. The prevalence of HCV in the diabetic population decreased over time (2013–2018), though the non-diabetic group showed similar HCV development for two cycles (2013–2016) and indicated an increasing tendency towards 2.3% from 2017 to 2018. In the diabetic group, the hepatitis B or C prevalence increased from 5.0% to 5.7% during 2013–2016, while in 2017–2018, a decreasing trend in the rate was observed (Table 2 and Figure S2).
3.4. Risk of Hepatitis Development in Diabetes Patients
In univariate and multivariate Cox regression analysis results, the non-poverty group showed a tendency to have a lower risk of hepatitis B or C prevalence than the poverty group (univariate: HR = 0.48; 95% CI, 0.31–0.74, p < 0.01; multivariate: HR = 0.50; 95% CI, 0.32–0.79, p < 0.01, respectively) (Table 3). In addition, diabetes patients who did not use illicit drugs had a lower risk of hepatitis B or C infection than illicit drug users (univariate: HR = 0.05; 95% CI, 0.03–0.08, p < 0.01; multivariate: HR = 0.05; 95% CI, 0.03–0.08, p < 0.01, respectively). In multivariate analysis, non-Hispanic Asian particularly showed a greater association in hepatitis B or C prevalence than other races among the diabetic group. (HR = 3.33; 95% CI, 1.05–10.60, p < 0.05).

Table 3.
Univariate and multivariate hazard ratio and 95% confidence intervals for the risk of hepatitis B or C with diabetes mellitus population.
3.5. Factors of Developing Hepatitis in Diabetes Patients
The results of the univariate and multivariate logistic regression analyses are summarized in Table 4. In the diabetic group, the univariate and multivariate logistic analyses showed that the non-poverty group was associated with a lower risk of hepatitis B or C development than the poverty group (univariate: OR 0.96; 95% CI, 0.94–0.99, p < 0.01; multivariate: OR 0.96; 95% CI, 0.94–0.99, p < 0.01, respectively). The diabetic group of non-users of illicit drugs was associated with reduced odds of hepatitis B or C infection compared with illicit drug users (univariate: OR 0.59; 95% CI, 0.55–0.62, p < 0.01; multivariate: OR 0.59; 95% CI, 0.56–0.62, p < 0.01, respectively). The non-Hispanic Asian group showed a tendency of higher correlation with hepatitis B or C infection than other races (univariate: OR 1.03; 95% CI, 0.97–1.09, p = 0.40; multivariate: OR 1.05; 95% CI, 0.99–1.11, p = 0.10, respectively).

Table 4.
Univariate and multivariate odds ratio and 95% confidence intervals for the association of hepatitis B or C with the diabetes mellitus population.
4. Discussion
The current study evaluated the prevalence of HBV and HCV infections in patients with diabetes and relevant factors. Our results showed that patients with diabetes had a higher prevalence of HBV and HCV infections than those without. Similarly, a previous cohort study conducted in the United Kingdom observed a higher prevalence of HBV in patients with diabetes than in those without diabetes [27]. Another previous study from Pakistan Hospital also indicated that patients with diabetes were more associated with HCV infection than the non-diabetic group (OR = 3.03; 95% CI, 2.64–3.48, p < 0.01) [28]. Furthermore, due to their weakened immune system [29], patients with diabetes may be more susceptible to HBV or HCV infections compared to the general population. This susceptibility may be exacerbated by frequent exposure to needles during blood glucose monitoring, which can contribute to virus transmission [30].
Our findings demonstrated that poverty was a potential contributor that might influence HBV and HCV infection development. Patients with diabetes living in poverty were more associated with a higher development of hepatitis than those without poverty. Consistent with these findings, a previous cross-sectional study in Canada [31], which reported the association between socioeconomic income and the prevalence of diabetes and related conditions, also showed that those with a low household income had a higher prevalence of diabetes than the population with a higher income. Greene et al. [32] analyzed surveillance data in New York City and reported that chronic hepatitis C was included in diseases related to severe poverty with low income. Furthermore, based on the American Association for the Study of Liver Disease (AASLD), HBV and HCV are the leading infectious diseases that are closely related to poverty [33]. Although various factors contributed to poverty, such as age or education levels, and are closely related to infectious disease spread [34], considering the low self-awareness of their condition among impoverished people, the poverty group could show a progressive increase in the risk of disease such as hepatitis [35]. In support of this, in Brazil, a previous study also showed hepatitis B susceptibility rates ranging from approximately 32% among individuals living with low income [36]. Therefore, poverty in people with complications such as DM is the cause of increasing HBV or HCV and is also likely to be an important factor in the incidence rate because there is a cost burden [37].
In our results, patients with diabetes without illicit drugs seem to have a lower risk of hepatitis infection than illicit drug users. Diabetic patients who are at risk of experiencing high stress and immune dysfunction may also be more susceptible to illegal drug use and HCV transmission [38,39]. For the association between HCV transmission and illicit drug use, Benjamin et al. [40] already demonstrated in a study of young American users of illegal drugs that 343 out of 714 participants were infected with HCV. Furthermore, the increase in the distribution of hepatitis caused by illegal drug use seems to be a global burden [41]. This is evidenced by the population-attributable fraction of hepatitis caused by illegal drug use in 2013, which was 10% for HBV in North America and 1% in Latin America and 81% for HCV in North America and 31% in Latin America [41]. This percentage has further increased since 1990, with HBV accounting for 6% in North America and 1% in Latin America and HCV accounting for 60% in North America and 19% in Latin America [41]. However, given the tendency for illicit drug users to frequently disregard physician advice, hepatitis infections in this population may become more severe or even go undetected [42]. Therefore, these findings can offer crucial insights to enhance screening protocols and identify a broader population of illegal drug users at high risk of acquiring HCV and HBV infections. This will aid in the early detection and treatment of the infections [43].
Among races, non-Hispanic Asians race seemed to significantly contribute to hepatitis in the diabetic group. Consistent with these findings, a previous study showed that non-Hispanic Asians were more associated with the prevalence of HBV infection than other races (OR = 3.85; 95% CI, 2.97–4.97, p < 0.05) [44]. In addition, according to 2011–2014 NHANES data, non-Hispanic Asian adults showed a higher prevalence of HBV infection (22.6%) than non-Hispanic White (2.6%), non-Hispanic Black (10.2%), and Hispanic (3.6%) adults [45]. Furthermore, in our study, the non-Hispanic Black race seemed to contribute to hepatitis infection without significance. Pathologically, according to Thomas et al., interleukin 28 B (IL28B), which plays a significant primary role in the resolution of HCV, is less likely to be present in the Black population [46]. Because of the lack of IL28B, the non-Hispanic Black group may have a higher risk of HCV infection [46]. However, our study cannot be confidential because race affects the risk of hepatitis; therefore, further studies are needed.
The current study had some limitations. First, our study did not distinguish between type 1 and type 2 diabetes mellitus. As type 2 diabetes accounts for more than 90% of diagnosed diabetes mellitus in the United States [47], our findings largely reflect the risk factors of hepatitis in patients with type 2 diabetes mellitus. Thus, we expect that more future studies will be conducted to distinguish the types of diabetes mellitus to identify the impact of diabetes and hepatitis. Second, we did not evaluate the economic costs of treating HCV infection and diabetes mellitus. Therefore, further studies are required to evaluate the economic impact of HBV or HCV infection in patients with diabetes. Third, we were unable to conduct further analysis on the correlation between hepatitis infection and CVD or diabetic comorbidities in the current study. This was because the important cardiovascular health metrics data, which could aid analysis of the correlation of hepatitis infection with complications such as cardiovascular disease or diabetic complications, were scarce in the NHANES [48], or the guidelines for evaluating criteria for high blood pressure and cholesterol risks were changed [49,50]. Thus, we hope that future studies can address these limitations. Forth, the NHANES data were limited to the United States. This may not provide global evidence for an association between diabetes and hepatitis. Thus, we expect further studies using merged data from various countries. Finally, as this study used a small sample size to identify illegal injection drug users, the number might have been underestimated [51]. It is difficult to obtain accurate data about illegal drug users. Thus, there may be inevitable non-responsive biases with illicit drug use, and our findings should be interpreted with caution.
5. Conclusions
The current study found that the risk of hepatitis B or C infection was higher in patients with diabetes than in those without. Therefore, the present study would help increase awareness regarding hepatitis prevention in patients with diabetes. Additionally, this study suggests that more attention should be paid to impoverished or illicit drug users among patients with diabetes regarding the threat of hepatitis infection. Finally, in addition to NHANES data, we expect that more global evidence will be provided through corresponding data from various countries.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20064962/s1, Table S1: Variables compare between including vs. excluding data with illegal drug use; Table S2: Association of diabetes mellitus status with hepatitis group; Table S3: Prevalence of hepatitis B or C with in the diabetes mellitus population according to FPG and HbA1c levels; Figure S1: Prevalence of hepatitis regarding the fast glucose or glycated hemoglobin levels; Figure S2: Prevalence of each hepatitis by the 2-year-cycle according to diabetes mellitus status from 2013 to 2018.
Author Contributions
Conceptualization: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Data curation: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Methodology: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Software: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Validation: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Formal analysis: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Investigation: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Resources: J.-Y.H., J.-H.K., S.-H.K. and H.L.; Writing—original draft: J.-Y.H., J.-H.K. and H.L.; Visualization: J.-Y.H., J.-H.K. and H.L.; Project administration: H.L.; Supervision: H.L.; Funding acquisition: H.L.; Writing—review and editing: H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The public-use NHANES data analyzed in this study are available at https://www.cdc.gov/nchs/nhanes/index.htm accessed on 10 September 2022.
Acknowledgement
This dissertation is the final outcome which was supported by the Korean Industrial Enterprise (Carboexpert, CE9A215 clinical study/202103040001).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ziaee, M.; Saljoughi, M.; Fardinfar, F.; Madarshahian, F.; Ebrahimzadeh, A. Comparison of Frequency Distribution of Diabetes in Hepatitis B and C Patients and Other People in Birjand. Mod. Care J. 2021, 18, e111024. [Google Scholar] [CrossRef]
- Singh, K.K.; Panda, S.K.; Shalimar; Acharya, S.K. Patients with Diabetes Mellitus are Prone to Develop Severe Hepatitis and Liver Failure due to Hepatitis Virus Infection. J. Clin. Exp. Hepatol. 2013, 3, 275–280. [Google Scholar] [CrossRef]
- CDC. Division of Preparedness and Emerging Infections (DPEI). 2021. Available online: https://www.cdc.gov/ncezid/dpei/eip/index.html (accessed on 1 March 2023).
- Centers for Disease Control and Prevention (CDC). Use of Hepatitis B Vaccination for Adults with Diabetes Mellitus: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb. Mortal. Wkly. Rep. 2011, 60, 1709–1711. [Google Scholar]
- Kirkman, M.S.; Schaffner, W. Another shot to protect people with diabetes: Add hepatitis B vaccination to the checklist. Diabetes Care 2012, 35, 941–942. [Google Scholar] [CrossRef]
- Schillie, S.; Wester, C.; Osborne, M.; Wesolowski, L.; Ryerson, A.B. CDC Recommendations for Hepatitis C Screening among Adults—United States, 2020. MMWR Recomm. Rep. 2020, 69, 1–17. [Google Scholar] [CrossRef]
- Silver, B.; Ramaiya, K.; Andrew, S.B.; Fredrick, O.; Bajaj, S.; Kalra, S.; Charlotte, B.M.; Claudine, K.; Makhoba, A. EADSG Guidelines: Insulin Therapy in Diabetes. Diabetes Ther. 2018, 9, 449–492. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Self-monitoring of blood glucose among adults with diabetes—United States, 1997–2006. MMWR. Morb. Mortal. Wkly. Rep. 2007, 56, 1133–1137. [Google Scholar]
- Guo, X.; Jin, M.; Yang, M.; Liu, K.; Li, J.W. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: A systematic review. Sci Rep. 2013, 3, 2981. [Google Scholar] [CrossRef]
- Ba-Essa, E.M.; Mobarak, E.I.; Al-Daghri, N.M. Hepatitis C virus infection among patients with diabetes mellitus in Dammam, Saudi Arabia. BMC Health Serv. Res. 2016, 16, 313. [Google Scholar] [CrossRef]
- Schillie, S.F.; Xing, J.; Murphy, T.V.; Hu, D.J. Prevalence of hepatitis B virus infection among persons with diagnosed diabetes mellitus in the United States, 1999–2010. J. Viral Hepat. 2012, 19, 674–676. [Google Scholar] [CrossRef]
- Balogun, W.O.; Adeleye, J.O.; Akinlade, K.S.; Kuti, M.; Otegbayo, J.A. Low prevalence of hepatitis-C viral seropositivity among patients with type-2 diabetes mellitus in a tertiary hospital. J. Natl. Med. Assoc. 2006, 98, 1805–1808. [Google Scholar]
- Neves, J.S.; Leitão, L.; Magriço, R.; Bigotte Vieira, M.; Viegas Dias, C.; Oliveira, A.; Carvalho, D.; Claggett, B. Caffeine Consumption and Mortality in Diabetes: An Analysis of NHANES 1999–2010. Front. Endocrinol. 2018, 9, 547. [Google Scholar] [CrossRef]
- National Health and Nutrition Examination Survey. Available online: http://www.cdc.gov/nchs/nhanes.htm (accessed on 10 September 2022).
- Zipf, G.; Chiappa, M.; Porter, K.S.; Ostchega, Y.; Lewis, B.G.; Dostal, J. National Health and Nutrition Examination Survey: Plan and Operations, 1999–2010; Vital and Health Statistics; Series 1; CDC: Atlanta, GA, USA, 2013; pp. 1–37. [Google Scholar]
- Huang, Y.Q.; Liu, L.; Huang, C.; Yu, Y.L.; Lo, K.; Huang, J.Y.; Chen, C.L.; Zhou, Y.L.; Feng, Y.Q. Impacts of Pre-Diabetes or Prehypertension on Subsequent Occurrence of Cardiovascular and All-Cause Mortality among Population without Cardiovascular Diseases. Diabetes Metab. Syndr. Obes. 2020, 13, 1743–1752. [Google Scholar] [CrossRef]
- Zeng, L.Y.; Lian, J.S.; Chen, J.Y.; Jia, H.Y.; Zhang, Y.M.; Xiang, D.R.; Yu, L.; Hu, J.H.; Lu, Y.F.; Zheng, L.; et al. Hepatitis B surface antigen levels during natural history of chronic hepatitis B: A Chinese perspective study. World J. Gastroenterol. 2014, 20, 9178–9184. [Google Scholar] [CrossRef]
- Moore, K.J.; Gauri, A.; Koru-Sengul, T. Prevalence and sociodemographic disparities of Hepatitis C in Baby Boomers and the US adult population. J. Infect. Public Health 2019, 12, 32–36. [Google Scholar] [CrossRef]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef]
- Fang, J.; Zhang, Z.; Ayala, C.; Thompson-Paul, A.M.; Loustalot, F. Cardiovascular Health among Non-Hispanic Asian Americans: NHANES, 2011–2016. J. Am. Heart Assoc. 2019, 8, e011324. [Google Scholar] [CrossRef]
- Ansu Baidoo, V.Y.; Zee, P.C.; Knutson, K.L. Racial and Ethnic Differences in Eating Duration and Meal Timing: Findings from NHANES 2011–2018. Nutrients 2022, 14, 2428. [Google Scholar] [CrossRef]
- Ellison-Barnes, A.; Johnson, S.; Gudzune, K. Trends in Obesity Prevalence among Adults Aged 18 through 25 Years, 1976–2018. JAMA 2021, 326, 2073–2074. [Google Scholar] [CrossRef]
- Shing, J.Z.; Ly, K.N.; Xing, J.; Teshale, E.H.; Jiles, R.B. Prevalence of Hepatitis B Virus Infection Among US Adults Aged 20–59 Years with a History of Injection Drug Use: National Health and Nutrition Examination Survey, 2001–2016. Clin. Infect. Dis. 2020, 70, 2619–2627. [Google Scholar] [CrossRef]
- Soares, L.; Latta, S.C.; Ricklefs, R.E. Neotropical migratory and resident birds occurring in sympatry during winter have distinct haemosporidian parasite assemblages. J. Biogeogr. 2019, 47, 748–759. [Google Scholar] [CrossRef]
- Barros, A.J.; Hirakata, V.N. Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med. Res. Methodol. 2003, 3, 21. [Google Scholar] [CrossRef]
- Mirel, L.B.; El Bural Félix, S.; Zhang, C.; Golden, C.; Cox, C.S. Comparative Analysis of the National Health Interview Survey Public-Use and Restricted-Use Linked Mortality Files; National Health Statistics Reports; CDC: Atlanta, GA, USA, 2020; pp. 1–32. [Google Scholar]
- Ferreira, G.L.C.; Marano, C.; De Moerlooze, L.; Guignard, A.; Feng, Y.; El Hahi, Y.; van Staa, T. Incidence and prevalence of hepatitis B in patients with diabetes mellitus in the UK: A population-based cohort study using the UK Clinical Practice Research Datalink. J. Viral Hepat. 2018, 25, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Jadoon, N.A.; Shahzad, M.A.; Yaqoob, R.; Hussain, M.; Ali, N. Seroprevalence of hepatitis C in type 2 diabetes: Evidence for a positive association. Virol. J. 2010, 7, 304. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.M.; Gorter, K.J.; Hak, E.; Goudzwaard, W.L.; Schellevis, F.G.; Hoepelman, A.I.; Rutten, G.E. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin. Infect. Dis. 2005, 41, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Villar, L.M.; Geloneze, B.; Vasques, A.C.J.; Pires, M.L.E.; Miguel, J.C.; da Silva, E.F.; Marques, V.A.; Scalioni, L.P.; Lampe, E. Prevalence of hepatitis B and hepatitis C among diabetes mellitus type 2 individuals. PLoS ONE 2019, 14, e0211193. [Google Scholar] [CrossRef]
- Bird, Y.; Lemstra, M.; Rogers, M.; Moraros, J. The relationship between socioeconomic status/income and prevalence of diabetes and associated conditions: A cross-sectional population-based study in Saskatchewan, Canada. Int. J. Equity Health 2015, 14, 93. [Google Scholar] [CrossRef]
- Greene, S.K.; Levin-Rector, A.; Hadler, J.L.; Fine, A.D. Disparities in Reportable Communicable Disease Incidence by Census Tract-Level Poverty, New York City, 2006–2013. Am. J. Public Health 2015, 105, e27–e34. [Google Scholar] [CrossRef]
- Ghany, M.G.; Morgan, T.R. Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. Hepatology 2020, 71, 686–721. [Google Scholar] [CrossRef]
- Janjua, P.Z.; Kamal, U.A. The Role of Education and Income in Poverty Alleviation: A Cross-Country Analysis. Lahore J. Econ. 2011, 16, 143–172. [Google Scholar] [CrossRef]
- Kim, H.S.; Rotundo, L.; Yang, J.D.; Kim, D.; Kothari, N.; Feurdean, M.; Ruhl, C.; Unalp-Arida, A. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J. Viral Hepat. 2017, 24, 1052–1066. [Google Scholar] [CrossRef]
- Scarponi, C.F.O.; Zolnikov, T.R.; Mol, M.P.G. Are waste pickers at risk for hepatitis B and C infections because of poverty or environmental exposures? Rev. Soc. Bras. Med. Trop 2019, 52, e20190123. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Fonseca, V.; Childs, B. Economic burden of diabetes-related hypoglycemia on patients, payors, and employers. J. Diabetes Its Complicat. 2021, 35, 107916. [Google Scholar] [CrossRef] [PubMed]
- Berbudi, A.; Rahmadika, N.; Tjahjadi, A.I.; Ruslami, R. Type 2 Diabetes and its Impact on the Immune System. Curr. Diabetes Rev. 2020, 16, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Aregbesola, A.; Onyeka, I.N.; Olubamwo, O.; Ronkainen, K.; Tiihonen, J.; Föhr, J.; Kauhanen, J. Diabetes hospitalizations and deaths in a cohort of treatment-seeking illicit drug users. SAGE Open Med. 2018, 6, 2050312118768164. [Google Scholar] [CrossRef]
- Eckhardt, B.; Winkelstein, E.R.; Shu, M.A.; Carden, M.R.; McKnight, C.; Des Jarlais, D.C.; Glesby, M.J.; Marks, K.; Edlin, B.R. Risk factors for hepatitis C seropositivity among young people who inject drugs in New York City: Implications for prevention. PLoS ONE 2017, 12, e0177341. [Google Scholar] [CrossRef]
- Degenhardt, L.; Charlson, F.; Stanaway, J.; Larney, S.; Alexander, L.T.; Hickman, M.; Cowie, B.; Hall, W.D.; Strang, J.; Whiteford, H.; et al. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: Findings from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 1385–1398. [Google Scholar] [CrossRef] [PubMed]
- Edlin, B.R.; Seal, K.H.; Lorvick, J.; Kral, A.H.; Ciccarone, D.H.; Moore, L.D.; Lo, B. Is it justifiable to withhold treatment for hepatitis C from illicit-drug users? N. Engl. J. Med. 2001, 345, 211–215. [Google Scholar] [CrossRef]
- Edlin, B.R. Prevention and treatment of hepatitis C in injection drug users. Hepatology 2002, 36 (Suppl. S1), S210–S219. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Cheung, R.; Henry, L.; Lok, A.S.; Nguyen, M.H. Chronic Hepatitis B Prevalence among Foreign-Born and U.S.-Born Adults in the United States, 1999–2016. Hepatology 2020, 71, 431–443. [Google Scholar] [CrossRef]
- Kruszon-Moran, D.; Paulose-Ram, R.; Denniston, M.; McQuillan, G. Viral Hepatitis among Non-Hispanic Asian Adults in the United States, 2011–2014; NCHS Data Brief; CDC: Atlanta, GA, USA, 2015; pp. 1–8. [Google Scholar]
- Thomas, D.L.; Thio, C.L.; Martin, M.P.; Qi, Y.; Ge, D.; O’Huigin, C.; Kidd, J.; Kidd, K.; Khakoo, S.I.; Alexander, G.; et al. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 2009, 461, 798–801. [Google Scholar] [CrossRef]
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type—United States, 2016. MMWR. Morb. Mortal. Wkly. Rep. 2018, 67, 359–361. [Google Scholar] [CrossRef]
- Lopez-Neyman, S.M.; Davis, K.; Zohoori, N.; Broughton, K.S.; Moore, C.E.; Miketinas, D. Racial disparities and prevalence of cardiovascular disease risk factors, cardiometabolic risk factors, and cardiovascular health metrics among US adults: NHANES 2011–2018. Sci. Rep. 2022, 12, 19475. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018, 71, e127–e248. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; de Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 3168–3209. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Lee, J.E.; Lee, S.; Lee, S.H.; Kang, J.S. Prevalence and patterns of illicit drug use in people with human immunodeficiency virus infection in Korea. PLoS ONE 2021, 16, e0249361. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).