Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment
Abstract
1. Introduction
2. Materials and Methods
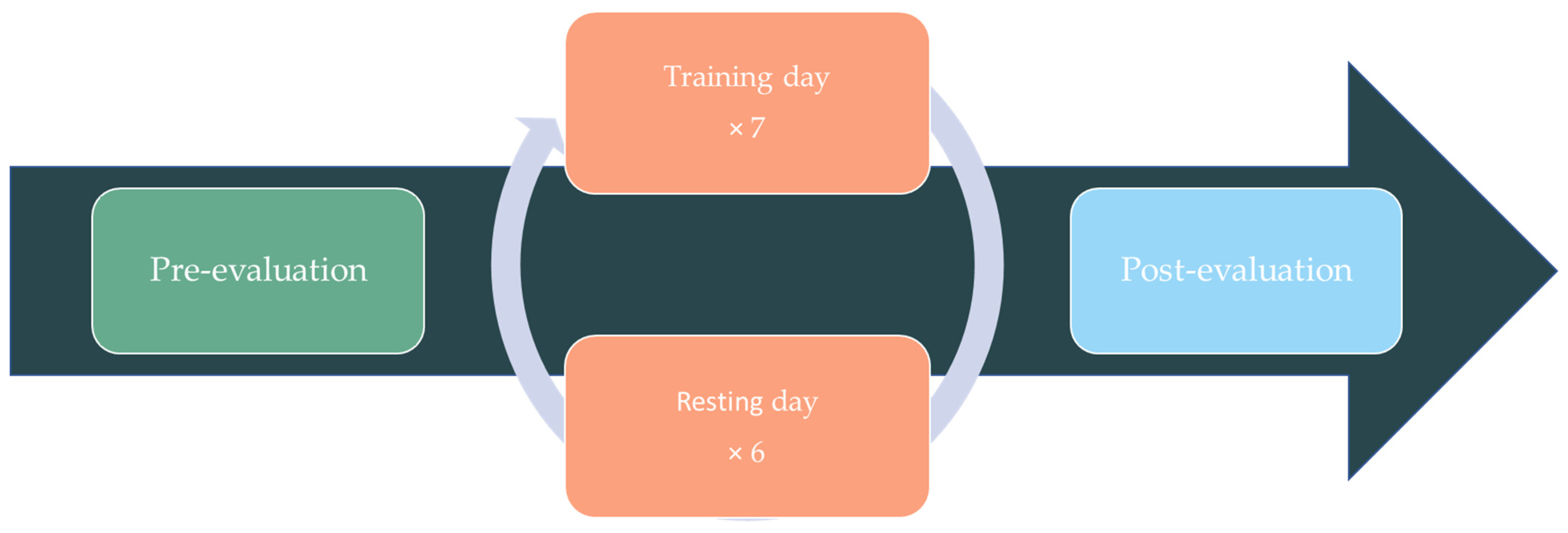
2.1. Study Design
2.2. Participants
2.3. Cognitive Stimulation Intervention
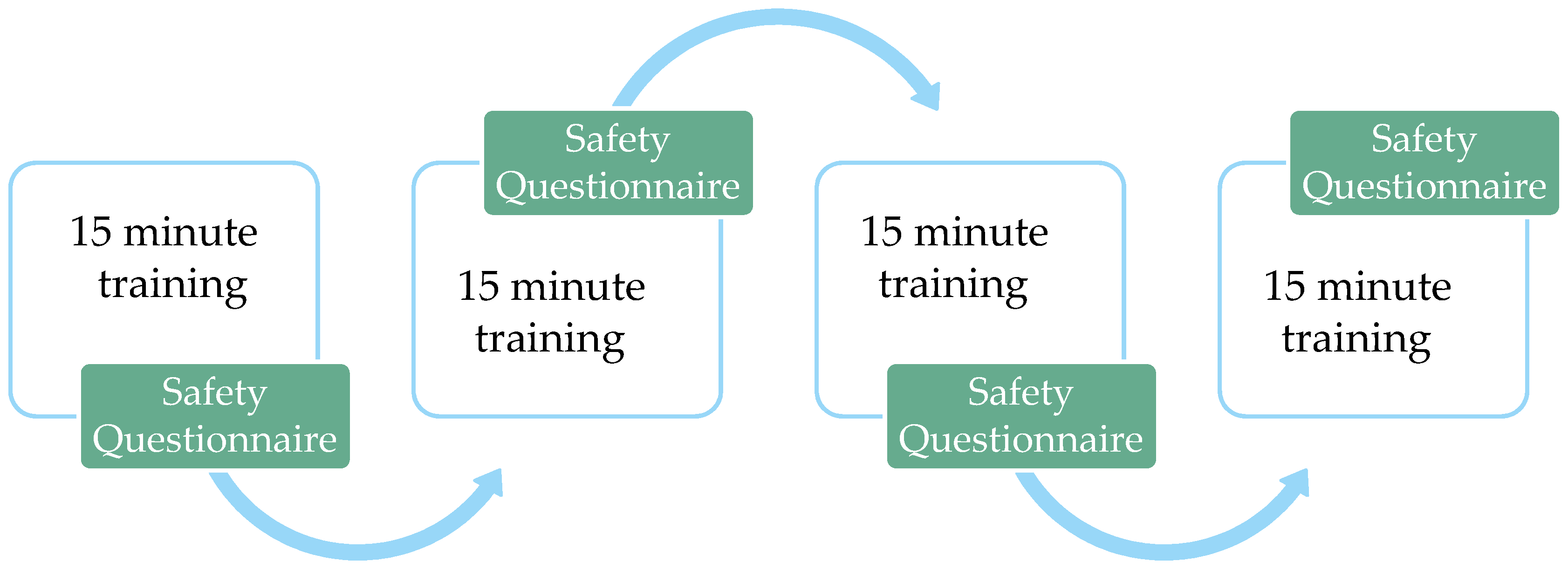
2.4. Procedure
2.5. Assessment Measures
2.6. Data Analyses
3. Results
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2022. CA A Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Curigliano, G.; Burstein, H.J.; Winer, E.P.; Gnant, M.; Dubsky, P.; Loibl, S.; Colleoni, M.; Regan, M.M.; Piccart-Gebhart, M.; Senn, H.-J.; et al. De-Escalating and Escalating Treatments for Early-Stage Breast Cancer: The St. Gallen International Expert Consensus Conference on the Primary Therapy of Early Breast Cancer 2017. Ann. Oncol. 2017, 28, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Anampa, J.; Makower, D.; Sparano, J.A. Progress in Adjuvant Chemotherapy for Breast Cancer: An Overview. BMC Med. 2015, 13, 195. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and Challenges of Radiotherapy for Treating Cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Warren, J.L.; Yabroff, K.R.; Meekins, A.; Topor, M.; Lamont, E.B.; Brown, M.L. Evaluation of Trends in the Cost of Initial Cancer Treatment. JNCI J. Natl. Cancer Inst. 2008, 100, 888–897. [Google Scholar] [CrossRef]
- Dackus, G.M.H.E.; Jóźwiak, K.; Sonke, G.S.; van der Wall, E.; van Diest, P.J.; Siesling, S.; Hauptmann, M.; Linn, S.C. Adjuvant Aromatase Inhibitors or Tamoxifen Following Chemotherapy for Perimenopausal Breast Cancer Patients. JNCI J. Natl. Cancer Inst. 2021, 113, 1506–1514. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment (Review). Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Kreuter, M.W.; Green, M.C.; Cappella, J.N.; Slater, M.D.; Wise, M.E.; Storey, D.; Clark, E.M.; O’Keefe, D.J.; Erwin, D.O.; Holmes, K.; et al. Narrative Communication in Cancer Prevention and Control: A Framework to Guide Research and Application. Ann. Behav. Med. 2007, 33, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Kalager, M.; Bretthauer, M. Improving Cancer Screening Programs. Science 2020, 367, 143–144. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early Detection of Cancer: Past, Present, and Future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Papanastasiou, A.; Seliniotaki, T.; Rizos, E.; Kampoli, K.; Ntavatzikos, A.; Arkadopoulos, N.; Tsionou, C.; Spandidos, D.A.; Koumarianou, A. Role of Stress, Age and Adjuvant Therapy in the Cognitive Function of Patients with Breast Cancer (Review). Oncol. Lett. 2019, 18, 507–517. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Ahles, T.A.; Mohile, S.G.; Mustian, K.M.; Palesh, O.; O’Mara, A.M.; Minasian, L.M.; Williams, A.M.; et al. Longitudinal Trajectory and Characterization of Cancer-Related Cognitive Impairment in a Nationwide Cohort Study. J. Clin. Oncol. 2018, 36, 3231–3239. [Google Scholar] [CrossRef] [PubMed]
- Koppelmans, V.; Breteler, M.M.B.; Boogerd, W.; Seynaeve, C.; Gundy, C.; Schagen, S.B. Neuropsychological Performance in Survivors of Breast Cancer More Than 20 Years After Adjuvant Chemotherapy. J. Clin. Oncol. 2012, 30, 1080–1086. [Google Scholar] [CrossRef]
- Van Dyk, K.; Bower, J.E.; Crespi, C.M.; Petersen, L.; Ganz, P.A. Cognitive Function Following Breast Cancer Treatment and Associations with Concurrent Symptoms. NPJ Breast Cancer 2018, 4, 25. [Google Scholar] [CrossRef]
- Ganz, P.A.; Van Dyk, K. Cognitive Impairment in Patients With Breast Cancer: Understanding the Impact of Chemotherapy and Endocrine Therapy. J. Clin. Oncol. 2020, 38, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Verma, S.S.; Aggarwal, S.; Gupta, S.C. Drug Repurposing for Breast Cancer Therapy: Old Weapon for New Battle. Semin. Cancer Biol. 2021, 68, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Rix, A.; Drude, N.I.; Mrugalla, A.; Baskaya, F.; Pak, K.Y.; Gray, B.; Kaiser, H.-J.; Tolba, R.H.; Fiegle, E.; Lederle, W.; et al. Assessment of Chemotherapy-Induced Organ Damage with Ga-68 Labeled Duramycin. Mol. Imaging Biol. 2020, 22, 623–633. [Google Scholar] [CrossRef]
- Allan, N.; Siller, C.; Breen, A. Anaesthetic Implications of Chemotherapy. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 52–56. [Google Scholar] [CrossRef]
- Glare, P.A.; Davies, P.S.; Finlay, E.; Gulati, A.; Lemanne, D.; Moryl, N.; Oeffinger, K.C.; Paice, J.A.; Stubblefield, M.D.; Syrjala, K.L. Pain in Cancer Survivors. J. Clin. Oncol. 2014, 32, 1739–1747. [Google Scholar] [CrossRef]
- Hardy, S.J.; Krull, K.R.; Wefel, J.S.; Janelsins, M. Cognitive Changes in Cancer Survivors. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 795–806. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Heckler, C.E.; Peppone, L.J.; Kamen, C.; Mustian, K.M.; Mohile, S.G.; Magnuson, A.; Kleckner, I.R.; Guido, J.J.; Young, K.L.; et al. Cognitive Complaints in Survivors of Breast Cancer After Chemotherapy Compared With Age-Matched Controls: An Analysis From a Nationwide, Multicenter, Prospective Longitudinal Study. J. Clin. Oncol. 2017, 35, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Joly, F.; Vardy, J.; Ahles, T.; Dubois, M.; Tron, L.; Winocur, G.; Ruiter, M.B.D.; Castel, H. Cancer-Related Cognitive Impairment: An Update on State of the Art, Detection, and Management Strategies in Cancer Survivors. Ann. Oncol. 2019, 30, 1925–1940. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, A.D.; Hosking, J.R.; Kichenadasse, G.; Mattiske, J.K.; Wilson, C. Objective and Subjective Cognitive Impairment Following Chemotherapy for Cancer: A Systematic Review. Cancer Treat. Rev. 2012, 38, 926–934. [Google Scholar] [CrossRef]
- Winocur, G.; Henkelman, M.; Wojtowicz, J.M.; Zhang, H.; Binns, M.A.; Tannock, I.F. The Effects of Chemotherapy on Cognitive Function in a Mouse Model: A Prospective Study. Clin. Cancer Res. 2012, 18, 3112–3121. [Google Scholar] [CrossRef]
- Apple, A.C.; Schroeder, M.P.; Ryals, A.J.; Wagner, L.I.; Cella, D.; Shih, P.-A.; Reilly, J.; Penedo, F.J.; Voss, J.L.; Wang, L. Hippocampal Functional Connectivity Is Related to Self-Reported Cognitive Concerns in Breast Cancer Patients Undergoing Adjuvant Therapy. NeuroImage Clin. 2018, 20, 110–118. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Ahles, T.A. Low Neuropsychologic Performance among Adult Cancer Survivors Treated with Chemotherapy. Curr. Neurol. Neurosci. Rep. 2003, 3, 215–222. [Google Scholar] [CrossRef]
- Rodríguez Martín, B.; Fernández Rodríguez, E.J.; Rihuete Galve, M.I.; Cruz Hernández, J.J. Study of Chemotherapy-Induced Cognitive Impairment in Women with Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 8896. [Google Scholar] [CrossRef] [PubMed]
- Sioka, C.; Kyritsis, A.P. Central and Peripheral Nervous System Toxicity of Common Chemotherapeutic Agents. Cancer Chemother Pharm. 2009, 63, 761–767. [Google Scholar] [CrossRef]
- Berndt, U.; Leplow, B.; Schoenfeld, R.; Lantzsch, T.; Grosse, R.; Thomssen, C. Memory and Spatial Cognition in Breast Cancer Patients Undergoing Adjuvant Endocrine Therapy. Breast Care 2016, 11, 240–246. [Google Scholar] [CrossRef]
- Whittaker, A.L.; George, R.P.; O’Malley, L. Prevalence of Cognitive Impairment Following Chemotherapy Treatment for Breast Cancer: A Systematic Review and Meta-Analysis. Sci. Rep. 2022, 12, 2135. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Cancer Statistics for the Year 2020: An Overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Mounier, N.M.; Abdel-Maged, A.E.-S.; Wahdan, S.A.; Gad, A.M.; Azab, S.S. Chemotherapy-Induced Cognitive Impairment (CICI): An Overview of Etiology and Pathogenesis. Life Sci. 2020, 258, 118071. [Google Scholar] [CrossRef] [PubMed]
- Gift, A.G.; Stommel, M.; Jablonski, A.; Given, W. A Cluster of Symptoms Over Time in Patients With Lung Cancer. Nurs. Res. 2003, 52, 393. [Google Scholar] [CrossRef]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of Life of Patients with Lung Cancer. OncoTargets Ther. 2016, 9, 1023–1028. [Google Scholar] [CrossRef]
- Berger, A.M.; Gerber, L.H.; Mayer, D.K. Cancer-Related Fatigue. Cancer 2012, 118, 2261–2269. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.J.; de Rijke, J.M.; Kessels, A.G.; Schouten, H.C.; van Kleef, M.; Patijn, J. Quality of Life and Non-Pain Symptoms in Patients with Cancer. J. Pain Symptom Manag. 2009, 38, 216–233. [Google Scholar] [CrossRef]
- Stone, P.C.; Minton, O. Cancer-Related Fatigue. Eur. J. Cancer 2008, 44, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Bolton, G.; Isaacs, A. Women’s Experiences of Cancer-Related Cognitive Impairment, Its Impact on Daily Life and Care Received for It Following Treatment for Breast Cancer. Psychol. Health Med. 2018, 23, 1261–1274. [Google Scholar] [CrossRef]
- Padgett, L.S.; Van Dyk, K.; Kelly, N.C.; Newman, R.; Hite, S.; Asher, A. Addressing Cancer-Related Cognitive Impairment in Cancer Survivorship. Oncol. Issues 2020, 35, 52–57. [Google Scholar] [CrossRef]
- Ono, M.; Ogilvie, J.M.; Wilson, J.S.; Green, H.J.; Chambers, S.K.; Ownsworth, T.; Shum, D.H.K. A Meta-Analysis of Cognitive Impairment and Decline Associated with Adjuvant Chemotherapy in Women with Breast Cancer. Front. Oncol. 2015, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, K.D.; Hutchinson, A.D.; Wilson, C.J.; Nettelbeck, T. A Meta-Analysis of the Effects of Chemotherapy on Cognition in Patients with Cancer. Cancer Treat. Rev. 2013, 39, 297–304. [Google Scholar] [CrossRef]
- Von Ah, D.; Crouch, A. Cognitive Rehabilitation for Cognitive Dysfunction after Cancer and Cancer Treatment: Implications for Nursing Practice. Semin. Oncol. Nurs. 2020, 36, 150977. [Google Scholar] [CrossRef] [PubMed]
- Iconomou, G.; Mega, V.; Koutras, A.; Iconomou, A.V.; Kalofonos, H.P. Prospective Assessment of Emotional Distress, Cognitive Function, and Quality of Life in Patients with Cancer Treated with Chemotherapy. Cancer 2004, 101, 404–411. [Google Scholar] [CrossRef]
- Bender, C.M.; Sereika, S.M.; Berga, S.L.; Vogel, V.G.; Brufsky, A.M.; Paraska, K.K.; Ryan, C.M. Cognitive Impairment Associated with Adjuvant Therapy in Breast Cancer. Psychooncology 2006, 15, 422–430. [Google Scholar] [CrossRef]
- Wefel, J.S.; Kayl, A.E.; Meyers, C.A. Neuropsychological Dysfunction Associated with Cancer and Cancer Therapies: A Conceptual Review of an Emerging Target. Br. J. Cancer 2004, 90, 1691–1696. [Google Scholar] [CrossRef]
- Castellon, S.A.; Ganz, P.A.; Bower, J.E.; Petersen, L.; Abraham, L.; Greendale, G.A. Neurocognitive Performance in Breast Cancer Survivors Exposed to Adjuvant Chemotherapy and Tamoxifen. J. Clin. Exp. Neuropsychol. 2004, 26, 955–969. [Google Scholar] [CrossRef]
- Boykoff, N.; Moieni, M.; Subramanian, S.K. Confronting Chemobrain: An in-Depth Look at Survivors’ Reports of Impact on Work, Social Networks, and Health Care Response. J. Cancer Surviv. 2009, 3, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Munir, F.; Burrows, J.; Yarker, J.; Kalawsky, K.; Bains, M. Women’s Perceptions of Chemotherapy-Induced Cognitive Side Affects on Work Ability: A Focus Group Study. J. Clin. Nurs. 2010, 19, 1362–1370. [Google Scholar] [CrossRef]
- Von Ah, D.; Storey, S.; Crouch, A. Relationship between Self-Reported Cognitive Function and Work-Related Outcomes in Breast Cancer Survivors. J. Cancer Surviv. 2018, 12, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Smidt, K.; Mackenzie, L.; Dhillon, H.; Vardy, J.; Lewis, J.; Loh, S.Y. The Perceptions of Australian Oncologists about Cognitive Changes in Cancer Survivors. Support Care Cancer 2016, 24, 4679–4687. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; DuPont-Reyes, M.J.; Balneaves, L.G.; Carlson, L.E.; Cohen, M.R.; Deng, G.; Johnson, J.A.; Mumber, M.; Seely, D.; Zick, S.M.; et al. Clinical Practice Guidelines on the Evidence-Based Use of Integrative Therapies during and after Breast Cancer Treatment. CA Cancer J. Clin. 2017, 67, 194–232. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines Survivorship. Available online: https://www.nccn.org/patientresources/patient-resources/guidelines-for-patients (accessed on 4 December 2022).
- Fernandes, H.A.; Richard, N.M.; Edelstein, K. Cognitive Rehabilitation for Cancer-Related Cognitive Dysfunction: A Systematic Review. Support Care Cancer 2019, 27, 3253–3279. [Google Scholar] [CrossRef] [PubMed]
- Stiles, J. Neural Plasticity and Cognitive Development. Dev. Neuropsychol. 2000, 18, 237–272. [Google Scholar] [CrossRef]
- Burke, S.N.; Barnes, C.A. Neural Plasticity in the Ageing Brain. Nat. Rev. Neurosci. 2006, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Thoenen, H. Neurotrophins and Neuronal Plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef]
- von Bernhardi, R.; Bernhardi, L.E.; Eugenín, J. What Is Neural Plasticity. In The Plastic Brain; von Bernhardi, R., Eugenín, J., Muller, K.J., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–15. ISBN 978-3-319-62817-2. [Google Scholar]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- de Azevedo, K.P.M.; de Oliveira Segundo, V.H.; de Medeiros, G.C.B.S.; de Sousa Mata, Á.N.; García, D.Á.; de Carvalho Leitão, J.C.G.; Knackfuss, M.I.; Piuvezam, G. Effects of Exercise on the Levels of BDNF and Executive Function in Adolescents. Medicine 2019, 98, e16445. [Google Scholar] [CrossRef]
- Bekinschtein, P.; Cammarota, M.; Medina, J.H. BDNF and Memory Processing. Neuropharmacology 2014, 76, 677–683. [Google Scholar] [CrossRef]
- Baraldi, T.; Schöwe, N.M.; Balthazar, J.; Monteiro-Silva, K.C.; Albuquerque, M.S.; Buck, H.S.; Viel, T.A. Cognitive Stimulation during Lifetime and in the Aged Phase Improved Spatial Memory, and Altered Neuroplasticity and Cholinergic Markers of Mice. Exp. Gerontol. 2013, 48, 831–838. [Google Scholar] [CrossRef]
- Quialheiro, A.; Bobinski, F.; Haefliger, J.d.G.; Del Antonio, R.; Lins, E.F.; Martins, D.F.; d’Orsi, E.; Xavier, A.J.; Peres, M.A. A Comprehensive Program of Cognitive Stimulation with Digital Inclusion, Physical Activity and Social Interaction Can Modify BDNF Levels and Improve Cognition in Adults over 50: A Randomized Controlled Pilot Study. Aging Ment. Health 2022, 26, 1979–1987. [Google Scholar] [CrossRef]
- Lu, Y.; Sareddy, G.R.; Wang, J.; Wang, R.; Li, Y.; Dong, Y.; Zhang, Q.; Liu, J.; O’Connor, J.C.; Xu, J.; et al. Neuron-Derived Estrogen Regulates Synaptic Plasticity and Memory. J. Neurosci. 2019, 39, 2792–2809. [Google Scholar] [CrossRef] [PubMed]
- Stoffel-Wagner, B.; Watzka, M.; Schramm, J.; Bidlingmaier, F.; Klingmüller, D. Expression of CYP19 (Aromatase) MRNA in Different Areas of the Human Brain. J. Steroid Biochem. Mol. Biol. 1999, 70, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Underwood, E.A.; Rochon, P.A.; Moineddin, R.; Lee, P.E.; Wu, W.; Pritchard, K.I.; Tierney, M.C. Cognitive Sequelae of Endocrine Therapy in Women Treated for Breast Cancer: A Meta-Analysis. Breast Cancer Res. Treat. 2018, 168, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Tuscher, J.J.; Szinte, J.S.; Starrett, J.R.; Krentzel, A.A.; Fortress, A.M.; Remage-Healey, L.; Frick, K.M. Inhibition of Local Estrogen Synthesis in the Hippocampus Impairs Hippocampal Memory Consolidation in Ovariectomized Female Mice. Horm. Behav. 2016, 83, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Flegal, K.E.; Ragland, J.D.; Ranganath, C. Adaptive Task Difficulty Influences Neural Plasticity and Transfer of Training. NeuroImage 2019, 188, 111–121. [Google Scholar] [CrossRef]
- Belleville, S.; Clément, F.; Mellah, S.; Gilbert, B.; Fontaine, F.; Gauthier, S. Training-Related Brain Plasticity in Subjects at Risk of Developing Alzheimer’s Disease. Brain 2011, 134, 1623–1634. [Google Scholar] [CrossRef]
- Mahncke, H.W.; Bronstone, A.; Merzenich, M.M. Brain Plasticity and Functional Losses in the Aged: Scientific Bases for a Novel Intervention. In Progress in Brain Research; Møller, A.R., Ed.; Reprogramming of the Brain; Elsevier: Amsterdam, The Netherlands, 2006; Volume 157, pp. 81–109. [Google Scholar]
- Pieramico, V.; Esposito, R.; Cesinaro, S.; Frazzini, V.; Sensi, S.L. Effects of Non-Pharmacological or Pharmacological Interventions on Cognition and Brain Plasticity of Aging Individuals. Front. Syst. Neurosci. 2014, 8, 153. [Google Scholar] [CrossRef]
- Smith, G.E.; Housen, P.; Yaffe, K.; Ruff, R.; Kennison, R.F.; Mahncke, H.W.; Zelinski, E.M. A Cognitive Training Program Based on Principles of Brain Plasticity: Results from the Improvement in Memory with Plasticity-Based Adaptive Cognitive Training (IMPACT) Study. J. Am. Geriatr. Soc. 2009, 57, 594–603. [Google Scholar] [CrossRef]
- Tapia, J.L.; Duñabeitia, J.A. Improving Language Acquisition and Processing With Cognitive Stimulation. Front. Psychol. 2021, 12, 663773. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.L.; Duñabeitia, J.A. Digital Therapeutics for Insomnia: Assessing the Effectiveness of a Computerized Home-Based Cognitive Stimulation Program. J. Integr. Neurosci. 2022, 22, 34. [Google Scholar] [CrossRef]
- Genevsky, A.; Garrett, C.T.; Alexander, P.P.; Vinogradov, S. Cognitive Training in Schizophrenia: A Neuroscience-Based Approach. Dialogues Clin. Neurosci. 2010, 12, 416–421. [Google Scholar] [CrossRef]
- Motter, J.N.; Pimontel, M.A.; Rindskopf, D.; Devanand, D.P.; Doraiswamy, P.M.; Sneed, J.R. Computerized Cognitive Training and Functional Recovery in Major Depressive Disorder: A Meta-Analysis. J. Affect. Disord. 2016, 189, 184–191. [Google Scholar] [CrossRef]
- Lampit, A.; Heine, J.; Finke, C.; Barnett, M.H.; Valenzuela, M.; Wolf, A.; Leung, I.H.K.; Hill, N.T.M. Computerized Cognitive Training in Multiple Sclerosis: A Systematic Review and Meta-Analysis. Neurorehabil. Neural. Repair. 2019, 33, 695–706. [Google Scholar] [CrossRef]
- Thompson, P.J.; Conn, H.; Baxendale, S.A.; Donnachie, E.; McGrath, K.; Geraldi, C.; Duncan, J.S. Optimizing Memory Function in Temporal Lobe Epilepsy. Seizure 2016, 38, 68–74. [Google Scholar] [CrossRef]
- Spector, A.; Woods, B.; Orrell, M. Cognitive Stimulation for the Treatment of Alzheimer’s Disease. Expert Rev. Neurother. 2008, 8, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Binarelli, G.; Joly, F.; Tron, L.; Lefevre Arbogast, S.; Lange, M. Management of Cancer-Related Cognitive Impairment: A Systematic Review of Computerized Cognitive Stimulation and Computerized Physical Activity. Cancers 2021, 13, 5161. [Google Scholar] [CrossRef]
- Torous, J.; Lipschitz, J.; Ng, M.; Firth, J. Dropout Rates in Clinical Trials of Smartphone Apps for Depressive Symptoms: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2020, 263, 413–419. [Google Scholar] [CrossRef]
- Lawlor-Savage, L.; Goghari, V.M. Working Memory Training in Schizophrenia and Healthy Populations. Behav. Sci. 2014, 4, 301–319. [Google Scholar] [CrossRef] [PubMed]
- Reijnders, J.; van Heugten, C.; van Boxtel, M. Cognitive Interventions in Healthy Older Adults and People with Mild Cognitive Impairment: A Systematic Review. Ageing Res. Rev. 2013, 12, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Spreij, L.A.; Visser-Meily, J.M.A.; van Heugten, C.M.; Nijboer, T.C.W. Novel Insights into the Rehabilitation of Memory Post Acquired Brain Injury: A Systematic Review. Front. Hum. Neurosci. 2014, 8, 993. [Google Scholar] [CrossRef]
- Whitney, J.; Easter, A.; Tchanturia, K. Service Users’ Feedback on Cognitive Training in the Treatment of Anorexia Nervosa: A Qualitative Study. Int. J. Eat. Disord. 2008, 41, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Haimov, I.; Shatil, E. Cognitive Training Improves Sleep Quality and Cognitive Function among Older Adults with Insomnia. PLoS ONE 2013, 8, e61390. [Google Scholar] [CrossRef]
- Ferguson, R.J.; Ahles, T.A.; Saykin, A.J.; McDonald, B.C.; Furstenberg, C.T.; Cole, B.F.; Mott, L.A. Cognitive-Behavioral Management of Chemotherapy-Related Cognitive Change. Psycho-Oncology 2007, 16, 772–777. [Google Scholar] [CrossRef]
- Becker, H.; Henneghan, A.M.; Volker, D.L.; Mikan, S.Q. A Pilot Study of a Cognitive-Behavioral Intervention for Breast Cancer Survivors. Oncol. Nurs. Forum. 2017, 44, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Abbadessa, G.; Brigo, F.; Clerico, M.; De Mercanti, S.; Trojsi, F.; Tedeschi, G.; Bonavita, S.; Lavorgna, L. Digital Therapeutics in Neurology. J. Neurol. 2022, 269, 1209–1224. [Google Scholar] [CrossRef]
- Torous, J.; Myrick, K.J.; Rauseo-Ricupero, N.; Firth, J. Digital Mental Health and COVID-19: Using Technology Today to Accelerate the Curve on Access and Quality Tomorrow. JMIR Ment. Health 2020, 7, e18848. [Google Scholar] [CrossRef]
- Webster, P. Virtual Health Care in the Era of COVID-19. Lancet 2020, 395, 1180–1181. [Google Scholar] [CrossRef] [PubMed]
- Duñabeitia, J.A.; Mera, F.; Baro, Ó.; Jadad-Garcia, T.; Jadad, A.R. Personalized Computerized Training for Cognitive Dysfunction after COVID-19: A Before-and-After Feasibility Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 3100. [Google Scholar] [CrossRef]
- Barak, A.; Hen, L.; Boniel-Nissim, M.; Shapira, N. A Comprehensive Review and a Meta-Analysis of the Effectiveness of Internet-Based Psychotherapeutic Interventions. J. Technol. Hum. Serv. 2008, 26, 109–160. [Google Scholar] [CrossRef]
- Triberti, S.; Savioni, L.; Sebri, V.; Pravettoni, G. EHealth for Improving Quality of Life in Breast Cancer Patients: A Systematic Review. Cancer Treat. Rev. 2019, 74, 1–14. [Google Scholar] [CrossRef]
- Vermeir, J.F.; White, M.J.; Johnson, D.; Crombez, G.; Ryckeghem, D.M.L.V. The Effects of Gamification on Computerized Cognitive Training: Systematic Review and Meta-Analysis. JMIR Serious Games 2020, 8, e18644. [Google Scholar] [CrossRef]
- Lin, Y.; Shih, W.J. Statistical Properties of the Traditional Algorithm-based Designs for Phase I Cancer Clinical Trials. Biostatistics 2001, 2, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, S.; Fisher, M.; de Villers-Sidani, E. Cognitive Training for Impaired Neural Systems in Neuropsychiatric Illness. Neuropsychopharmacol 2012, 37, 43–76. [Google Scholar] [CrossRef]
- Tetlow, A.M.; Edwards, J.D. Systematic Literature Review and Meta-Analysis of Commercially Available Computerized Cognitive Training Among Older Adults. J. Cogn. Enhanc. 2017, 1, 559–575. [Google Scholar] [CrossRef]
- Test y Juegos; Version 4.4.1; CogniFit Inc.: San Francisco, CA, USA, 2022.
- de Vries, S.T.; Mol, P.G.M.; de Zeeuw, D.; Haaijer-Ruskamp, F.M.; Denig, P. Development and Initial Validation of a Patient-Reported Adverse Drug Event Questionnaire. Drug Saf. 2013, 36, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Andreu Vaillo, Y.; Murgui Pérez, S.; Martínez López, P.; Romero Retes, R. Mini-Mental Adjustment to Cancer Scale: Construct Validation in Spanish Breast Cancer Patients. J. Psychosom. Res. 2018, 114, 38–44. [Google Scholar] [CrossRef]
- Wagner, L.I.; Sweet, J.; Butt, Z.; Lai, J.; Cella, D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy–Cognitive Function Instrument. J. Support Oncol. 2009, 7, W32–W39. [Google Scholar]
- Mendoza, T.R.; Wang, X.S.; Cleeland, C.S.; Morrissey, M.; Johnson, B.A.; Wendt, J.K.; Huber, S.L. The Rapid Assessment of Fatigue Severity in Cancer Patients. Cancer 1999, 85, 1186–1196. [Google Scholar] [CrossRef]
- Beck, A.; Steer, R.; Brown, G. Manual for the Beck Depression Inventory–II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.; Vagg, P.R.; Jacobs, G.A. Manual for the State-Trait Anxiety Inventory; Consulting Psychologists Press: Palo Alto, CA, USA, 1983. [Google Scholar] [CrossRef]
- The WHOQOL GROUP. Development of the World Health Organization WHOQOL-BREF Quality of Life Assessment. Psychol. Med. 1998, 28, 551–558. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R.; RStudio, PBC.: Boston, MA, USA, 2020. [Google Scholar]
- JASP; Version 0.17; JASP Team: Amsterdam, The Netherlands, 2023.
- Woods, B.; Rai, H.K.; Elliott, E.; Aguirre, E.; Orrell, M.; Spector, A. Cognitive Stimulation to Improve Cognitive Functioning in People with Dementia. Cochrane Database Syst. Rev. 2023, 1, 1–4. [Google Scholar] [CrossRef]
- Saragih, I.D.; Tonapa, S.I.; Saragih, I.S.; Lee, B.-O. Effects of Cognitive Stimulation Therapy for People with Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. Int. J. Nurs. Stud. 2022, 128, 104181. [Google Scholar] [CrossRef]
- Cafferata, R.M.T.; Hicks, B.; von Bastian, C.C. Effectiveness of Cognitive Stimulation for Dementia: A Systematic Review and Meta-Analysis. Psychol. Bull. 2021, 147, 455–476. [Google Scholar] [CrossRef] [PubMed]
- LeMoult, J.; Gotlib, I.H. Depression: A Cognitive Perspective. Clin. Psychol. Rev. 2019, 69, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Perini, G.; Cotta Ramusino, M.; Sinforiani, E.; Bernini, S.; Petrachi, R.; Costa, A. Cognitive Impairment in Depression: Recent Advances and Novel Treatments. Neuropsychiatr. Dis. Treat. 2019, 15, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Semkovska, M.; Quinlivan, L.; O’Grady, T.; Johnson, R.; Collins, A.; O’Connor, J.; Knittle, H.; Ahern, E.; Gload, T. Cognitive Function Following a Major Depressive Episode: A Systematic Review and Meta-Analysis. Lancet Psychiatry 2019, 6, 851–861. [Google Scholar] [CrossRef]
- Koster, E.H.W.; Hoorelbeke, K.; Onraedt, T.; Owens, M.; Derakshan, N. Cognitive Control Interventions for Depression: A Systematic Review of Findings from Training Studies. Clin. Psychol. Rev. 2017, 53, 79–92. [Google Scholar] [CrossRef]
- Moreno, A.; Wall, K.J.; Thangavelu, K.; Craven, L.; Ward, E.; Dissanayaka, N.N. A Systematic Review of the Use of Virtual Reality and Its Effects on Cognition in Individuals with Neurocognitive Disorders. Alzheimer Dement. Transl. Res. Clin. Interv. 2019, 5, 834–850. [Google Scholar] [CrossRef]
- da Cruz, G.P.; Pereira, L.S.; Raymundo, T.M. Cognitive Training for Elderly People without Cognitive Impairment: An Occupational Therapy Intervention during the COVID-19 Pandemic. Cad. Bras. Ter. Ocup. 2022, 30, 1–18. [Google Scholar] [CrossRef]
- Jang, H.; Yeo, M.; Cho, J.; Kim, S.; Chin, J.; Kim, H.J.; Seo, S.W.; Na, D.L. Effects of Smartphone Application-Based Cognitive Training at Home on Cognition in Community-Dwelling Non-Demented Elderly Individuals: A Randomized Controlled Trial. Alzheimer Dement. Transl. Res. Clin. Interv. 2021, 7, e12209. [Google Scholar] [CrossRef]
- Gibbor, L.; Yates, L.; Volkmer, A.; Spector, A. Cognitive Stimulation Therapy (CST) for Dementia: A Systematic Review of Qualitative Research. Aging Ment. Health 2021, 25, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.; Hadi Hosseini, S.M.; Heckler, C.; Janelsins, M.; Palesh, O.; Mustian, K.; Morrow, G. Cognitive Training for Improving Executive Function in Chemotherapy-Treated Breast Cancer Survivors. Clin. Breast Cancer 2013, 13, 299–306. [Google Scholar] [CrossRef]
- Damholdt, M.; Mehlsen, M.; O’Toole, M.; Andreasen, R.; Pedersen, A.; Zachariae, R. Web-Based Cognitive Training for Breast Cancer Survivors with Cognitive Complaints—A Randomized Controlled Trial. Psycho-Oncology 2016, 25, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Von Ah, D.; McDonald, B.C.; Crouch, A.D.; Ofner, S.; Perkins, S.; Storey, S.; Considine, R.; Unverzagt, F. Randomized Double-Masked Controlled Trial of Cognitive Training in Breast Cancer Survivors: A Preliminary Study. Support Care Cancer 2022, 30, 7457–7467. [Google Scholar] [CrossRef]
- Sala, G.; Gobet, F. Cognitive Training Does Not Enhance General Cognition. Trends Cogn. Sci. 2019, 23, 9–20. [Google Scholar] [CrossRef]
- Von Ah, D.; Carpenter, J.S.; Saykin, A.; Monahan, P.; Wu, J.; Yu, M.; Rebok, G.; Ball, K.; Schneider, B.; Weaver, M.; et al. Advanced Cognitive Training for Breast Cancer Survivors: A Randomized Controlled Trial. Breast Cancer Res. Treat. 2012, 135, 799–809. [Google Scholar] [CrossRef]
- Ferguson, R.J.; McDonald, B.C.; Rocque, M.A.; Furstenberg, C.T.; Horrigan, S.; Ahles, T.A.; Saykin, A.J. Development of CBT for Chemotherapy-Related Cognitive Change: Results of a Waitlist Control Trial. Psychooncology 2012, 21, 176–186. [Google Scholar] [CrossRef]
- Zokaei, N.; MacKellar, C.; Čepukaitytė, G.; Patai, E.Z.; Nobre, A.C. Cognitive Training in the Elderly: Bottlenecks and New Avenues. J. Cogn. Neurosci. 2017, 29, 1473–1482. [Google Scholar] [CrossRef]
- Seiler, A.; Jenewein, J. Resilience in Cancer Patients. Front. Psychiatry 2019, 10, 208. [Google Scholar] [CrossRef]
- Niveau, N.; New, B.; Beaudoin, M. How Should Self-Esteem Be Considered in Cancer Patients? Front. Psychol. 2021, 12, 763900. [Google Scholar] [CrossRef] [PubMed]
- Macía, P.; Barranco, M.; Gorbeña, S.; Iraurgi, I. Expression of Resilience, Coping and Quality of Life in People with Cancer. PLoS ONE 2020, 15, e0236572. [Google Scholar] [CrossRef] [PubMed]
- Ośmiałowska, E.; Misiąg, W.; Chabowski, M.; Jankowska-Polańska, B. Coping Strategies, Pain, and Quality of Life in Patients with Breast Cancer. J. Clin. Med. 2021, 10, 4469. [Google Scholar] [CrossRef] [PubMed]
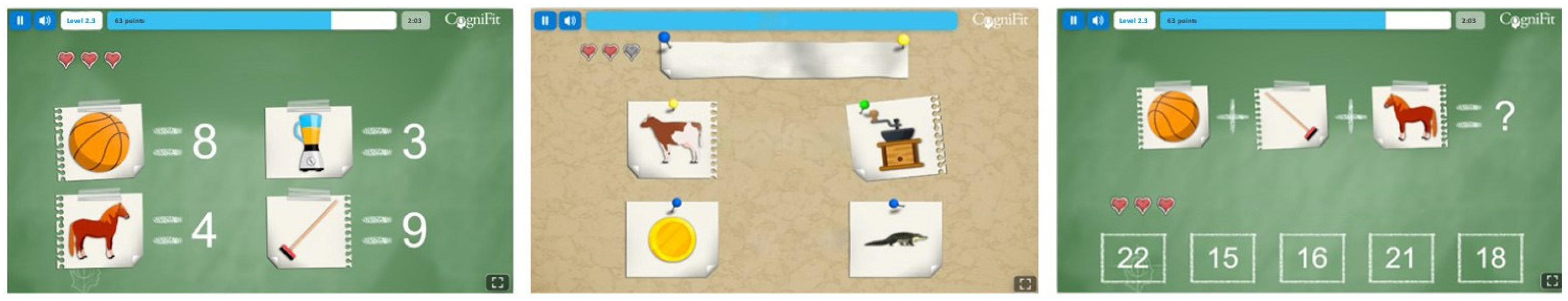
| Age | Phase Participation | Marital Status | Education | Employment |
|---|---|---|---|---|
| 64 | Phase I | Divorced | High School | Retired |
| 54 | Phase I | Married | General education | Active worker |
| 37 | Phase I | Married | General education | Active worker |
| 65 | Phase II | Married | General education | Retired |
| 47 | Phase II | Married | Bachelor’s Degree | Active worker |
| 47 | Phase II | Single | General education | Sick leave |
| 38 | Phase II | Married | High School | Unemployed |
| 48 | Phase II | Married | Bachelor’s Degree | Active worker |
| 67 | Phase II | Widow | General education | Retired |
| 66 | Phase II | Married | High School | Retired |
| 42 | Phase II | Married | General education | Active worker |
| 54 | Phase II | Married | General education | Sick leave |
| 33 | Phase II | Married | Bachelor’s Degree | Sick leave |
| Cycle-I | Cycle-II | Cycle-III | Cycle-IV | |
|---|---|---|---|---|
| Participant 1 | 2 | 3 | 5 | 9 |
| Participant 2 | 1 | 4 | 7 | 9 |
| Participant 3 | 3 | 5 | 7 | 10 |
| Mean (SD) Pre-Evaluation | Mean (SD) Post-Evaluation | |
|---|---|---|
| Mini-MAC | 84.50 (6.11) | 86.90 (6.17) |
| FACT-COG | 80.30 (25.31) | 86.10 (38.15) |
| BFI | 21.00 (12.98) | 17.60 (11.74) |
| STAI | 45.90 (5.08) | 43.40 (5.33) |
| BDI-II | 36.20 (6.77) | 31.80 (6.90) |
| WHOQOL-BREF | 90.80 (9.41) | 91.40 (8.93) |
| CAB-CF | 325.70 (123.37) | 567.20 (96.38) |
| 95% CI for Mean Difference | |||||||
|---|---|---|---|---|---|---|---|
| t | p | Mean Difference | SE Difference | Lower | Upper | Cohen’s d | |
| Mini-MAC | 2.44 | 0.037 * | −2.40 | 0.98 | −4.61 | −0.18 | −0.77 |
| FACT-COG | 0.86 | 0.411 | −5.80 | 6.72 | −21.00 | 9.40 | −0.27 |
| BFI | 1.15 | 0.278 | 3.40 | 2.94 | −3.26 | 10.06 | 0.36 |
| STAI | 1.95 | 0.082 | 2.50 | 1.27 | −0.38 | 5.38 | 0.62 |
| BDI-II | 2.52 | 0.032 * | 4.40 | 1.74 | 0.46 | 8.33 | 0.80 |
| WHOQOL-BREF | 0.21 | 0.838 | −0.60 | 2.84 | −7.04 | 5.84 | −0.06 |
| CAB-CF | 7.11 | <0.001 *** | −241.50 | 33.95 | −318.30 | −164.69 | −2.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, J.L.; Taberner-Bonastre, M.T.; Collado-Martínez, D.; Pouptsis, A.; Núñez-Abad, M.; Duñabeitia, J.A. Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment. Int. J. Environ. Res. Public Health 2023, 20, 4953. https://doi.org/10.3390/ijerph20064953
Tapia JL, Taberner-Bonastre MT, Collado-Martínez D, Pouptsis A, Núñez-Abad M, Duñabeitia JA. Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment. International Journal of Environmental Research and Public Health. 2023; 20(6):4953. https://doi.org/10.3390/ijerph20064953
Chicago/Turabian StyleTapia, Jose L., María Teresa Taberner-Bonastre, David Collado-Martínez, Athanasios Pouptsis, Martín Núñez-Abad, and Jon Andoni Duñabeitia. 2023. "Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment" International Journal of Environmental Research and Public Health 20, no. 6: 4953. https://doi.org/10.3390/ijerph20064953
APA StyleTapia, J. L., Taberner-Bonastre, M. T., Collado-Martínez, D., Pouptsis, A., Núñez-Abad, M., & Duñabeitia, J. A. (2023). Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment. International Journal of Environmental Research and Public Health, 20(6), 4953. https://doi.org/10.3390/ijerph20064953









